Degradation of Atrazine in Water by Dielectric Barrier Discharge Combined with Periodate Oxidation: Enhanced Performance, Degradation Pathways, and Toxicity Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
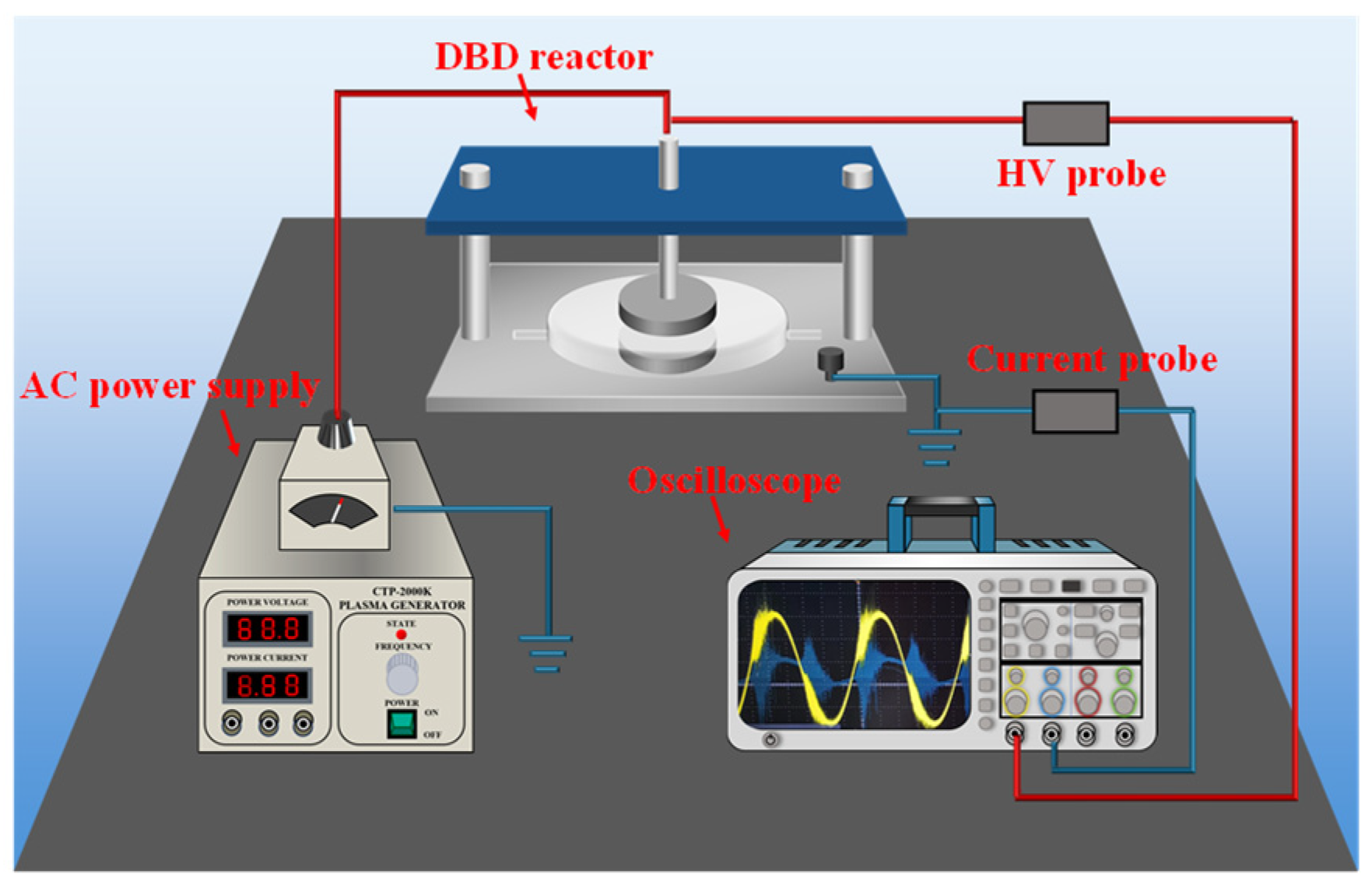
2.2. Experimental Apparatus and Procedure
2.3. Analytical Methods
3. Results and Discussion
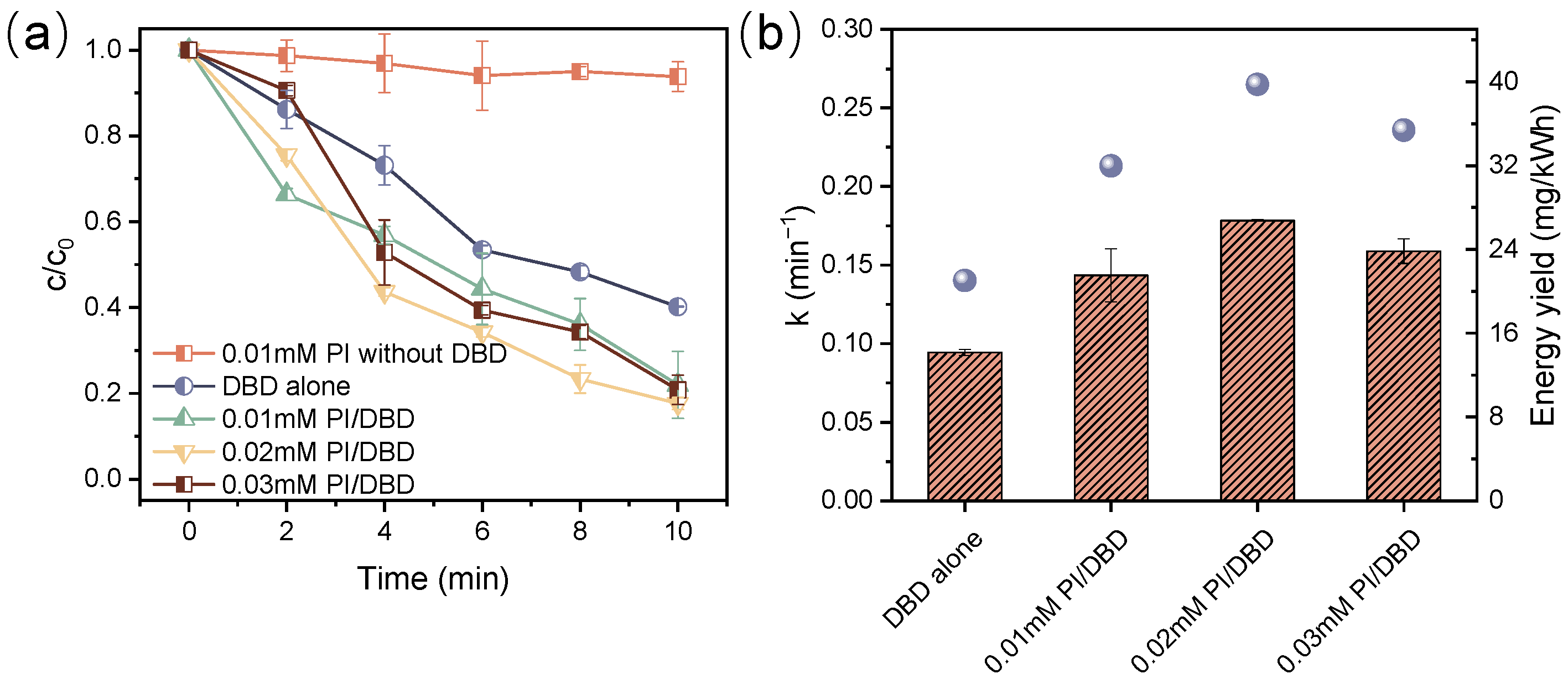
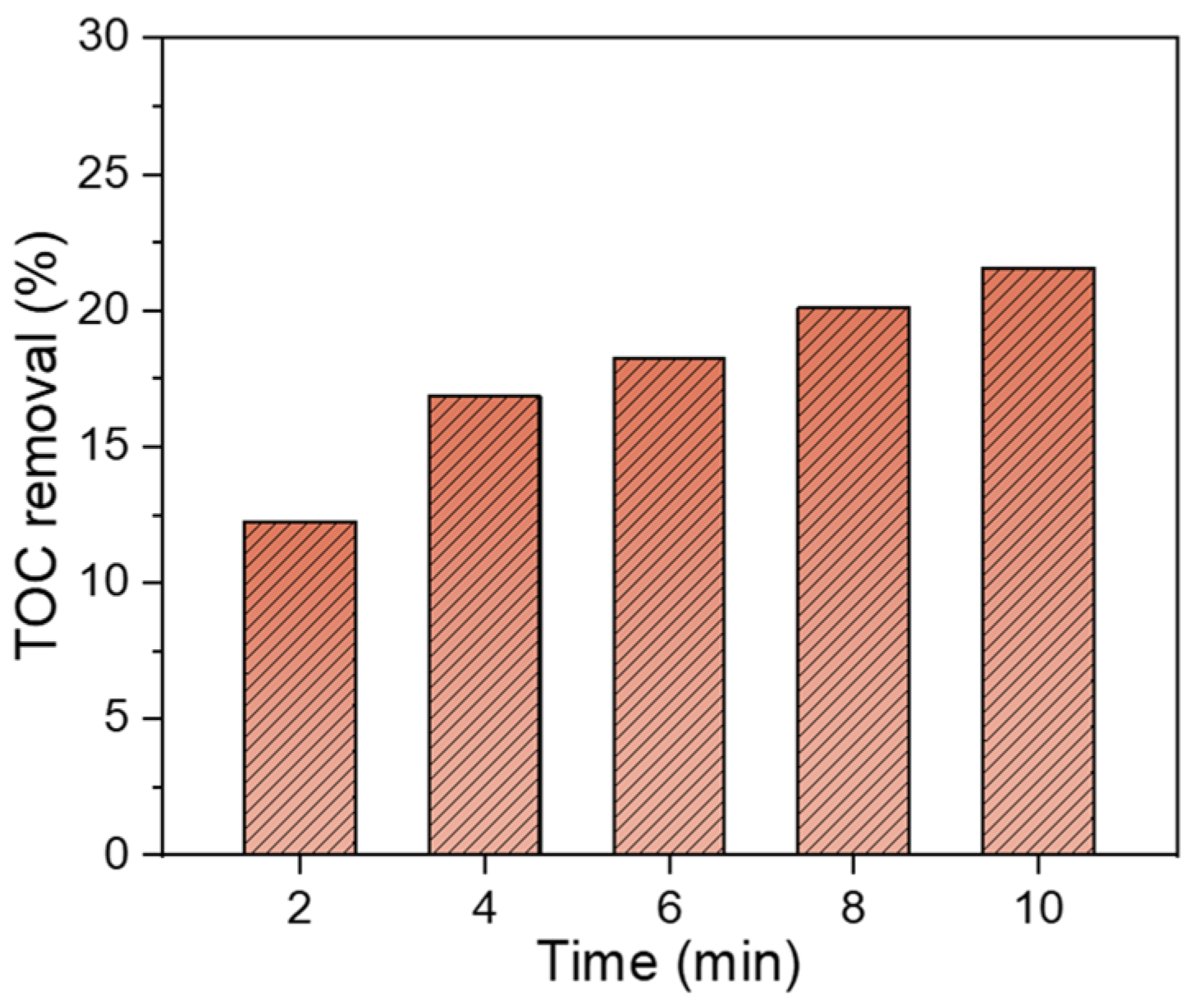
3.1. Decomposition of ATZ by DBD Combined with PI
3.2. Effect of Discharge Power
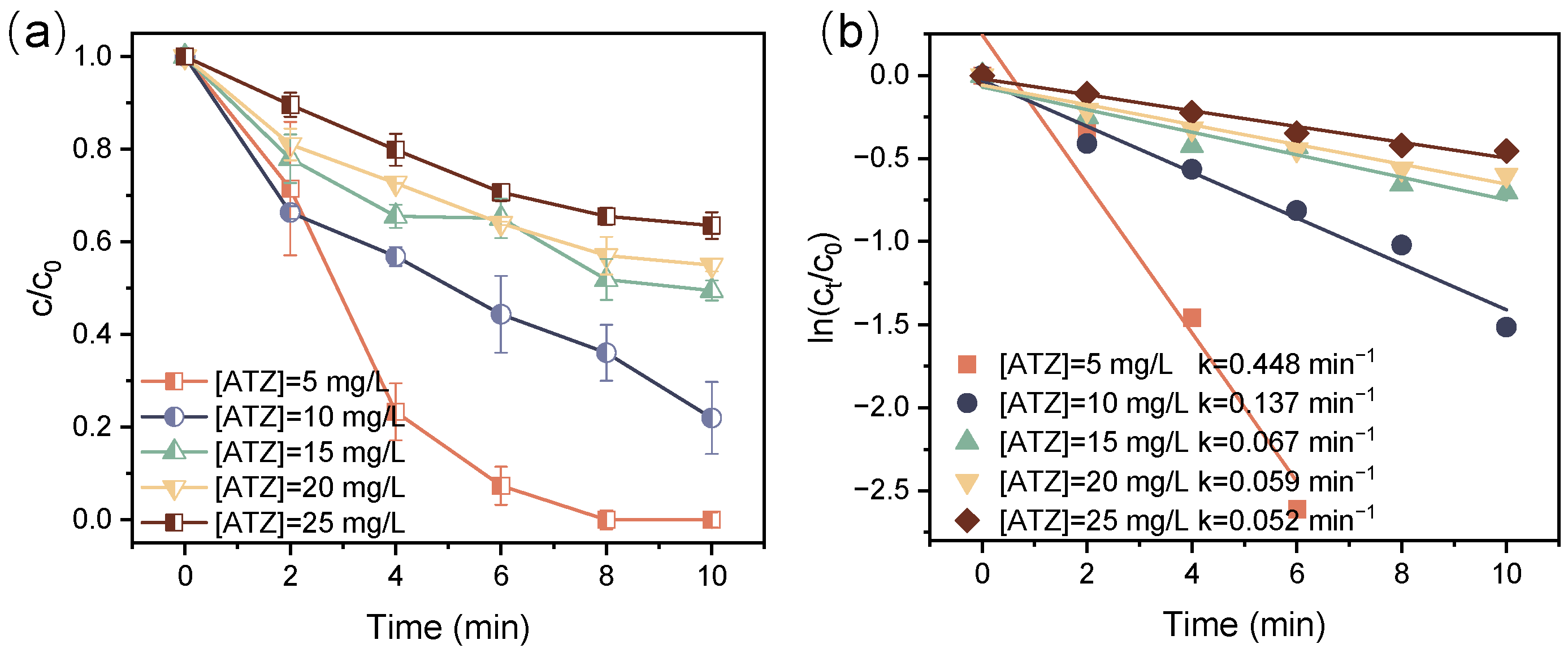
3.3. Effect of Initial ATZ Concentration
3.4. Effect of Solution pH
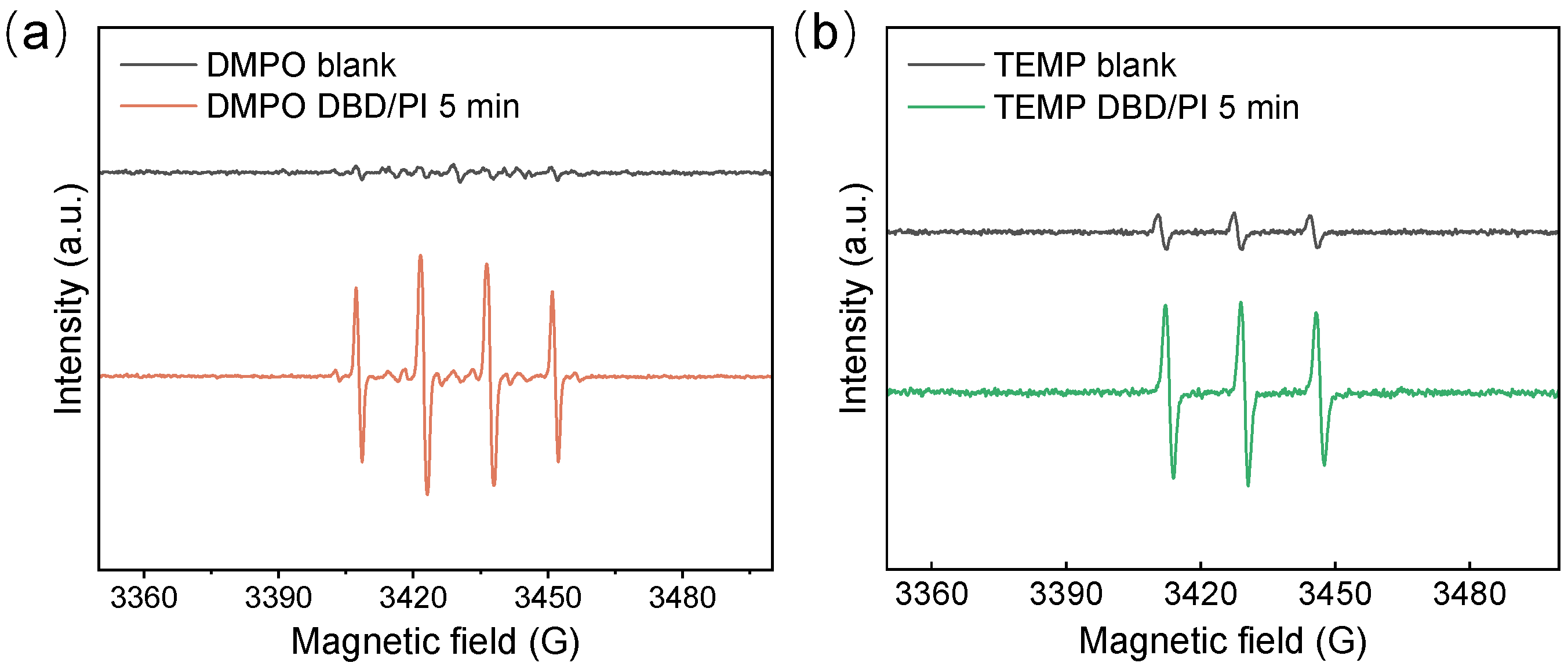
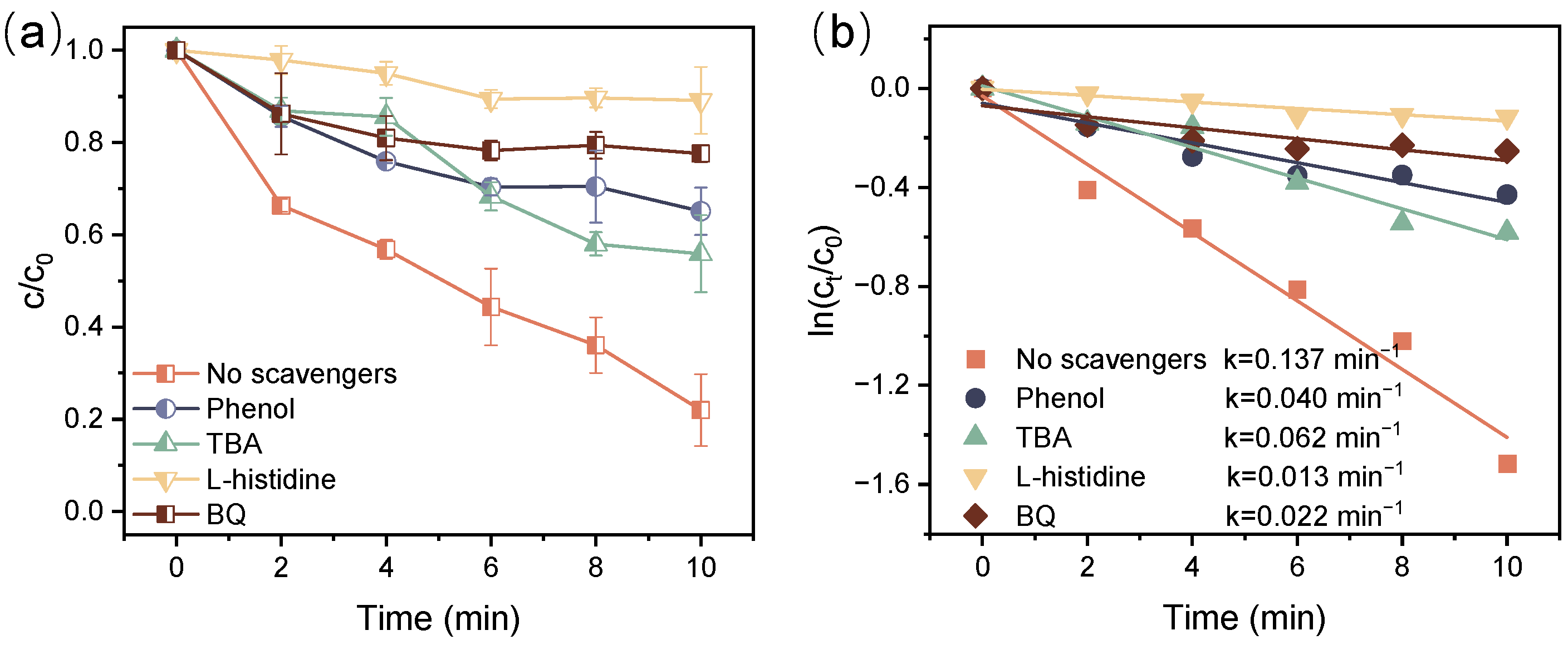
3.5. The Roles of Active Species
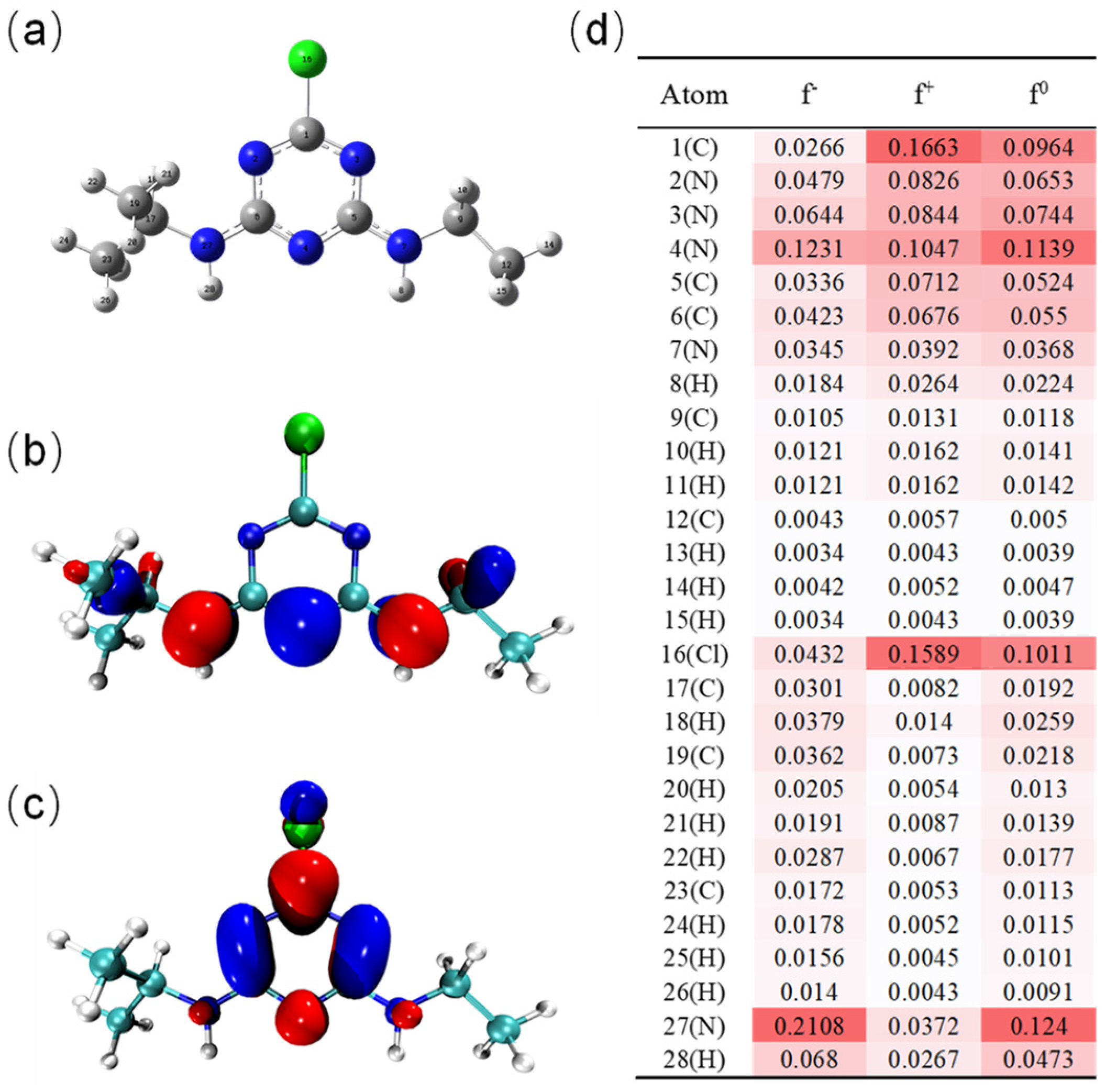
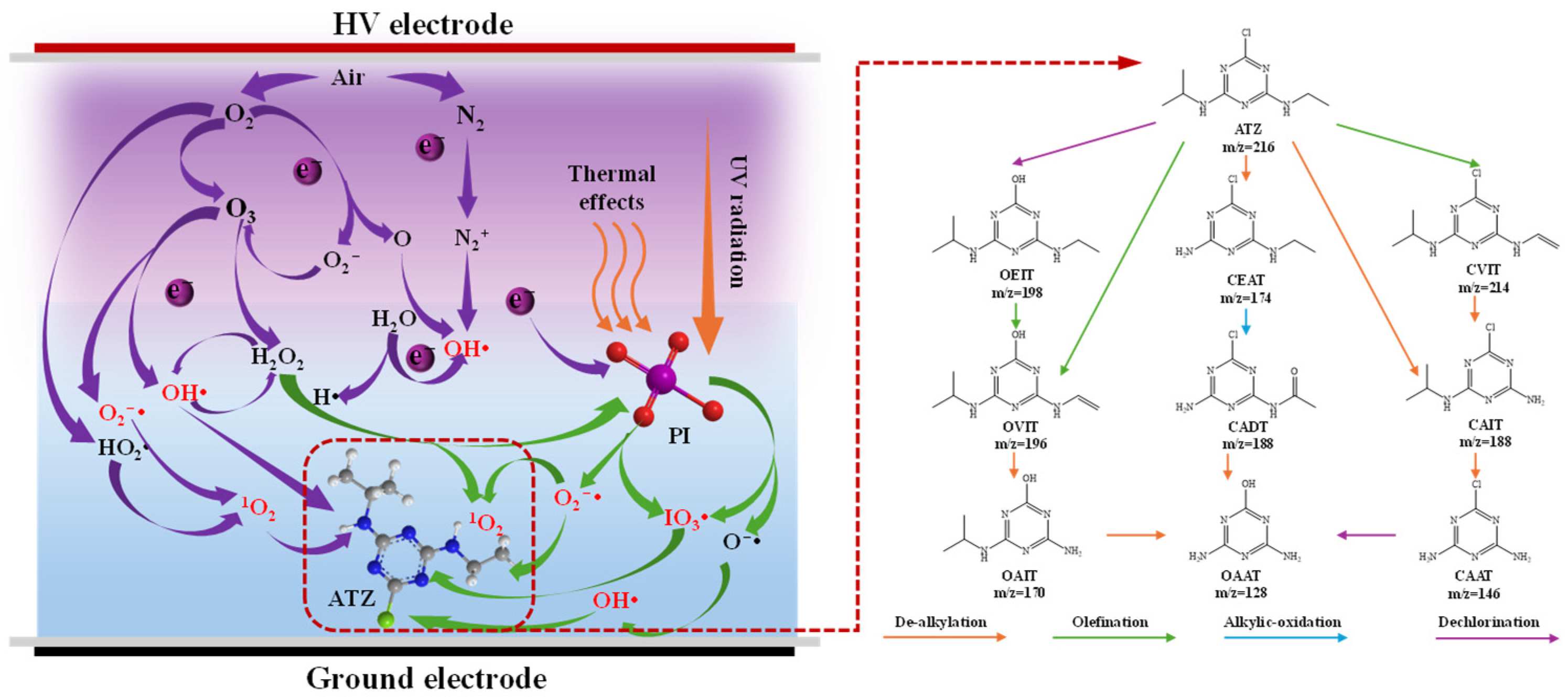
3.6. Degradation Mechanism of ATZ
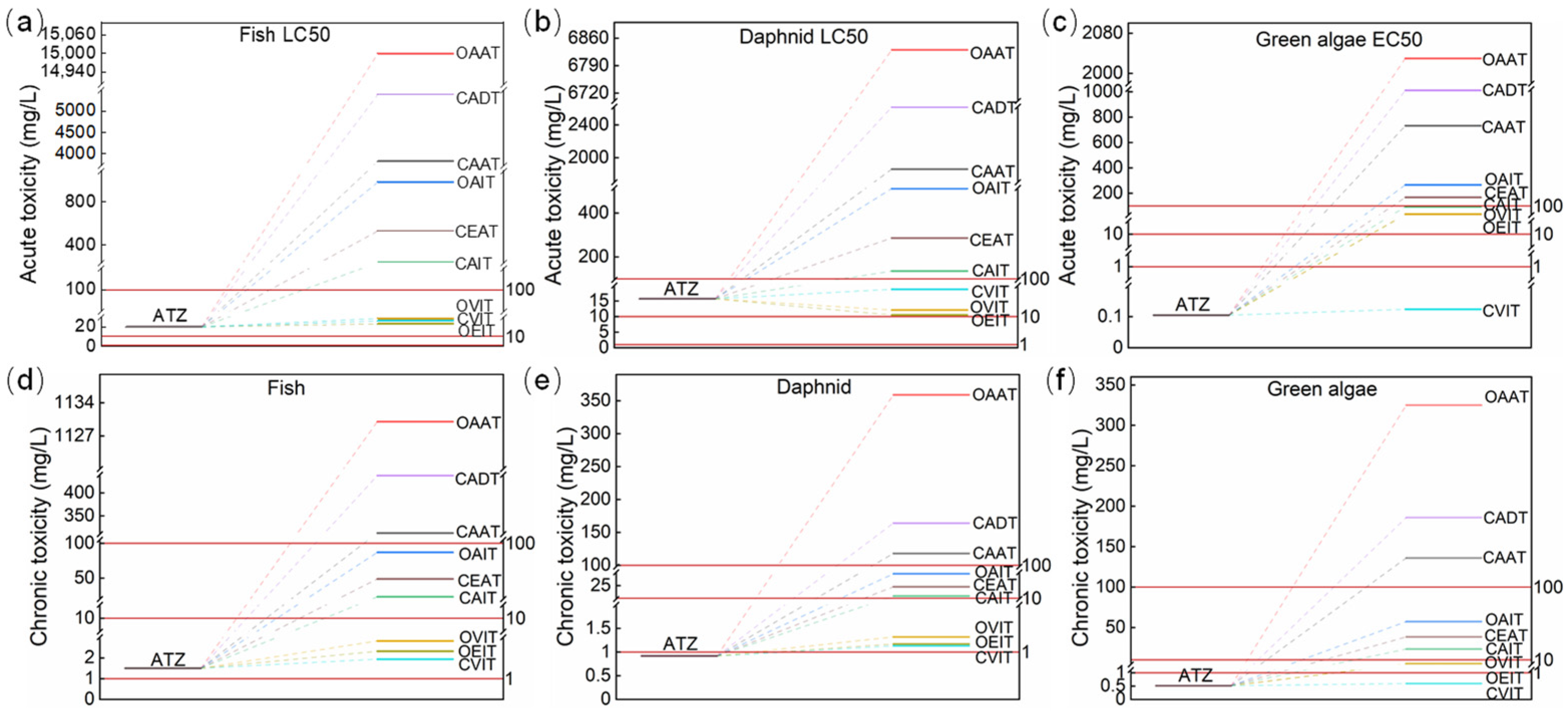
3.7. Toxicity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, S.J.; Chen, C.R.; Wang, Y.; Liu, S.Q.; Zhao, J.Y.; Cao, B.; Jiang, D.; Jiang, Z.; Zhang, Y. Advances in Understanding and Mitigating Atrazine’s Environmental and Health Impact: A Comprehensive Review. J. Environ. Manag. 2024, 365, 121530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Z.; Feng, Y.L.; Wang, W.Z.; Ru, S.G.; Zhao, L.C.; Ma, Y.Q.; Song, X.K.; Liu, L.J.; Wang, J. Pollution Level and Ecological Risk Assessment of Triazine Herbicides in Laizhou Bay and Derivation of Seawater Quality Criteria. J. Hazard. Mater. 2024, 477, 135270. [Google Scholar] [CrossRef] [PubMed]
- Gajendra, G.; Pulimi, M.; Natarajan, C.; Mukherjee, A. Occurrence, Toxicodynamics, and Mechanistic Insights for Atrazine Degradation in the Environment. Water Air Soil Pollut. 2024, 235, 649. [Google Scholar] [CrossRef]
- Stradtman, S.C.; Freeman, J.L. Mechanisms of Neurotoxicity Associated with Exposure to the Herbicide Atrazine. Toxics 2021, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Vahidi-Kolur, R.; Yazdanbakhsh, A.; Hosseini, S.A.; Sheikhmohammadi, A. Photoreduction of Atrazine from Aqueous Solution Using Sulfite/Iodide/UV Process, Degradation, Kinetics and by-Products Pathway. Sci. Rep. 2024, 14, 5217. [Google Scholar] [CrossRef]
- Roy, D.; Neogi, S.; De, S. Visible Light Assisted Activation of Peroxymonosulfate by Bimetallic MOF Based Heterojunction MIL-53(Fe/Co)/CeO2 for Atrazine Degradation: Pivotal Roles of Dual Redox Cycle for Reactive Species Generation. Chem. Eng. J. 2022, 430, 133069. [Google Scholar] [CrossRef]
- Wang, T.C.; Qu, G.Z.; Yin, X.Q.; Sun, Q.H.; Liang, D.L.; Guo, X.T.; Jia, H.Z. Dimethyl Phthalate Elimination from Micro-Polluted Source Water by Surface Discharge Plasma: Performance, Active Species Roles and Mechanisms. J. Hazard. Mater. 2018, 357, 279–288. [Google Scholar] [CrossRef]
- Chu, W.; Chan, K.H.; Kwan, C.Y.; Choi, K.Y. Degradation of Atrazine by Modified Stepwise-Fenton’s Processes. Chemosphere 2007, 67, 755–761. [Google Scholar] [CrossRef]
- Chan, K.H.; Chu, W. Model Applications and Mechanism Study on the Degradation of Atrazine by Fenton’s System. J. Hazard. Mater. 2005, 118, 227–237. [Google Scholar] [CrossRef]
- Poonia, K.; Hasija, V.; Singh, P.; Parwaz Khan, A.A.; Thakur, S.; Thakur, V.K.; Mukherjee, S.; Ahamad, T.; Alshehri, S.M.; Raizada, P. Photocatalytic Degradation Aspects of Atrazine in Water: Enhancement Strategies and Mechanistic Insights. J. Cleaner Prod. 2022, 367, 133087. [Google Scholar] [CrossRef]
- Zhu, M.; Dong, B.; Yu, Y.H.; Bu, L.J.; Deng, J.; Zhou, S.Q. Heterogeneous Catalysis of Ozone Using Ordered Mesoporous Fe3O4 for Degradation of Atrazine. Chem. Eng. J. 2017, 328, 527–535. [Google Scholar] [CrossRef]
- Saylor, G.L.; Zhao, C.; Kupferle, M.J. Synergistic Enhancement of Oxidative Degradation of Atrazine Using Combined Electrolysis and Ozonation. J. Water Process Eng. 2018, 21, 154–162. [Google Scholar] [CrossRef]
- Yang, Z.C.; Yu, A.Q.; Shan, C.; Gao, G.D.; Pan, B.C. Enhanced Fe(III)-Mediated Fenton Oxidation of Atrazine in the Presence of Functionalized Multi-Walled Carbon Nanotubes. Water Res. 2018, 137, 37–46. [Google Scholar] [CrossRef] [PubMed]
- He, H.J.; Liu, Y.P.; You, S.H.; Liu, J.; Xiao, H.; Tu, Z.H. A Review on Recent Treatment Technology for Herbicide Atrazine in Contaminated Environment. Int. J. Environ. Res. Public Health 2019, 16, 5129. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, X.Y.; Shen, C.J.; Wang, Y.; Li, Z.; Cao, B.; Wang, S. Non-Thermal Plasma Degradation of Dye Wastewater Assisted by Reverse Osmosis Process through Interfacial Mass Transfer Enhancement. Chem. Eng. Sci. 2023, 282, 119221. [Google Scholar] [CrossRef]
- Magureanu, M.; Bilea, F.; Bradu, C.; Hong, D. A Review on Non-Thermal Plasma Treatment of Water Contaminated with Antibiotics. J. Hazard. Mater. 2021, 417, 125481. [Google Scholar] [CrossRef] [PubMed]
- Aggelopoulos, C.A.; Meropoulis, S.; Hatzisymeon, M.; Lada, Z.G.; Rassias, G. Degradation of Antibiotic Enrofloxacin in Water by Gas-Liquid Nsp-DBD Plasma: Parametric Analysis, Effect of H2O2 and CaO2 Additives and Exploration of Degradation Mechanisms. Chem. Eng. J. 2020, 398, 125622. [Google Scholar] [CrossRef]
- Huang, J.W.; Han, J.G.; Guo, H. Fe2+/Fe3+ Cycle Promoting Hydroxylamine Activation in Dielectric Barrier Discharge System for Efficient Degradation of Antibiotics: Insight into Performance and Activation Mechanism. Sep. Purif. Technol. 2024, 355, 129709. [Google Scholar] [CrossRef]
- Jiang, W.X.; Zhang, J.W.; Guo, H. Sulfite Activation by Non-Thermal Plasma Coupled with Fe2+ for Ibuprofen Degradation: In-Depth Insight into Activation Energy Barrier and Mechanism. Sep. Purif. Technol. 2024, 351, 128042. [Google Scholar] [CrossRef]
- Shen, T.Y.; Wang, X.J.; Xu, P.; Yang, C.Y.; Li, J.Q.; Wang, P.; Zhang, G.S. Effect of Dielectric Barrier Discharge Plasma on Persulfate Activation for Rapid Degradation of Atrazine: Optimization, Mechanism and Energy Consumption. Environ. Res. 2022, 212, 113287. [Google Scholar] [CrossRef]
- Wang, B.W.; Zhang, Y.J.; Wang, Y. Synergistic Degradation Levofloxacin through Dielectric Barrier Discharge and Sodium Persulfate. J. Environ. Chem. Eng. 2023, 11, 111158. [Google Scholar] [CrossRef]
- Wang, Y.W.; Xiang, L.R.; Li, Z.; Han, J.G.; Guo, H. Sulfite Activation by Water Film Dielectric Barrier Discharge Plasma for Ibuprofen Degradation: Efficiency, Comparison of Persulfate, Mechanism, Active Substances Dominant to Pathway, and Toxicity Evaluation. Sep. Purif. Technol. 2024, 330, 125531. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; He, J.L.; Luo, Y.X.; Clement Miruka, A.; Zhang, A.; Liu, Y.N. Synergistic Performance and Mechanisms of Plasma and Peracetic Acid for Antibiotic Degradation in Water. Chem. Eng. J. 2024, 498, 155096. [Google Scholar] [CrossRef]
- Yang, Y.X.; Wang, Y.C.; Jiang, W.X.; Guo, H. Efficient Degradation of Bisphenol A by a Novel Ternary Synergistic Dielectric Barrier Discharge Plasma Advanced Oxidation Process: The Role of Peracetic Acid and Ferrous Ions. Sep. Purif. Technol. 2025, 354, 129568. [Google Scholar] [CrossRef]
- Li, R.X.; Wang, J.Q.; Wu, H.; Zhu, Z.Y.; Guo, H.G. Periodate Activation for Degradation of Organic Contaminants: Processes, Performance and Mechanism. Sep. Purif. Technol. 2022, 292, 120928. [Google Scholar] [CrossRef]
- Zhang, K.T.; Zhang, S.Q.; Ye, C.S.; Ou, R.W.; Zeng, H.B.; Yu, X.; Feng, M.B. Sunlight-Activated Periodate Oxidation: A Novel and Versatile Strategy for Highly Efficient Water Decontamination. Chem. Eng. J. 2023, 451, 138642. [Google Scholar] [CrossRef]
- Choi, Y.J.; Yoon, H.-I.; Lee, C.H.; Vetráková, L.; Heger, D.; Kim, K.; Kim, J.W. Activation of Periodate by Freezing for the Degradation of Aqueous Organic Pollutants. Environ. Sci. Technol. 2018, 52, 5378–5385. [Google Scholar] [CrossRef]
- Kayan, B.; Gözmen, B.; Demirel, M.; Gizir, A.M. Degradation of Acid Red 97 Dye in Aqueous Medium Using Wet Oxidation and Electro-Fenton Techniques. J. Hazard. Mater. 2010, 177, 95–102. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W.Y. Singlet-Oxygen Generation in Alkaline Periodate Solution. Environ. Sci. Technol. 2015, 49, 14392–14400. [Google Scholar] [CrossRef]
- Chen, T.S.; Sun, Y.K.; Dong, H.Y.; Chen, J.; Yu, Y.H.; Ao, Z.M.; Guan, X.H. Understanding the Importance of Periodate Species in the pH-Dependent Degradation of Organic Contaminants in the H2O2/Periodate Process. Environ. Sci. Technol. 2022, 56, 10372–10380. [Google Scholar] [CrossRef]
- Bendjama, H.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M. Efficient Degradation Method of Emerging Organic Pollutants in Marine Environment Using UV/Periodate Process: Case of Chlorazol Black. Mar. Pollut. Bull. 2018, 126, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Ghodbane, H.; Hamdaoui, O. Degradation of Anthraquinonic Dye in Water by Photoactivated Periodate. Desalin. Water Treat. 2016, 57, 4100–4109. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Merouani, S. Improvement of Sonochemical Degradation of Brilliant Blue R in Water Using Periodate Ions: Implication of Iodine Radicals in the Oxidation Process. Ultrason. Sonochem. 2017, 37, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Chen, M.-J.; Huang, C.-P.; Kuo, J.; Lo, S.-L. Efficient Sonochemical Degradation of Perfluorooctanoic Acid Using Periodate. Ultrason. Sonochem. 2016, 31, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.J.; An, L.L.; Zhang, K.T.; Chen, Q.; Yu, X.; Zhang, M.L.; Feng, M.B. Synergistic Oxidation of Organic Micropollutants by Mn(VII)/Periodate System: Performance and Mechanism. Sep. Purif. Technol. 2022, 297, 121554. [Google Scholar] [CrossRef]
- Zong, Y.; Shao, Y.F.; Zeng, Y.; Shao, B.B.; Xu, L.; Zhao, Z.Y.; Liu, W.; Wu, D.L. Enhanced Oxidation of Organic Contaminants by Iron(II)-Activated Periodate: The Significance of High-Valent Iron–Oxo Species. Environ. Sci. Technol. 2021, 55, 7634–7642. [Google Scholar] [CrossRef]
- Liu, Y.N.; Wang, C.H.; Shen, X.; Zhang, A.; Yan, S.W.; Li, X.; Miruka, A.C.; Wu, S.M.; Guo, Y.; Ognier, S. Degradation of Glucocorticoids in Aqueous Solution by Dielectric Barrier Discharge: Kinetics, Mechanisms, and Degradation Pathways. Chem. Eng. J. 2019, 374, 412–428. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.02; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Melnikov, F.; Kostal, J.; Voutchkova-Kostal, A.; Zimmerman, J.B.; Anastas, P.T. Assessment of Predictive Models for Estimating the Acute Aquatic Toxicity of Organic Chemicals. Green Chem. 2016, 18, 4432–4445. [Google Scholar] [CrossRef]
- Sukhatskiy, Y.; Shepida, M.; Sozanskyi, M.; Znak, Z.; Gogate, P.R. Periodate-Based Advanced Oxidation Processes for Wastewater Treatment: A Review. Sep. Purif. Technol. 2023, 304, 122305. [Google Scholar] [CrossRef]
- Puyang, C.D.; Han, J.G.; Guo, H. Degradation of Emerging Contaminants in Water by a Novel Non-Thermal Plasma/Periodate Advanced Oxidation Process: Performance and Mechanisms. Chem. Eng. J. 2024, 483, 149194. [Google Scholar] [CrossRef]
- Yang, L.; He, L.; Ma, Y.F.; Wu, L.; Zheng, L.; Wang, J.; Chen, Y.L.; Li, Y.L.; Zhang, Z.L. Periodate-Based Oxidation Focusing on Activation, Multivariate-Controlled Performance and Mechanisms for Water Treatment and Purification. Sep. Purif. Technol. 2022, 289, 120746. [Google Scholar] [CrossRef]
- Wang, X.J.; Wang, P.; Liu, X.M.; Hu, L.M.; Wang, Q.; Xu, P.; Zhang, G.S. Enhanced Degradation of PFOA in Water by Dielectric Barrier Discharge Plasma in a Coaxial Cylindrical Structure with the Assistance of Peroxymonosulfate. Chem. Eng. J. 2020, 389, 124381. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, L.; Yu, Z.B.; Wang, L.; Xiang, G.L.; Wan, S.G. Synergistic Degradation Performance and Mechanism of 17β-Estradiol by Dielectric Barrier Discharge Non-Thermal Plasma Combined with Pt–TiO2. Sep. Purif. Technol. 2015, 152, 46–54. [Google Scholar] [CrossRef]
- Cheng, J.S.; Fan, Y.Y.; Pei, X.Y.; Tian, D.; Liu, Z.W.; Yang, L.Z.; Feng, E.; Ji, H.F.; Chen, Q. An Energy Efficient Process for Degrading Perfluorooctanoic Acid (PFOA) Using Strip Fountain Dielectric Barrier Discharge Plasma. Water 2022, 14, 2420. [Google Scholar] [CrossRef]
- Cao, M.H.; Xu, P.; Shi, F.; Li, G.; Zhang, X.X.; Qi, K.X.; Zheng, Q.Z.; Qu, J.H.; Zhang, G.S. Ultra-Fast Atrazine Degradation through Synergistic Enhancement: Exploring the Synergistic Mechanism of CoFe2O4/MXene and Thermal on Peroxymonosulfate Activation. Chem. Eng. J. 2024, 497, 154388. [Google Scholar] [CrossRef]
- Hu, S.H.; Yan, W.W.; Yu, J.M.; Zhu, B.; Lan, Y.; Xi, W.H.; Xu, Z.M.; Han, W.; Cheng, C. Degradation of Sulfamethoxazole in Water by Dielectric Barrier Discharge Plasma Jet: Influencing Parameters, Degradation Pathway, Toxicity Evaluation. Plasma Sci. Technol. 2023, 25, 035510. [Google Scholar] [CrossRef]
- Fang, C.; Wang, S.H.; Shao, C.S.; Liu, C.; Wu, Y.H.; Huang, Q. Study of Detoxification of Methyl Parathion by Dielectric Barrier Discharge (DBD) Non-Thermal Plasma at Gas-Liquid Interface: Mechanism and Bio-Toxicity Evaluation. Chemosphere 2022, 307, 135620. [Google Scholar] [CrossRef]
- Du, J.K.; Xiao, G.F.; Xi, Y.X.; Zhu, X.W.; Su, F.; Kim, S.H. Periodate Activation with Manganese Oxides for Sulfanilamide Degradation. Water Res. 2020, 169, 115278. [Google Scholar] [CrossRef]
- Scaria, J.; Nidheesh, P.V. Comparison of Hydroxyl-Radical-Based Advanced Oxidation Processes with Sulfate Radical-Based Advanced Oxidation Processes. Curr. Opin. Chem. Eng. 2022, 36, 100830. [Google Scholar] [CrossRef]
- Wang, Q.C.; Zhang, A.; Li, P.; Héroux, P.; Zhang, H.; Yu, X.; Liu, Y.N. Degradation of Aqueous Atrazine Using Persulfate Activated by Electrochemical Plasma Coupling with Microbubbles: Removal Mechanisms and Potential Applications. J. Hazard. Mater 2021, 403, 124087. [Google Scholar] [CrossRef] [PubMed]
- Aggelopoulos, C.A.; Tataraki, D.; Rassias, G. Degradation of Atrazine in Soil by Dielectric Barrier Discharge Plasma–Potential Singlet Oxygen Mediation. Chem. Eng. J. 2018, 347, 682–694. [Google Scholar] [CrossRef]
- Yue, J.H.; Guo, W.; Zhu, Y.H.; Li, D.Y.; Liang, S.; Cao, R.; Wang, A.D.; Li, J. Insights on the Degradation Mechanism of Neotame Using UV/Periodate: Roles of Reactive Species, Kinetics, and Pathways. Chem. Eng. J. 2024, 495, 153059. [Google Scholar] [CrossRef]
- Al-Nu’airat, J.; Oluwoye, I.; Zeinali, N.; Altarawneh, M.; Dlugogorski, B.Z. Review of Chemical Reactivity of Singlet Oxygen with Organic Fuels and Contaminants. Chem. Rec. 2021, 21, 315–342. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Ji, J.; Zhao, Y.; Guo, J.; Duan, A.; Yuan, X.; Wang, H.; Zhou, S.; Li, X. Rational Design of Waste Anode Graphite-Derived Carbon Catalyst to Activate Peroxymonosulfate for Atrazine Degradation. Environ. Res. 2024, 257, 119296. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Duan, J.; Luo, P.; Zhu, L.; Liu, Y. Degradation of Atrazine in Water by Dielectric Barrier Discharge Combined with Periodate Oxidation: Enhanced Performance, Degradation Pathways, and Toxicity Assessment. Toxics 2024, 12, 746. https://doi.org/10.3390/toxics12100746
Zhang H, Duan J, Luo P, Zhu L, Liu Y. Degradation of Atrazine in Water by Dielectric Barrier Discharge Combined with Periodate Oxidation: Enhanced Performance, Degradation Pathways, and Toxicity Assessment. Toxics. 2024; 12(10):746. https://doi.org/10.3390/toxics12100746
Chicago/Turabian StyleZhang, Han, Jinping Duan, Pengcheng Luo, Luxiang Zhu, and Yanan Liu. 2024. "Degradation of Atrazine in Water by Dielectric Barrier Discharge Combined with Periodate Oxidation: Enhanced Performance, Degradation Pathways, and Toxicity Assessment" Toxics 12, no. 10: 746. https://doi.org/10.3390/toxics12100746
APA StyleZhang, H., Duan, J., Luo, P., Zhu, L., & Liu, Y. (2024). Degradation of Atrazine in Water by Dielectric Barrier Discharge Combined with Periodate Oxidation: Enhanced Performance, Degradation Pathways, and Toxicity Assessment. Toxics, 12(10), 746. https://doi.org/10.3390/toxics12100746





