The Effect of Urinary Polycyclic Aromatic Hydrocarbon Metabolites on Lipid Profiles: Does Oxidative Stress Play a Crucial Mediation Role?
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Demographic Information
2.2. Chemical Analyses on Urinary PAH Metabolites
2.3. Analyses of the Biomarkers
2.3.1. Urinary 8-OHdG Analysis
2.3.2. Correction of Urinary PAH Metabolites and 8-OHdG by Creatinine
2.3.3. Lipid Profiles Analysis
2.4. Statistical Analysis
3. Results
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, M.; Sahu, S.K.; Pandit, G.G. Distribution of PAHs in different compartment of creek ecosystem: Ecotoxicological concern and human health risk. Environ. Toxicol. Pharmacol. 2017, 50, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, R.; Arnoldsson, K.; Lejon, C.; Hagglund, L.; Wingfors, H. Field evaluation and calibration of a small axial passive air sampler for gaseous and particle bound polycyclic aromatic hydrocarbons (PAHs) and oxygenated PAHs. Environ. Pollut. 2016, 216, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Luderer, U.; Christensen, F.; Johnson, W.O.; She, J.; Ip, H.S.; Zhou, J.; Alvaran, J.; Krieg, E.F., Jr.; Kesner, J.S. Associations between urinary biomarkers of polycyclic aromatic hydrocarbon exposure and reproductive function during menstrual cycles in women. Environ. Int. 2017, 100, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Olaniran, A.O. Neurotoxicity of polycyclic aromatic hydrocarbons: A systematic mapping and review of neuropathological mechanisms. Toxics 2022, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- Everett, C.J.; King, D.E.; Player, M.S.; Matheson, E.M.; Post, R.E.; Mainous, A.G., 3rd. Association of urinary polycyclic aromatic hydrocarbons and serum C-reactive protein. Environ. Res. 2010, 110, 79–82. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, W.L.; Lin, C.M.; Lai, C.H.; Loh, C.H.; Chen, H.I.; Liou, S.H. The relationship between plasma and urinary 8-hydroxy-2-deoxyguanosine biomarkers measured by liquid chromatography tandem mass spectrometry. Environ. Sci. Pollut. Res. Int. 2016, 23, 17496–17502. [Google Scholar] [CrossRef]
- Da Broi, M.G.; de Albuquerque, F.O.; de Andrade, A.Z.; Cardoso, R.L.; Jordao Junior, A.A.; Navarro, P.A. Increased concentration of 8-hydroxy-2’-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res. 2016, 366, 231–242. [Google Scholar] [CrossRef]
- Yost, A.D.; Joshi, S.G. Atmospheric Nonthermal Plasma-Treated PBS Inactivates Escherichia coli by Oxidative DNA Damage. PLoS ONE 2015, 10, e0139903. [Google Scholar] [CrossRef]
- Wang, I.J.; Karmaus, W.J.; Yang, C.C. Polycyclic aromatic hydrocarbons exposure, oxidative stress, and asthma in children. Int. Arch. Occup. Environ. Health 2017, 90, 297–303. [Google Scholar] [CrossRef]
- Nilsson, R.; Nordlinder, R.; Moen, B.E.; Ovrebo, S.; Bleie, K.; Skorve, A.H.; Hollund, B.E.; Tagesson, C. Increased urinary excretion of 8-hydroxydeoxyguanosine in engine room personnel exposed to polycyclic aromatic hydrocarbons. Occup. Environ. Med. 2004, 61, 692–696. [Google Scholar] [CrossRef]
- Huang, H.B.; Lai, C.H.; Chen, G.W.; Lin, Y.Y.; Jaakkola, J.J.; Liou, S.H.; Wang, S.L. Traffic-related air pollution and DNA damage: A longitudinal study in Taiwanese traffic conductors. PLoS ONE 2012, 7, e37412. [Google Scholar] [CrossRef] [PubMed]
- Dehbi, H.M.; Blangiardo, M.; Gulliver, J.; Fecht, D.; de Hoogh, K.; Al-Kanaani, Z.; Tillin, T.; Hardy, R.; Chaturvedi, N.; Hansell, A.L. Air pollution and cardiovascular mortality with over 25years follow-up: A combined analysis of two British cohorts. Environ. Int. 2017, 99, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Hampel, R.; Franck, U.; Wiedensohler, A.; Cyrys, J.; Pan, X.; Wichmann, H.E.; Peters, A.; Schneider, A.; Breitner, S. Assessing responses of cardiovascular mortality to particulate matter air pollution for pre-, during- and post-2008 Olympics periods. Environ. Res. 2015, 142, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cook, R.L.; Ilacqua, V.A.; Kan, H.; Talbott, E.O.; Kearney, G. Studying associations between urinary metabolites of polycyclic aromatic hydrocarbons (PAHs) and cardiovascular diseases in the United States. Sci. Total Environ. 2010, 408, 4943–4948. [Google Scholar] [CrossRef] [PubMed]
- Tofighi, A.; Rahmani, F.; Jamali Qarakhanlou, B.; Babaei, S. The effect of regular aerobic exercise on reverse cholesterol transport A1 and apo lipoprotein a-I gene expression in inactive women. Iran. Red. Crescent Med. J. 2015, 17, e26321. [Google Scholar] [CrossRef]
- Watson, C.E.; Weissbach, N.; Kjems, L.; Ayalasomayajula, S.; Zhang, Y.; Chang, I.; Navab, M.; Hama, S.; Hough, G.; Reddy, S.T.; et al. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J. Lipid Res. 2011, 52, 361–373. [Google Scholar] [CrossRef]
- Gigante, B.; Leander, K.; Vikstrom, M.; Frumento, P.; Carlsson, A.C.; Bottai, M.; de Faire, U. Elevated ApoB serum levels strongly predict early cardiovascular events. Heart 2012, 98, 1242–1245. [Google Scholar] [CrossRef]
- Lu, S.Y.; Li, Y.X.; Zhang, J.Q.; Zhang, T.; Liu, G.H.; Huang, M.Z.; Li, X.; Ruan, J.J.; Kannan, K.; Qiu, R.L. Associations between polycyclic aromatic hydrocarbon (PAH) exposure and oxidative stress in people living near e-waste recycling facilities in China. Environ. Int. 2016, 94, 161–169. [Google Scholar] [CrossRef]
- Lin, Y.; Qiu, X.; Yu, N.; Yang, Q.; Araujo, J.A.; Zhu, Y. Urinary Metabolites of Polycyclic Aromatic Hydrocarbons and the Association with Lipid Peroxidation: A Biomarker-Based Study between Los Angeles and Beijing. Environ. Sci. Technol. 2016, 50, 3738–3745. [Google Scholar] [CrossRef]
- Kawano, Y.; Nishiumi, S.; Tanaka, S.; Nobutani, K.; Miki, A.; Yano, Y.; Seo, Y.; Kutsumi, H.; Ashida, H.; Azuma, T. Activation of the aryl hydrocarbon receptor induces hepatic steatosis via the upregulation of fatty acid transport. Arch. Biochem. Biophys. 2010, 504, 221–227. [Google Scholar] [CrossRef]
- Jin, Y.; Miao, W.; Lin, X.; Wu, T.; Shen, H.; Chen, S.; Li, Y.; Pan, Q.; Fu, Z. Sub-chronically exposing mice to a polycyclic aromatic hydrocarbon increases lipid accumulation in their livers. Environ. Toxicol. Pharmacol. 2014, 38, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xiang, B.; Jin, Y.; Li, C.; Li, J.; Ren, S.; Huang, H.; Luo, Q. Dysregulation of lipid metabolism induced by airway exposure to polycyclic aromatic hydrocarbons in C57BL/6 mice. Environ. Pollut. 2019, 245, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sandau, C.D.; Romanoff, L.C.; Caudill, S.P.; Sjodin, A.; Needham, L.L.; Patterson, D.G., Jr. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ. Res. 2008, 107, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, Y.; Liu, Y.; Xiao, L.; Cen, X.; Li, W.; Guo, Y.; Kim, M.; Yuan, J.; Chen, W. Association between urinary polycyclic aromatic hydrocarbon metabolites and dyslipidemias in the Chinese general population: A cross-sectional study. Environ. Pollut. 2019, 245, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millennium. Commun. Monogr. 2009, 76, 408–420. [Google Scholar] [CrossRef]
- Hayes, A.F.; Preacher, K.J. Statistical mediation analysis with a multicategorical independent variable. Br. J. Math. Stat. Psychol. 2014, 67, 451–470. [Google Scholar] [CrossRef]
- Consultation, W.H.O.E. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Zhang, P.D.; He, L.Y.; Guo, Y.; Liu, P.; Li, G.X.; Wang, L.Z.; Liu, Y.F. Blood lipid profiles and factors associated with dyslipidemia assessed by a point-of-care testing device in an outpatient setting: A large-scale cross-sectional study in Southern China. Clin. Biochem. 2015, 48, 586–589. [Google Scholar] [CrossRef]
- Wu, D.M.; Pai, L.; Sun, P.K.; Hsu, L.L.; Sun, C.A. Joint effects of alcohol consumption and cigarette smoking on atherogenic lipid and lipoprotein profiles: Results from a study of Chinese male population in Taiwan. Eur. J. Epidemiol. 2001, 17, 629–635. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Q.; Wang, H.; Wang, B.; Wang, X.; Ren, A.; Tao, S. Risk of human exposure to polycyclic aromatic hydrocarbons: A case study in Beijing, China. Environ. Pollut. 2015, 205, 70–77. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, H.; Xie, J.; Song, Y.; Liu, Y.; Huang, X.; Zhou, T.; Rong, Y.; Wu, T.; Yuan, J.; et al. Urinary Polycyclic Aromatic Hydrocarbon Metabolites and Altered Lung Function in Wuhan, China. Am. J. Respir. Crit. Care Med. 2016, 193, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, J.; Shen, G.; Zhong, J.; Wang, C.; Wei, S.; Chen, C.; Chen, Y.; Lu, Y.; Shen, H.; et al. Dietary and inhalation exposure to polycyclic aromatic hydrocarbons and urinary excretion of monohydroxy metabolites--a controlled case study in Beijing, China. Environ. Pollut. 2014, 184, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Yoshinaga, J. Inhalation and dietary exposure to polycyclic aromatic hydrocarbons and urinary 1-hydroxypyrene in non-smoking university students. Int. Arch. Occup. Environ. Health 2007, 81, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Senthilkumar, K.; Alomirah, H.; Moon, H.B.; Minh, T.B.; Mohd, M.A.; Nakata, H.; Kannan, K. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ. Sci. Technol. 2013, 47, 2932–2938. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, R.; Lu, S.; Zhang, D.; Zhou, Y.; Lv, Y. Exposure to polycyclic aromatic hydrocarbons could cause their oxidative DNA damage: A case study for college students in Guangzhou, China. Environ. Sci. Pollut. Res. Int. 2015, 22, 1770–1777. [Google Scholar] [CrossRef]
- Yang, L.; Yan, K.; Zeng, D.; Lai, X.; Chen, X.; Fang, Q.; Guo, H.; Wu, T.; Zhang, X. Association of polycyclic aromatic hydrocarbons metabolites and risk of diabetes in coke oven workers. Environ. Pollut. 2017, 223, 305–310. [Google Scholar] [CrossRef]
- Oliveira, M.; Slezakova, K.; Alves, M.J.; Fernandes, A.; Teixeira, J.P.; Delerue-Matos, C.; Pereira, M.D.; Morais, S. Polycyclic aromatic hydrocarbons at fire stations: Firefighters’ exposure monitoring and biomonitoring, and assessment of the contribution to total internal dose. J. Hazard. Mater. 2017, 323, 184–194. [Google Scholar] [CrossRef]
- Riediger, N.D.; Bruce, S.G.; Young, T.K. Cardiovascular risk according to plasma apolipoprotein and lipid profiles in a Canadian First Nation. Prev. Chronic Dis. 2011, 8, A05. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Varbo, A. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635. [Google Scholar] [CrossRef]
- Kato, S.; Yoshimura, K.; Kimata, T.; Mine, K.; Uchiyama, T.; Kaneko, K. Urinary 8-Hydroxy-2′-Deoxyguanosine: A Biomarker for Radiation-Induced Oxidative DNA Damage in Pediatric Cardiac Catheterization. J. Pediatr. 2015, 167, 1369–1374.e1. [Google Scholar] [CrossRef]
- Ferguson, K.K.; McElrath, T.F.; Chen, Y.H.; Loch-Caruso, R.; Mukherjee, B.; Meeker, J.D. Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. Am. J. Obstet. Gynecol. 2015, 212, 208.e1–208.e8. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.; Zhou, M.; Wang, B.; Cao, L.; Yang, S.; Qiu, W.; Nie, X.; Ye, Z.; Zhou, Y.; Chen, W. Personal PM2.5 exposure and lung function: Potential mediating role of systematic inflammation and oxidative damage in urban adults from the general population. Sci. Total Environ. 2021, 755, 142522. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Hou, J.; Zhou, Y.; Yang, Y.; Cheng, J.; Xu, T.; Xiao, L.; Chen, W.; Yuan, J. Dose-response relationship between urinary polycyclic aromatic hydrocarbons metabolites and urinary 8-hydroxy-2′-deoxyguanosine in a Chinese general population. Chemosphere 2017, 174, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, K.K.; McElrath, T.F.; Pace, G.G.; Weller, D.; Zeng, L.; Pennathur, S.; Cantonwine, D.E.; Meeker, J.D. Urinary Polycyclic Aromatic Hydrocarbon Metabolite Associations with Biomarkers of Inflammation, Angiogenesis, and Oxidative Stress in Pregnant Women. Environ. Sci. Technol. 2017, 51, 4652–4660. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Dai, X.; Guo, H.; Huang, S.; Kuang, D.; Feng, J.; Wang, T.; Zhang, W.; Huang, K.; Hu, D.; et al. Polycyclic aromatic hydrocarbons-associated microRNAs and their interactions with the environment: Influences on oxidative DNA damage and lipid peroxidation in coke oven workers. Environ. Sci. Technol. 2014, 48, 4120–4128. [Google Scholar] [CrossRef]
- Gierach, M.; Gierach, J.; Junik, R. Evaluation of lipid profiles in patients with metabolic syndrome according to cardiovascular risk calculated on the basis of the SCORE chart. Endokrynol. Pol. 2016, 67, 265–270. [Google Scholar] [CrossRef][Green Version]
- Patel, C.J.; Cullen, M.R.; Ioannidis, J.P.; Butte, A.J. Systematic evaluation of environmental factors: Persistent pollutants and nutrients correlated with serum lipid levels. Int. J. Epidemiol. 2012, 41, 828–843. [Google Scholar] [CrossRef]
- Yang, Q.; Qiu, X.; Li, R.; Ma, J.; Li, K.; Li, G. Polycyclic aromatic hydrocarbon (PAH) exposure and oxidative stress for a rural population from the North China Plain. Environ. Sci. Pollut. Res. Int. 2015, 22, 1760–1769. [Google Scholar] [CrossRef]
- Al-Aubaidy, H.A.; Jelinek, H.F. Oxidative stress and triglycerides as predictors of subclinical atherosclerosis in prediabetes. Redox Rep. 2014, 19, 87–91. [Google Scholar] [CrossRef]
- Xu, G.W.; Yao, Q.H.; Weng, Q.F.; Su, B.L.; Zhang, X.; Xiong, J.H. Study of urinary 8-hydroxydeoxyguanosine as a biomarker of oxidative DNA damage in diabetic nephropathy patients. J. Pharm. Biomed. Anal. 2004, 36, 101–104. [Google Scholar] [CrossRef]
- Peng, C.; Bind, M.C.; Colicino, E.; Kloog, I.; Byun, H.M.; Cantone, L.; Trevisi, L.; Zhong, J.; Brennan, K.; Dereix, A.E.; et al. Particulate Air Pollution and Fasting Blood Glucose in Nondiabetic Individuals: Associations and Epigenetic Mediation in the Normative Aging Study, 2000–2011. Environ. Health Perspect. 2016, 124, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- van Strien, T.; Winkens, L.; Toft, M.B.; Pedersen, S.; Brouwer, I.; Visser, M.; Lahteenmaki, L. The mediation effect of emotional eating between depression and body mass index in the two European countries Denmark and Spain. Appetite 2016, 105, 500–508. [Google Scholar] [CrossRef] [PubMed]
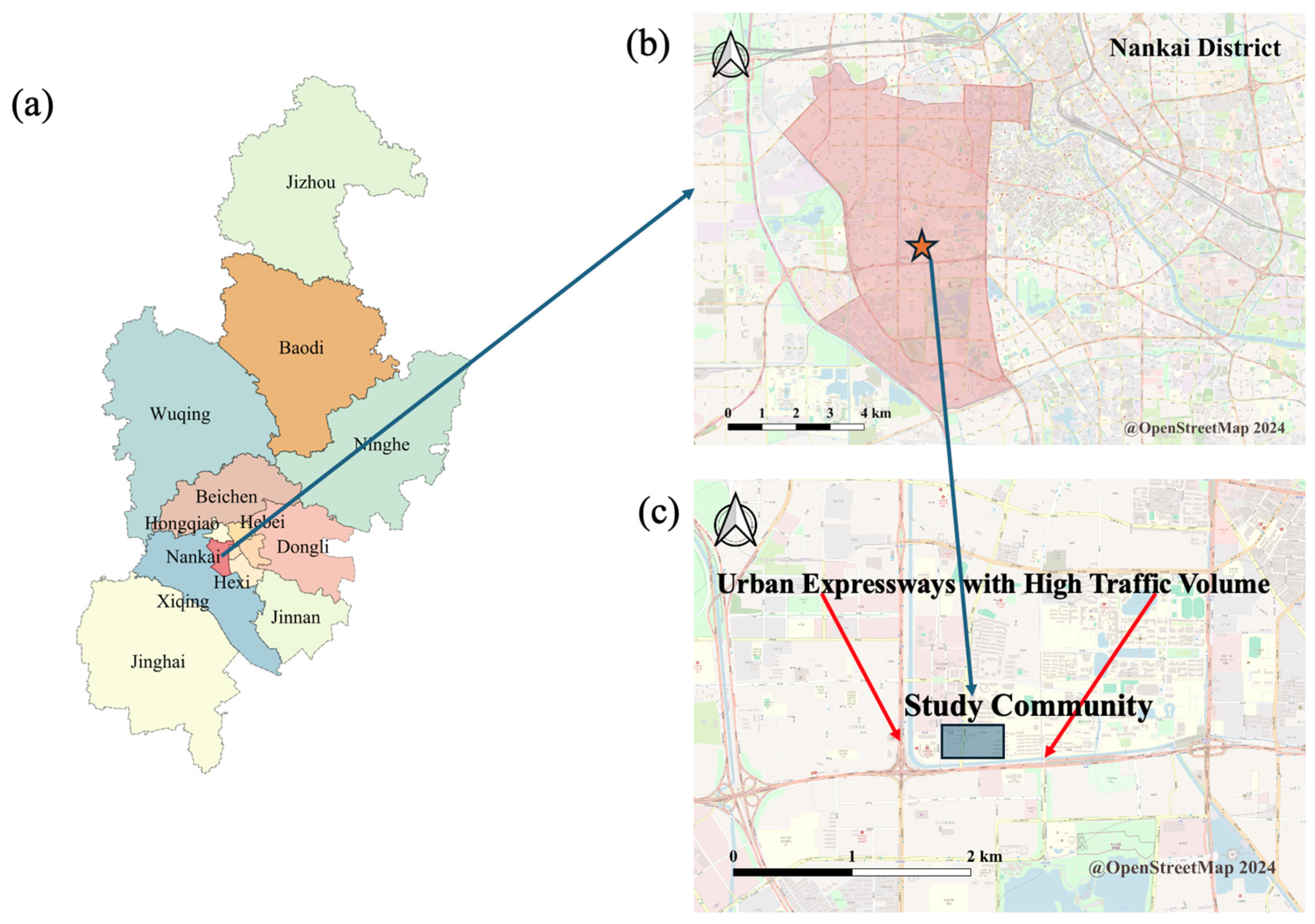

| Variable | Male (n = 46) | Female (n = 63) | Total (n = 109) | F | p |
|---|---|---|---|---|---|
| Age (mean ± SD) | 69 ± 7.0 | 67 ± 5.0 | 68 ± 6.0 | 1.73 | 0.87 |
| BMI (kg/m2) (mean ± SD) | 25.61 ± 3.34 | 24.60 ± 3.09 | 25.03 ± 3.22 | 1.62 | 0.11 |
| Smoking status | 54% | 16% | 32% | 18.05 | <0.01 |
| Alcohol status | 44% | 5% | 21% | 23.94 | <0.01 |
| Variables | Median (P25, P75) | Z | p | ||
|---|---|---|---|---|---|
| Male (n = 46) | Female (n = 63) | Total (n = 109) | |||
| TC (mmol/L) | 5.07 (4.54, 5.71) | 5.61 (5.17, 6.26) | 5.42 (4.86, 5.95) | −2.88 | 0.004 |
| TG (mmol/L) | 1.31 (0.92, 1.94) | 1.44 (1.18, 1.85) | 1.41 (1.03, 1.90) | −1.13 | 0.260 |
| HDL-C (mmol/L) | 1.14 (1.03, 1.31) | 1.31 (1.17, 1.48) | 1.26 (1.09, 1.43) | −2.87 | 0.004 |
| LDL-C (mmol/L) | 3.06 (2.63, 3.57) | 3.41 (2.90, 3.79) | 3.24 (2.74, 3.64) | −2.11 | 0.035 |
| Apo A1(g/L) | 1.24 (1.17, 1.29) | 1.32 (1.26, 1.39) | 1.29 (1.21, 1.36) | −3.87 | <0.001 |
| Apo B (g/L) | 0.89 (0.77, 0.98) | 0.94 (0.88, 1.05) | 0.92 (0.81, 1.04) | −2.02 | 0.043 |
| Apo B/Apo A1 | 0.72 (0.61, 0.83) | 0.72 (0.65, 0.81) | 0.72 (0.62, 0.82) | −0.09 | 0.929 |
| 8-OHdG (ng/mg Cr) | 4.98 (3.20, 7.17) | 5.64 (3.15, 8.92) | 5.44 (3.15, 8.48) | −0.92 | 0.357 |
| lg (8OHdG) | lg (1-OHNap) | lg (2-OHNap) | lg (9-OHFlu) | lg (3-OHFlu) | lg (2-OHFlu) | lg (1-OHPyr) | lg (2-OHBcPhe) | lg (6-OHChr) |
|---|---|---|---|---|---|---|---|---|
| Model 1 # | 16.8 * (1.8–31.8) | 30.9 * (14.0–47.8) | 43.4 * (25.3–61.4) | 47.5 * (31.0–63.9) | 48.0 * (29.5–66.4) | 51.4 * (28.5–74.3) | 9.7 (−12.3–31.8) | 33.9 * (15.0–52.7) |
| Model 2 & | 14.4 (−1.0–29.9) | 30.5 * (13.1–47.9) | 42.0 * (23.3–60.7) | 45.6 * (28.5–52.7) | 46.7 * (27.6–65.8) | 49.6 * (26.0–73.2) | 8.4 (−13.9–30.6) | 32.4 * (12.9–51.9) |
| lg(8OHdG) | lg(1-OHNap) | lg(2-OHNap) | lg(9-OHFlu) | lg(3-OHFlu) | lg(2-OHFlu) | lg(1-OHPyr) | lg(2-OHBcPhe) | lg(6-OHChr) |
|---|---|---|---|---|---|---|---|---|
| lg(TC) (*10−2) | ||||||||
| Model 1 # | −0.7 (−3.9–2.5) | 0.1 (−3.8–4.0) | 2.7 (−1.8–7.2) | 1.6 (−2.6–5.7) | 3.7 (−1.2–8.5) | 2.9 (−2.8–8.5) | 2.7 (−3.0–8.5) | 2.8 (−2.6–8.2) |
| Model 2 & | −0.9 (−4.1–2.4) | 0.3 (−3.8–4.3) | 2.8 (−1.8–7.5) | 1.3 (−3.0–5.6) | 3.2 (−1.8–8.2) | 3.9 (−1.9–9.7) | 2.8 (−3.3–8.9) | 2.7 (−2.8–8.3) |
| lg(TG) | ||||||||
| Model 1 | −4.2 (−13.4–5.0) | −2.5 (−14.6–9.6) | 9.1 (−3.3–21.4) | 6.1 (−6.1–18.4) | 0.10.3 (−3.0–23.7) | 8.1 (−8.0–24.3) | 5.3 (−11.3–21.8) | 8.3 (−7.7–24.2) |
| Model 2 | −3.2 (−12.6–6.1) | −1.2 (−13.6–11.2) | 11.8 (−0.9–24.5) | 8.7 (−3.9–21.3) | 12.4 (−1.2–26.1) | 11.2 (−5.2–27.7) | 4.1 (−13.1–21.3) | 11.7 (−4.4–27.9) |
| lg(HDL-C) (*10−2) | ||||||||
| Model 1 | −0.1 (−5.3–5.0) | 1.5 (−5.3–8.3) | −1.7 (−8.5–5.0) | −1.8 (−8.6–5.0) | −1.5 (−8.8–5.8) | −1.0 (−10.4–8.4) | 0.0 (−8.7–8.6) | −2.0 (−10.7–6.6) |
| Model 2 | −1.4 (−6.3–3.5) | 0.3 (−6.4–7.0) | −4.2 (−10.9–2.6) | −4.1 (−10.8–2.6) | −3.8 (−10.9–3.3) | −2.7 (−12.2–6.7) | 0.2 (−8.2–8.7) | −4.9 (−13.1–3.3) |
| lg(LDL-C) (*10−2) | ||||||||
| Model 1 | 1.0 (−3.2–5.2) | 0.3 (−4.9–5.5) | 4.9 (−1.2–10.9) | 3.3 (−2.1–8.8) | 6.0 (−0.6–12.6) | 6.2 (−1.1–13.5) | 5.0 (−2.6–12.5) | 4.3 (−2.8–11.5) |
| Model 2 | 1.4 (−2.9–5.7) | 1.2 (−4.0–6.4) | 6.1 (−0.1–12.3) | 3.9 (−1.7–9.5) | 6.3 (−0.4–12.9) | 8.7 * (1.5–15.9) | 5.4 (−2.4–13.2) | 5.2 (−2.0–12.4) |
| lg(Apo A1) (*10−2) | ||||||||
| Model 1 | −1.7* (−3.2–−0.2) | −1.2 (−3.3–0.8) | −0.3 (−2.7–2.1) | −0.6 (−2.7–1.5) | −0.9 (−3.2–1.4) | −2.8 * (−5.5–−0.1) | −0.7 (−3.4–2.0) | −0.7 (−3.3–1.8) |
| Model 2 | −2.0* (−3.5–−0.5) | −1.7 (−3.8–0.3) | −1.0 (−30.5–1.4) | −1.2 (−3.3–0.9) | −1.6 (−3.8–0.7) | −3.4 * (−6.1–−0.6) | −0.9 (−3.6–1.9) | −1.3 (−3.9–1.2) |
| lg(Apo B) (*10−2) | ||||||||
| Model 1 | 1.0 (−2.4–4.4) | −2.3 (−6.4–1.8) | −0.5 (−5.4–4.4) | −2.1 (−6.5–2.3) | 0.8 (−4.5–6.1) | 1.9 (−4.3–8.0) | 6.2 * (0.5–11.9) | −1.9 (−7.7–3.9) |
| Model 2 | 1.1 (−2.4–4.6) | −2.2 (−6.4–2.1) | −0.4 (−5.4–4.7) | −2.2 (−6.8–2.4) | 0.5 (−4.9–5.9) | 2.8 (−3.6–9.2) | 6.0 * (0.0–11.9) | −1.4 (−7.4–4.6) |
| lg(Apo B/Apo A1) (*10−2) | ||||||||
| Model 1 | 2.7 (−1.3–6.6) | −1.1 (−6.0–3.8) | −0.2 (−5.6–5.2) | −1.5 (−6.6–3.7) | 1.7 (−4.2–7.6) | 4.7 (−2.5–11.9) | 6.9 * (0.4–13.3) | −1.2 (−7.9–5.6) |
| Model 2 | 3.1 (−0.9–7.1) | −0.4 (−5.4–4.5) | 0.6 (−5.0–6.3) | −1.0 (−6.3–4.3) | 2.1 (−3.9–8.0) | 6.2 (−1.3–13.6) | 6.9 * (0.2–13.6) | 0.0 (−6.8–6.8) |
| lg(8OHdG) | lg(TC) | lg(TG) | lg(HDL-C) | lg(LDL-C) | lg(Apo A1) | lg(Apo B) | lg(Apo B/Apo A1) |
|---|---|---|---|---|---|---|---|
| Model 1 # | 1.1 (−4.4–6.6) | 3.1 (−11.1–17.4) | 1.0 (−6.7–8.7) | 1.8 (−5.6–9.1) | −0.3 (−3.0–2.4) | −3.5 (−9.3–2.2) | −3.2 (−9.6–3.1) |
| Model 2 & | 1.0 (−4.7–6.7) | 5.9 (−8.5-.20.3) | −1.4 (−8.9–6.2) | 2.6 (−5.0–10.1) | −0.9 (−3.6–1.8) | −3.2 (−9.1–2.7) | 0.4 (−0.2–1.1) |
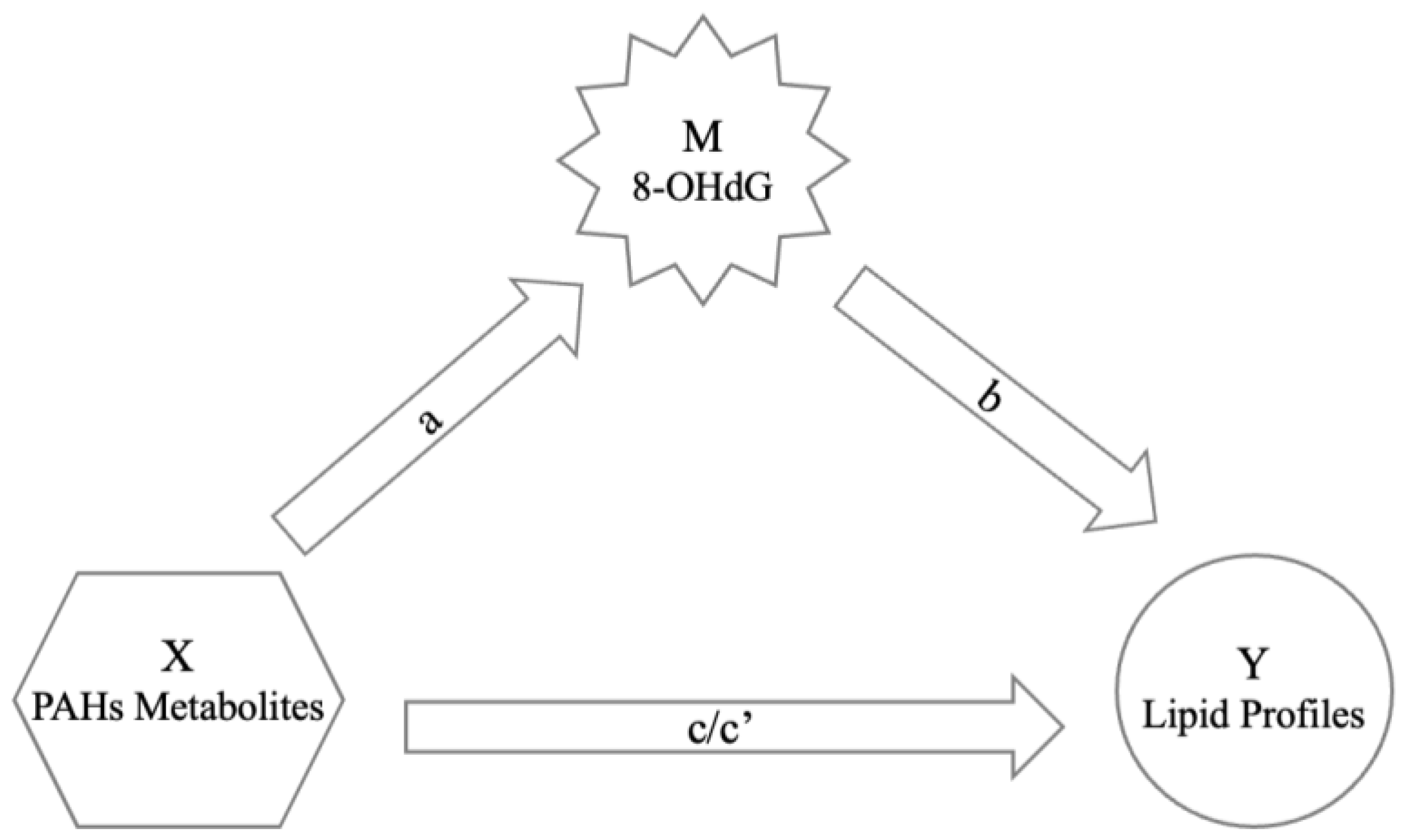
| Mediating Process (8-OHdG) | a 1 | b 2 | c 3 | c’ 4 | a × b |
|---|---|---|---|---|---|
| 1-OHNap-Apo A1 | 14.4 (−1.0, 29.9) | 0.4 (−2.0, 2.8) | −2.4 * (−4.0, −0.9) | −2.5 * (−4.1, −0.9) | 0.1 (−0.3, 0.6) |
| 1-OHPyr-LDL-C | 49.6 * (26.0, 73.2) | −2.1 (−12.2, 8.0) | 9.6 * (1.5, 17.8) | 10.7 * (1.1, 20.3) | −1.0 (−5.6, 3.7) |
| 1-OHPyr-Apo A1 | 49.6 * (26.0, 73.2) | −0.1 (−3.9, 3.8) | −3.1 (−6.1, 0.1) | −3.1 (−6.7, 0.6) | −0.1 (−1.7, 2.3) |
| 2-OHBcPhe-Apo B | 8.4 (−13.9, 30.6) | −10.2 * (−19.4, −1.1) | 6;1 (−0.6, 12.9) | 7.0 * (0.5, 13.5) | −0.9 (−3.1, 1.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, J.; Yang, L.; Zhang, N.; Zhang, L.; Han, B. The Effect of Urinary Polycyclic Aromatic Hydrocarbon Metabolites on Lipid Profiles: Does Oxidative Stress Play a Crucial Mediation Role? Toxics 2024, 12, 748. https://doi.org/10.3390/toxics12100748
Wang Y, Xu J, Yang L, Zhang N, Zhang L, Han B. The Effect of Urinary Polycyclic Aromatic Hydrocarbon Metabolites on Lipid Profiles: Does Oxidative Stress Play a Crucial Mediation Role? Toxics. 2024; 12(10):748. https://doi.org/10.3390/toxics12100748
Chicago/Turabian StyleWang, Yuting, Jia Xu, Liujie Yang, Nan Zhang, Liwen Zhang, and Bin Han. 2024. "The Effect of Urinary Polycyclic Aromatic Hydrocarbon Metabolites on Lipid Profiles: Does Oxidative Stress Play a Crucial Mediation Role?" Toxics 12, no. 10: 748. https://doi.org/10.3390/toxics12100748
APA StyleWang, Y., Xu, J., Yang, L., Zhang, N., Zhang, L., & Han, B. (2024). The Effect of Urinary Polycyclic Aromatic Hydrocarbon Metabolites on Lipid Profiles: Does Oxidative Stress Play a Crucial Mediation Role? Toxics, 12(10), 748. https://doi.org/10.3390/toxics12100748








