A Long-Term Assessment of Nitrogen Removal Performance and Microecosystem Evolution in Bioretention Columns Modified with Sponge Iron
Abstract
:1. Introduction
2. Materials and Methods
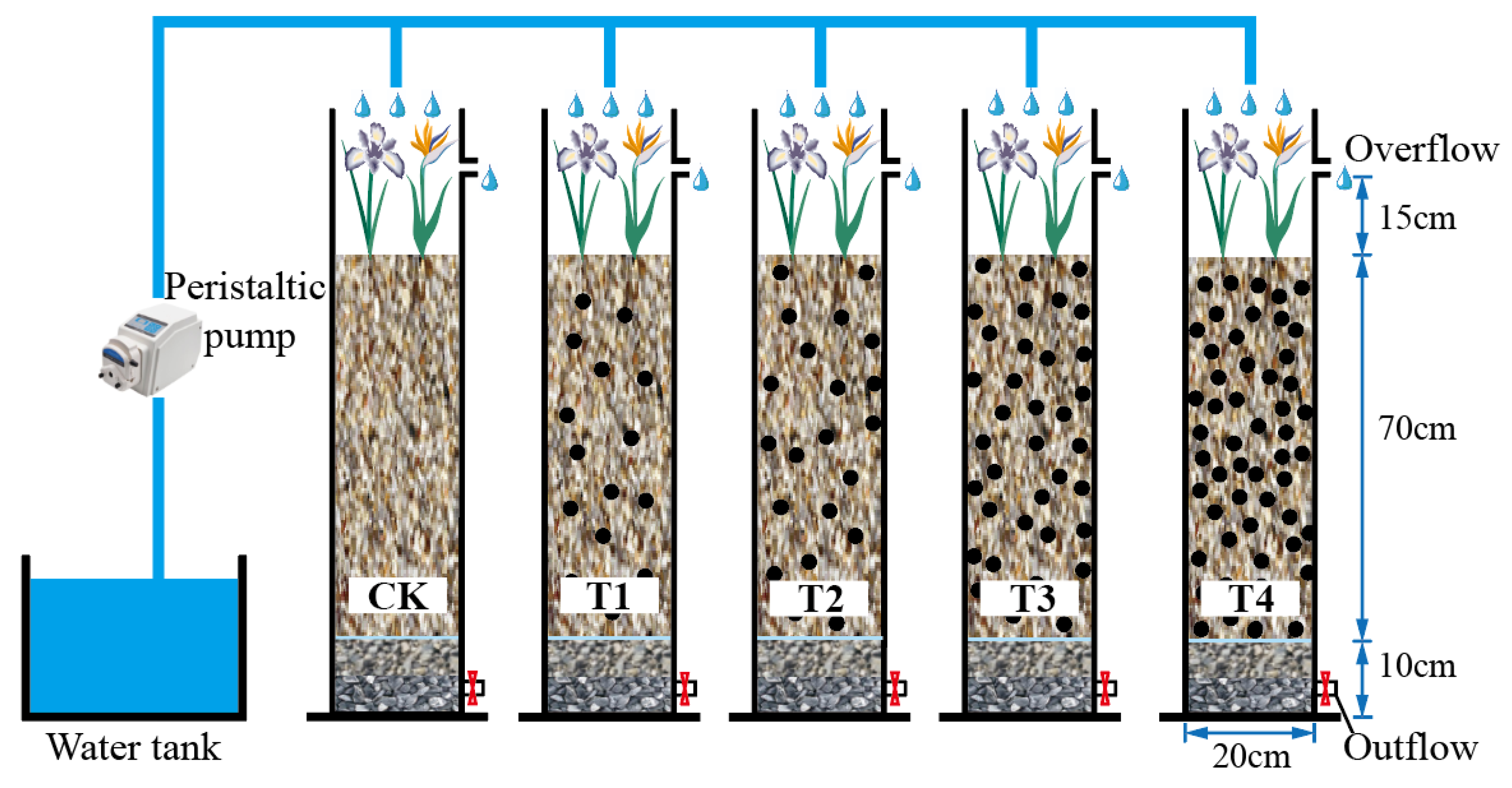
2.1. Experimental Device
2.2. Experimental Scheme
2.3. Sample Analysis
3. Results
3.1. Nitrogen Removal Performance
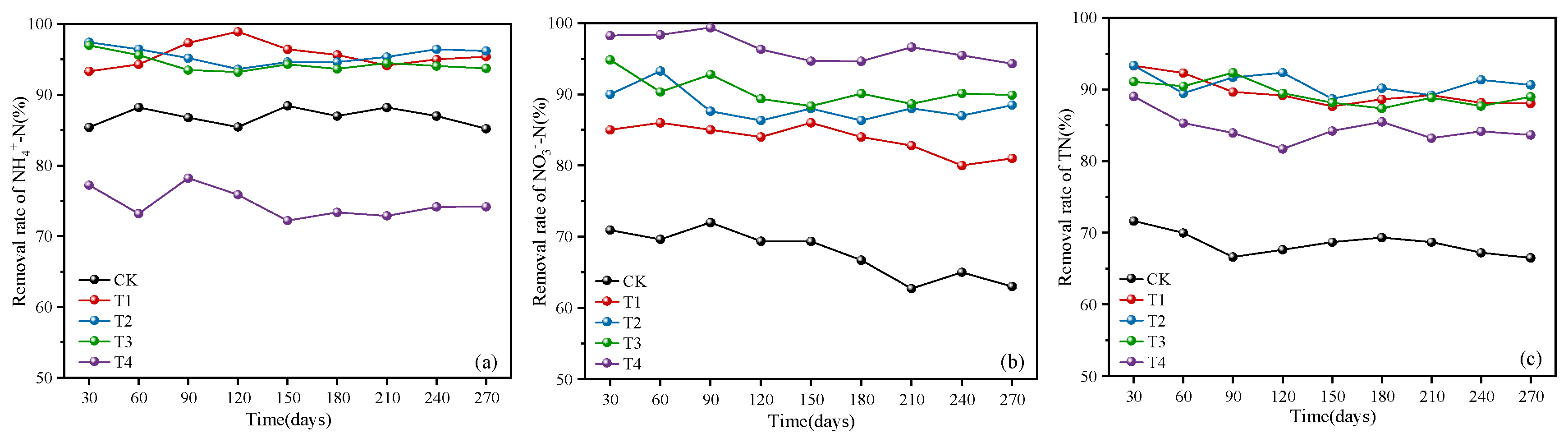
3.1.1. Removal of Ammonia Nitrogen, Nitrate Nitrogen, and Total Nitrogen
3.1.2. Denitrification Efficiency
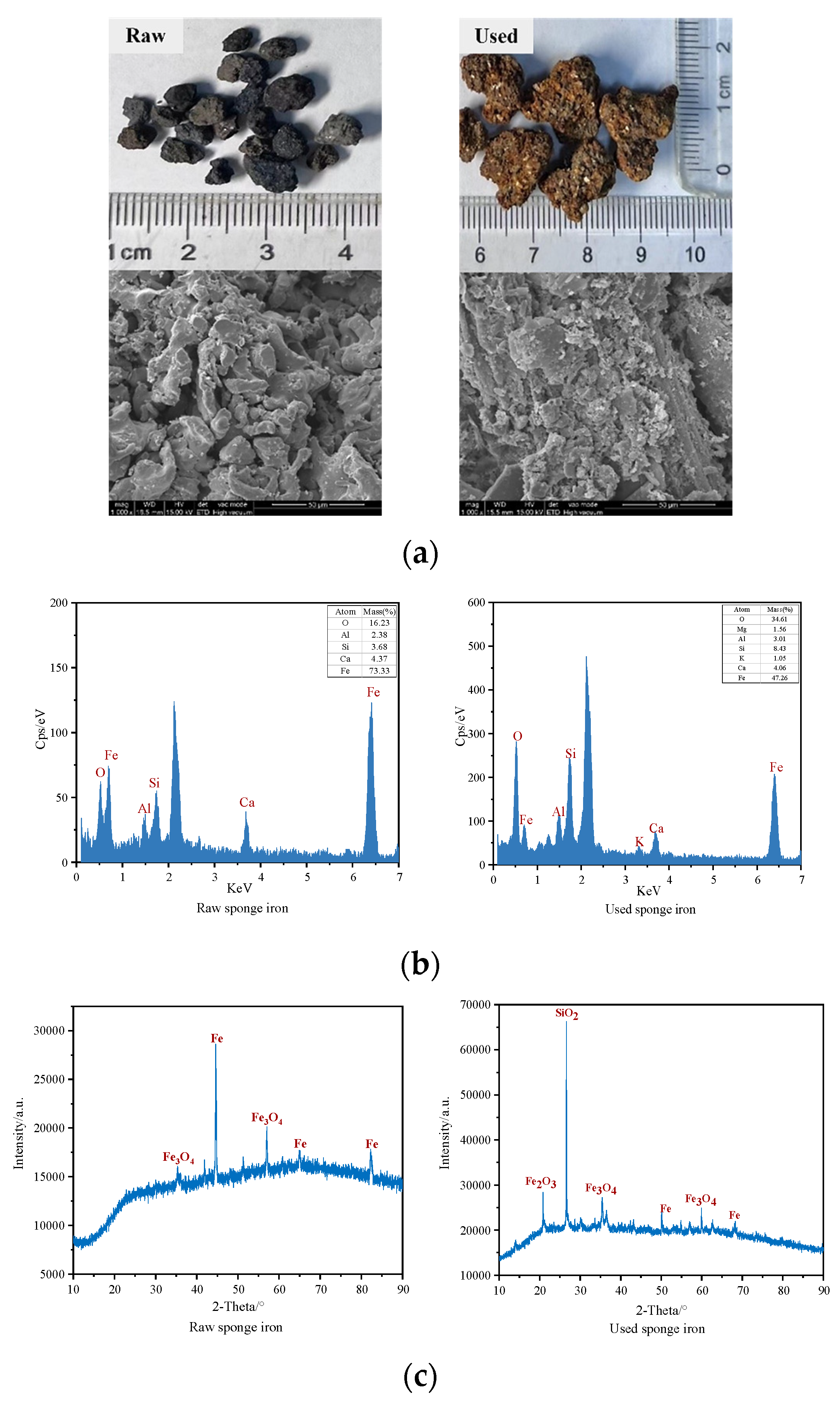
3.2. Changes in Surface Morphology and Element Composition of Sponge Iron
3.3. Changes in Plants’ Physiological Characteristics
3.3.1. MDA, H2O2, and Chlorophyll
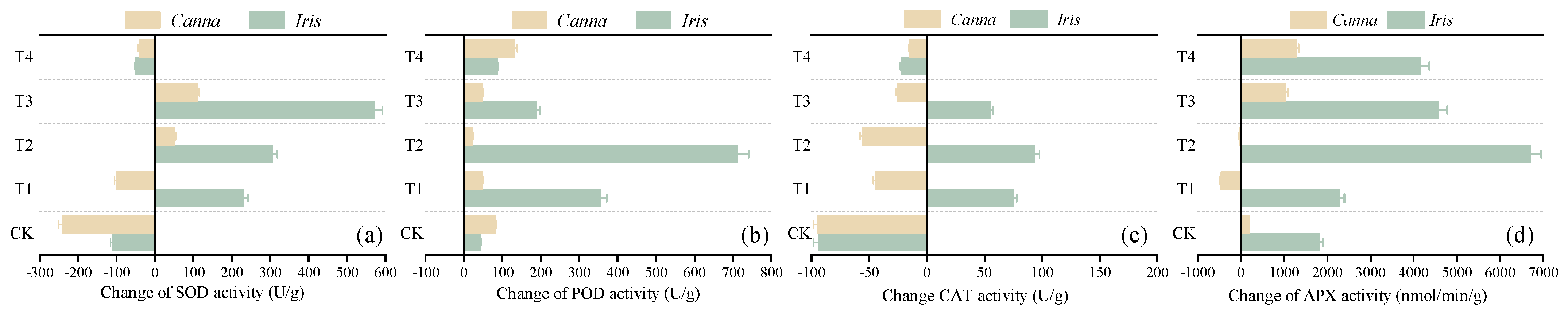
3.3.2. Changes in Enzyme Activity
3.3.3. Accumulation of Iron in Plants
3.4. Changes in Functional Microorganisms for Nitrogen Removal
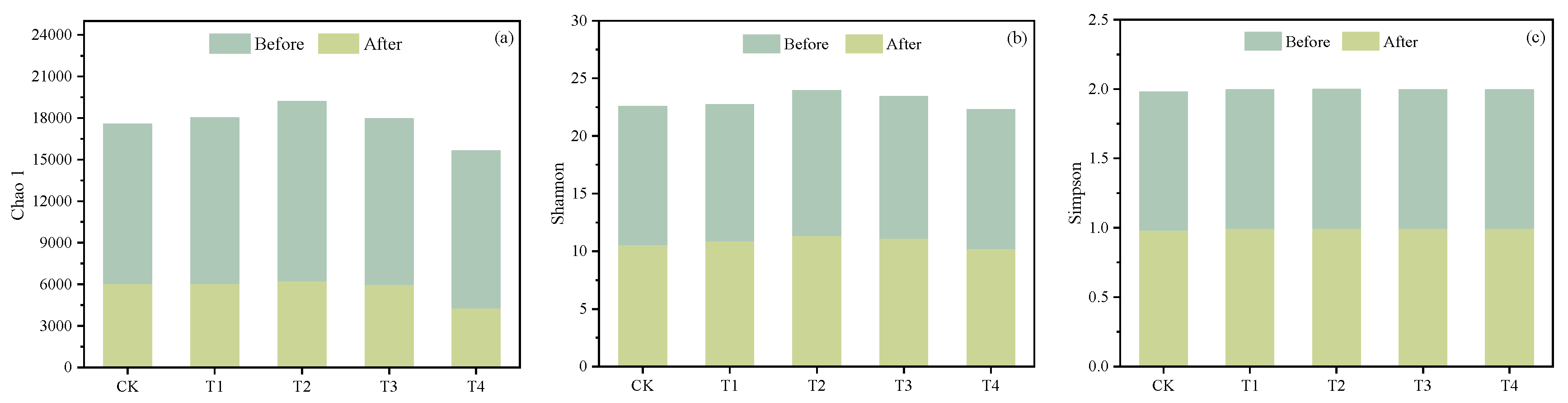
3.4.1. Microbial Diversity
3.4.2. Microbial Community Structure at the Phylum and Genus Levels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tedoldi, D.; Chebbo, G.; Pierlot, D.; Kovacs, Y.; Gromaire, M.C. Impact of runoff infiltration on contaminant accumulation and transport in the soil/filter media of Sustainable Urban Drainage Systems: A literature review. Sci. Total Environ. 2016, 569, 904–926. [Google Scholar] [CrossRef]
- Fu, D.; Pan, T.; Xu, C.; Zhang, J. Control performance of bioretention system on non-point source pollution in typical Chinese rural areas of Southeastern China. Ecol. Eng. 2023, 190, 106934. [Google Scholar] [CrossRef]
- He, Q.; Lin, Z.; Dong, P.; Tang, W. Decontamination performance of a bioretention system using a simple sand-based filler proportioning method. Environ. Technol. 2022, 43, 709–717. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Liu, J.; Zhang, L.; Li, G.; Zhang, Z.; Gong, Y.; Li, H.; Li, J. Coal gangue modified bioretention system for runoff pollutants removal and the biological characteristics. J. Environ. Manag. 2022, 314, 115044. [Google Scholar] [CrossRef]
- Zhang, H.; Ahmad, Z.; Shao, Y.; Yang, Z.; Jia, Y.; Zhong, H. Bioretention for removal of nitrogen: Processes, operational conditions, and strategies for improvement. Environ. Sci. Pollut. Res. 2021, 28, 10519–10535. [Google Scholar] [CrossRef]
- He, Q.; Feng, M.; Lin, Z.; Shi, Q. Experimental Study on the Pollutant Removal Performance and Cleaning Characteristics of Six Sand-Based Bioretention Systems. J. Environ. Eng. 2022, 148, 04022055. [Google Scholar] [CrossRef]
- Palmer, E.T.; Poor, C.J.; Hinman, C.; Stark, J.D. Nitrate and phosphate removal through enhanced bioretention media: Mesocosm study. Water Environ. Res. 2013, 85, 823–832. [Google Scholar] [CrossRef]
- Chen, J.; Xie, Y.; Sun, S.; Zhang, M.; Yan, P.; Xu, F.; Tang, L.; He, S. Efficient nitrogen removal through coupling biochar with zero-valent iron by different packing modes in bioretention system. Environ. Res. 2023, 223, 115375. [Google Scholar] [CrossRef]
- Si, Z.; Song, X.; Wang, Y.; Cao, X.; Wang, Y.; Zhao, Y.; Ge, X.; Sand, W. Untangling the nitrate removal pathways for a constructed wetland-sponge iron coupled system and the impacts of sponge iron on a wetland ecosystem. J. Hazard. Mater. 2020, 393, 122407. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Z.; Zhang, Z.; Yang, Y.; Xu, X. Kinetics of nitrate reductive denitrification by nanoscale zero-valent iron. Process Saf. Environ. 2010, 88, 439–445. [Google Scholar] [CrossRef]
- Kiskira, K.; Papirio, S.; Van Hullebusch, E.; Esposito, G. Fe(II)-mediated autotrophic denitrification: A new bioprocess for iron bioprecipitation/biorecovery and simultaneous treatment of nitrate-containing wastewaters. Int. Biodeterior. Biodegrad. 2017, 119, 631–648. [Google Scholar] [CrossRef]
- Liu, J.; Su, J.; Ali, A.; Wang, Z.; Chen, C.; Xu, L. Role of porous polymer carriers and iron-carbon bioreactor combined micro-electrolysis and biological denitrification in efficient removal of nitrate from wastewater under low carbon to nitrogen ratio. Bioresour. Technol. 2021, 321, 124447. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Hou, J.; Wang, P.; You, G.; Miao, L.; Lv, B.; Yang, Y.; Zhang, F. Application of zero valent iron coupling with biological process for wastewater treatment: A review. Rev. Environ. Sci. Bio/Technol. 2017, 16, 667–693. [Google Scholar] [CrossRef]
- Ma, B.; Chu, M.; Zhang, H.; Chen, K.; Li, F.; Liu, X.; Kosolapov, D.B.; Zhi, W.; Chen, Z.; Yang, J.; et al. Mixotrophic aerobic denitrification facilitated by denitrifying bacterial-fungal communities assisted with iron in micro-polluted water: Performance, metabolic activity, functional genes abundance, and community co-occurrence. J. Hazard. Mater. 2024, 476, 135057. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dai, W.; Zheng, P.; Zheng, X.; He, S.; Zhao, M. Iron scraps enhance simultaneous nitrogen and phosphorus removal in subsurface flow constructed wetlands. J. Hazard. Mater. 2020, 395, 122612. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, S.; Wang, W.; Meng, L.; Guo, J. Applications of sponge iron and effects of organic carbon source on sulfate-reducing ammonium oxidation process. Int. J. Environ. Res. Public Health 2022, 19, 2283. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Cheng, W.; Wang, M.; Wan, T.; Ren, J.; Lv, T.; Li, Y. Effect of substrate on operation performance of ecological floating bed for treating simulated tailwater from wastewater treatment plant. Chem. Ecol. 2021, 37, 715–728. [Google Scholar] [CrossRef]
- Pereira, E.G.; Oliva, M.A.; Rosado-Souza, L.; Mendes, G.C.; Colares, D.S.; Stopato, C.H.; Almeida, A.M. Iron excess affects rice photosynthesis through stomatal and non-stomatal limitations. Plant Sci. 2013, 201, 81–92. [Google Scholar] [CrossRef]
- Weinand, T.; Asch, J.; Asch, F. Effects of endophytic Bacillus spp. on accumulation and distribution of iron in the shoots of lowland rice grown under iron toxic conditions. J. Plant Nutr. Soil Sci. 2023, 186, 351–363. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Hao, S.; Dou, Q.; Lan, S.; Peng, Y. Anammox-synchronous zero-valent iron oxidation promoting synergistic nitrogen and phosphorus removal from wastewater. Bioresour. Technol. 2022, 347, 126365. [Google Scholar] [CrossRef]
- Li, L.; Davis, A.P. Urban stormwater runoff nitrogen composition and fate in bioretention systems. Environ. Sci. Technol. 2014, 48, 3403–3410. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Z.; Cai, G.; Zhan, J. Experimental study on the selection of common adsorption substrates for extensive green roofs (EGRs). Water Sci. Technol. 2021, 83, 961–974. [Google Scholar] [CrossRef]
- Hu, K.; Li, W.; Mu, H.; Ren, S.; Zhu, H.; Zeng, K.; Wang, B.; Liang, J.; Zhang, Q.; Yang, L.; et al. In-situ anaerobic treatment removes the passivation layer of sponge iron to restore the nitrogen and phosphorus removal performance of SBR. Process Saf. Environ. 2023, 174, 79–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Douglas, G.B.; Kaksonen, A.H.; Cui, L.; Ye, Z. Microbial reduction of nitrate in the presence of zero-valent iron. Sci. Total Environ. 2019, 646, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Roberts, K.L.; Grace, M.R.; Kessler, A.J.; Cook, P.L. Role of organic carbon, nitrate and ferrous iron on the partitioning between denitrification and DNRA in constructed stormwater urban wetlands. Sci. Total Environ. 2019, 666, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Y.; Goto, N.; Fujie, K. Effect of pH on the reduction of nitrite in water by metallic iron. Water Res. 2001, 35, 2789–2793. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, G.; Liu, Y.; Wang, X.F.; Gao, W.; Zhang, S.; You, C. Simple Phenotypic Sensor for Visibly Tracking H2O2 Fluctuation to Detect Plant Health Status. J. Agric. Food Chem. 2022, 70, 10058–10064. [Google Scholar] [CrossRef]
- Wang, X.; Shi, G.; Xu, Q.; Hu, J. Exogenous polyamines enhance copper tolerance of Nymphoides peltatum. J. Plant Physiol. 2007, 164, 1062–1070. [Google Scholar] [CrossRef]
- Prakash, V.; Singh, V.; Tripathi, D.; Sharma, S.; Corpas, F.J. Nitric oxide (NO) and salicylic acid (SA): A framework for their relationship in plant development under abiotic stress. Plant Biol. 2021, 23, 39–49. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, J.; Guo, Y.; Fan, J.; Hu, Z. Physiological responses of Potamogeton crispus to different levels of ammonia nitrogen in constructed wetland. Water Air Soil Pollut. 2016, 227, 65. [Google Scholar] [CrossRef]
- Suthar, S.; Chand, N.; Singh, V. Fate and toxicity of triclosan in tidal flow constructed wetlands amended with cow dung biochar. Chemosphere 2023, 311, 136875. [Google Scholar] [CrossRef] [PubMed]
- Rios, G.R.; Rios, C.O.; de Araújo, T.O.; Siqueira-Silva, A.I.; Pereira, E.G. Iron excess and nitrogen deprivation influence photosynthetic metabolism in grasses used for mineland rehabilitation. Theor. Exp. Plant Physiol. 2023, 35, 427–442. [Google Scholar] [CrossRef]
- Kathuria, H.; Giri, J.; Nataraja, K.N.; Murata, N.; Udayakumar, M.; Tyagi, A.K. Glycinebetaine-induced water-stress tolerance in codA-expressing transgenic indica rice is associated with up-regulation of several stress responsive genes. Plant Biotechnol. J. 2009, 7, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska, D.; Grudkowska, M.; Zagdańska, B. Drought-responsive antioxidant enzymes in potato (Solanum tuberosum L.). Potato Res. 2010, 53, 373–382. [Google Scholar] [CrossRef]
- Aziz, A.; Wahid, A.; Farooq, M. Leaf age and seasonality determines the extent of oxidative stress and induction of antioxidants in lemongrass. Pak. J. Agric. Sci. 2014, 51, 659–664. [Google Scholar]
- Li, M.; Zhang, P.; Adeel, M.; Guo, Z.; Chetwynd, A.J.; Ma, C.; Bai, T.; Hao, Y.; Rui, Y. Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as Fe fertilizers. Environ. Pollut. 2021, 269, 116134. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin increased maize (Zea mays L.) seedling drought tolerance by alleviating drought-induced photosynthetic inhibition and oxidative damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Zhang, S.H.; Yang, Q.; Ma, R.C. Erwinia carotovora ssp. carotovora infection induced “defense lignin” accumulation and lignin biosynthetic gene expression in Chinese cabbage (Brassica rapa L. ssp. pekinensis). J. Integr. Plant Biol. 2007, 49, 993–1002. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Hou, J.; Li, W.; Zhang, W.J. Metabolic adaptations to ammonia-induced oxidative stress in leaves of the submerged macrophyte Vallisneria natans (Lour.) Hara. Aquat. Toxicol. 2008, 87, 88–98. [Google Scholar] [CrossRef]
- Jeyasubramanian, K.; Thoppey, U.U.G.; Hikku, G.S.; Selvakumar, N.; Subramania, A.; Krishnamoorthy, K. Enhancement in growth rate and productivity of spinach grown in hydroponics with iron oxide nanoparticles. RSC Adv. 2016, 6, 15451–15459. [Google Scholar] [CrossRef]
- Thongbai, P.; Goodman, B.A. Free radical generation and post-anoxic injury in rice grown in an iron-toxic soil. J. Plant Nutr. 2000, 23, 1887–1900. [Google Scholar] [CrossRef]
- Ma, J.F.; Nomoto, K. Effective regulation of iron acquisition in graminaceous plants. The role of mugineic acids as phytosiderophores. Physiol. Plant. 2010, 97, 609–617. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, X.; Zhao, Q.; Zhao, T. Plant growth, antioxidative enzyme, and cadmium tolerance responses to cadmium stress in Canna orchioides. Hortic. Plant J. 2021, 7, 256–266. [Google Scholar] [CrossRef]
- Mahender, A.; Swamy, B.P.M.; Anandan, A.; Ali, J. Tolerance of Iron-Deficient and -Toxic Soil Conditions in Rice. Plants 2019, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Li, Y.; Wang, D.; Zhang, J.; Zhao, L. Assessment on the cumulative effect of pollutants and the evolution of micro-ecosystems in bioretention systems with different media. Ecotoxicol. Environ. Saf. 2021, 228, 112957. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Li, Y.; Wang, P.; Wang, C. Background nutrients and bacterial community evolution determine 13C-17β-estradiol mineralization in lake sediment microcosms. Sci. Total Environ. 2019, 651, 2304–2311. [Google Scholar] [CrossRef]
- Ramdat, N.; Wang, Z.J.; Huang, J.C.; Wang, Y.; Chachar, A.; Zhou, C.; Wang, Z. Effects of Enrofloxacin on Nutrient Removal by a Floating Treatment Wetland Planted with Iris pseudacorus: Response and Resilience of Rhizosphere Microbial Communities. Sustainability 2022, 14, 3358. [Google Scholar] [CrossRef]
- Liu, J.; Sample, D.J.; Bell, C.; Guan, Y. Review and research needs of bioretention used for the treatment of urban stormwater. Water 2014, 6, 1069–1099. [Google Scholar] [CrossRef]
- Zuo, X.; Guo, Z.; Wu, X.; Yu, J. Diversity and metabolism effects of microorganisms in bioretention systems with sand, soil and fly ash. Sci. Total Environ. 2019, 676, 447–454. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Biological nitrate removal from water and wastewater by solid-phase denitrification process. Biotechnol. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef]



| Proportion (%) | CK | T1 | T2 | T3 | T4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Before | After | Before | After | Before | After | Before | After | Before | After | |
| Phylum level | Proteobacteria | 36.32 | 30.37 | 27.99 | 25.68 | 31.49 | 32.62 | 37.69 | 28.94 | 42.52 | 26.28 |
| Bacteroidetes | 18.23 | 9.30 | 13.81 | 22.31 | 12.74 | 11.89 | 10.73 | 15.09 | 6.43 | 19.91 | |
| Patescibacteria | 7.69 | 16.42 | 20.76 | 11.73 | 10.93 | 6.27 | 10.99 | 9.17 | 3.68 | 4.12 | |
| Planctomycetes | 6.77 | 9.49 | 6.21 | 8.56 | 5.97 | 8.53 | 6.35 | 9.27 | 7.63 | 9.86 | |
| Acidobacteria | 6.37 | 8.88 | 6.43 | 5.53 | 8.02 | 8.40 | 6.58 | 7.30 | 5.03 | 3.94 | |
| Actinobacteria | 1.72 | 2.86 | 2.44 | 2.79 | 2.78 | 4.85 | 5.16 | 3.01 | 13.82 | 6.85 | |
| Chloroflexi | 3.72 | 6.83 | 3.64 | 6.27 | 4.52 | 8.65 | 3.91 | 9.05 | 4.13 | 4.27 | |
| Firmicutes | 0.58 | 1.61 | 0.55 | 1.76 | 0.94 | 2.24 | 0.62 | 2.73 | 1.24 | 11.61 | |
| Genus level | Sphingorhabdus | 7.09 | - | 0.50 | - | 0.29 | - | 0.82 | - | 0.74 | - |
| Terrimonas | 2.22 | 1.75 | 1.91 | 0.96 | 1.39 | 1.79 | 1.47 | 2.23 | 0.62 | 0.58 | |
| Acidibacter | 1.83 | 1.46 | 0.94 | 1.03 | 0.93 | 0.93 | 0.74 | 0.86 | 0.30 | 0.25 | |
| Nitrospira | 0.62 | 0.45 | 0.78 | 0.52 | 0.90 | 0.92 | 0.95 | 1.87 | 0.71 | 1.18 | |
| Thiobacillus | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.13 | 0.01 | 0.04 | 0.14 | 0.13 | |
| Thauera | 0.02 | 0.21 | 0.13 | 0.08 | 0.01 | 0.32 | 0.06 | 0.19 | 0.09 | 0.65 | |
| Pseudomonas | 0.62 | 0.78 | 0.38 | 0.21 | 0.28 | 0.13 | 0.26 | 0.09 | 0.14 | 0.09 | |
| Dechloromonas | 0.10 | 0.23 | 0.22 | 0.17 | 0.07 | 0.15 | 0.21 | 0.13 | 0.27 | 0.02 | |
| Hydrogenophaga | 0.22 | 0.15 | 0.57 | 0.61 | 0.78 | 0.67 | 1.02 | 0.97 | 0.88 | 0.91 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Shi, Q.; He, Q. A Long-Term Assessment of Nitrogen Removal Performance and Microecosystem Evolution in Bioretention Columns Modified with Sponge Iron. Toxics 2024, 12, 727. https://doi.org/10.3390/toxics12100727
Lin Z, Shi Q, He Q. A Long-Term Assessment of Nitrogen Removal Performance and Microecosystem Evolution in Bioretention Columns Modified with Sponge Iron. Toxics. 2024; 12(10):727. https://doi.org/10.3390/toxics12100727
Chicago/Turabian StyleLin, Zizeng, Qinghuan Shi, and Qiumei He. 2024. "A Long-Term Assessment of Nitrogen Removal Performance and Microecosystem Evolution in Bioretention Columns Modified with Sponge Iron" Toxics 12, no. 10: 727. https://doi.org/10.3390/toxics12100727






