The Dual Role of Natural Organic Matter in the Degradation of Organic Pollutants by Persulfate-Based Advanced Oxidation Processes: A Mini-Review
Abstract
1. Introduction
| Reference | Degradation Efficiency of Pollutants | NOM Effects | PS-AOP Type | NOM Type | Target Pollutant |
|---|---|---|---|---|---|
| [45] | The removal efficiency of oxytetracycline was reduced by approximately 20%, and the reaction rate constant kobs decreased from 0.182 to 0.038 min−1 with the addition of 10 mg·L−1 HA. | Inhibiting effect | 3DP-HPC@CoAl-LDH 1/PMS | Humic acid (HA) | Oxytetracycline |
| [46] | As the NOM concentration increased from 1 to 5 mg·L−1, the removal rate of 4-chloro-3,5-dimethylphenol decreased from 3.85 × 10−4 to 1.38 × 10−4 s−1. | UV/PDS | Humic acid (HA) | 4-Chloro-3,5-dimethylphenol | |
| [47] | The degradation rate of ofloxacin in the GA/Fe(III)/PS system increased by more than 80% compared to the control without GA addition. | Promoting effect | GA/Fe(III)/PDS | Gallic acid (GA) | Ofloxacin |
| [34] | The removal rate of naphthalene reached 71.78% in the Ilex extra/Fe(II)/PDS system, which was 1.86-fold higher than for the Fe(II)/PDS system (38.58%). | Ilex extra/Fe(II)/PDS | Ilex extra | Naphthalene | |
| [48] | The degradation of bisphenol S by PMS was significantly enhanced by EGCG at pH 3.0–7.0, but inhibited at pH 8.0–10.0. | Dual effects | EGCG/PMS | Epigallocatechin-3-gallate (EGCG) | Bisphenol S |
| [49] | The removal rate of ibuprofen increased from 71.9% to 77.3% as HA concentration increased from 0 to 5 mg·g−1. However, a decrease (74.8% to 56.8%) was observed when HA concentration increased from 10 to 50 mg·g−1. | Fe(II)-SP 2/PS | Humic acid (HA) | Ibuprofen |
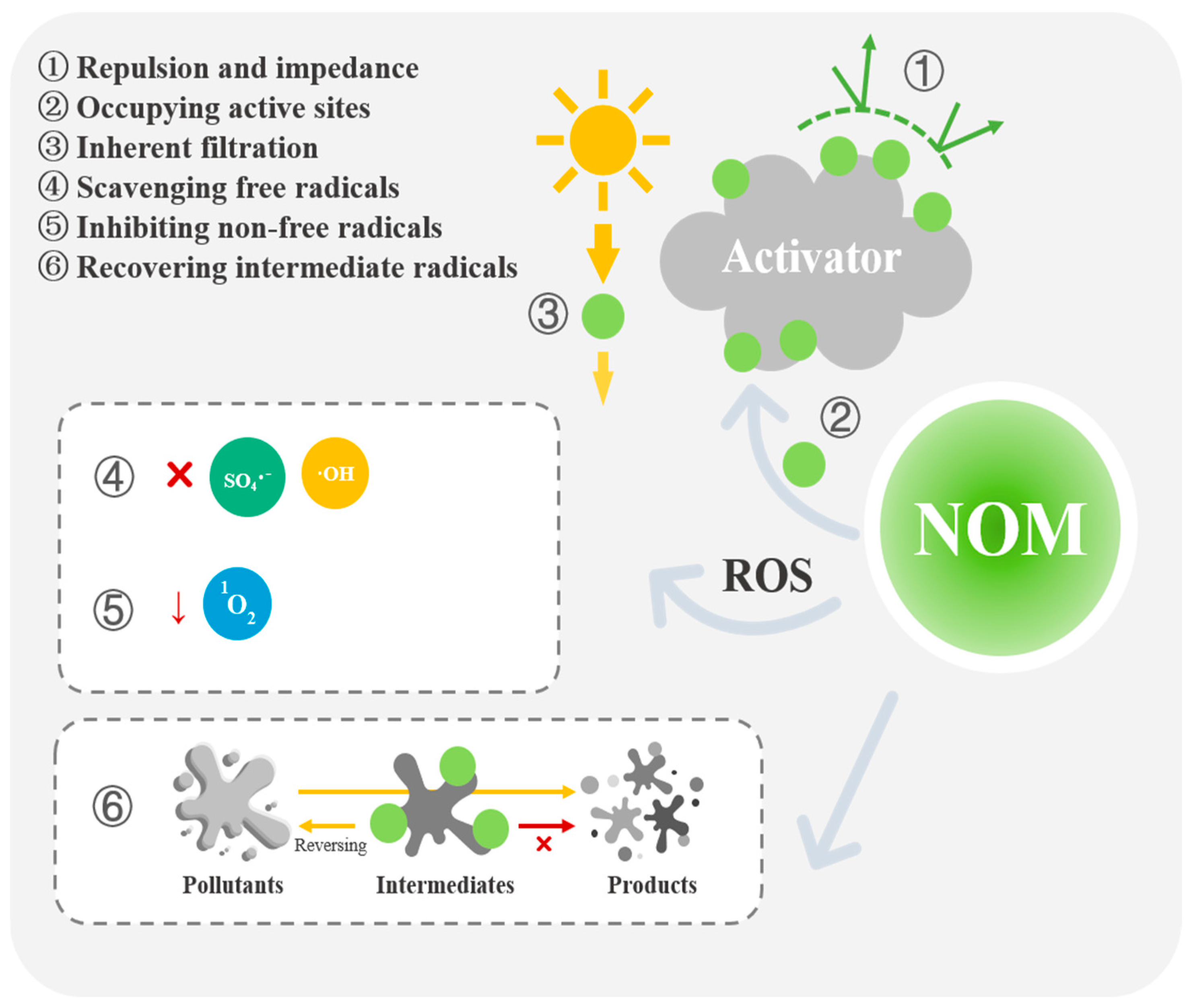
2. The Inhibitory Effects and Mechanisms of NOM on OP Removal by PS-AOPs
2.1. NOM Prevents the Activation of PS
2.1.1. Electrostatic Repulsion and Spatial Impedance
2.1.2. The Active Sites of Solid Catalysts Occupied by NOM
2.1.3. The Inherent Filtration Impact of NOM
2.2. Suppression of ROS by NOM
2.2.1. NOM Effectively Scavenges Free Radicals and ROS
2.2.2. Inhibition of Non-Free-Radical Active Substance Generation by NOM
2.3. Conversion of Pollutant Intermediates to Their Parent Compounds by NOM
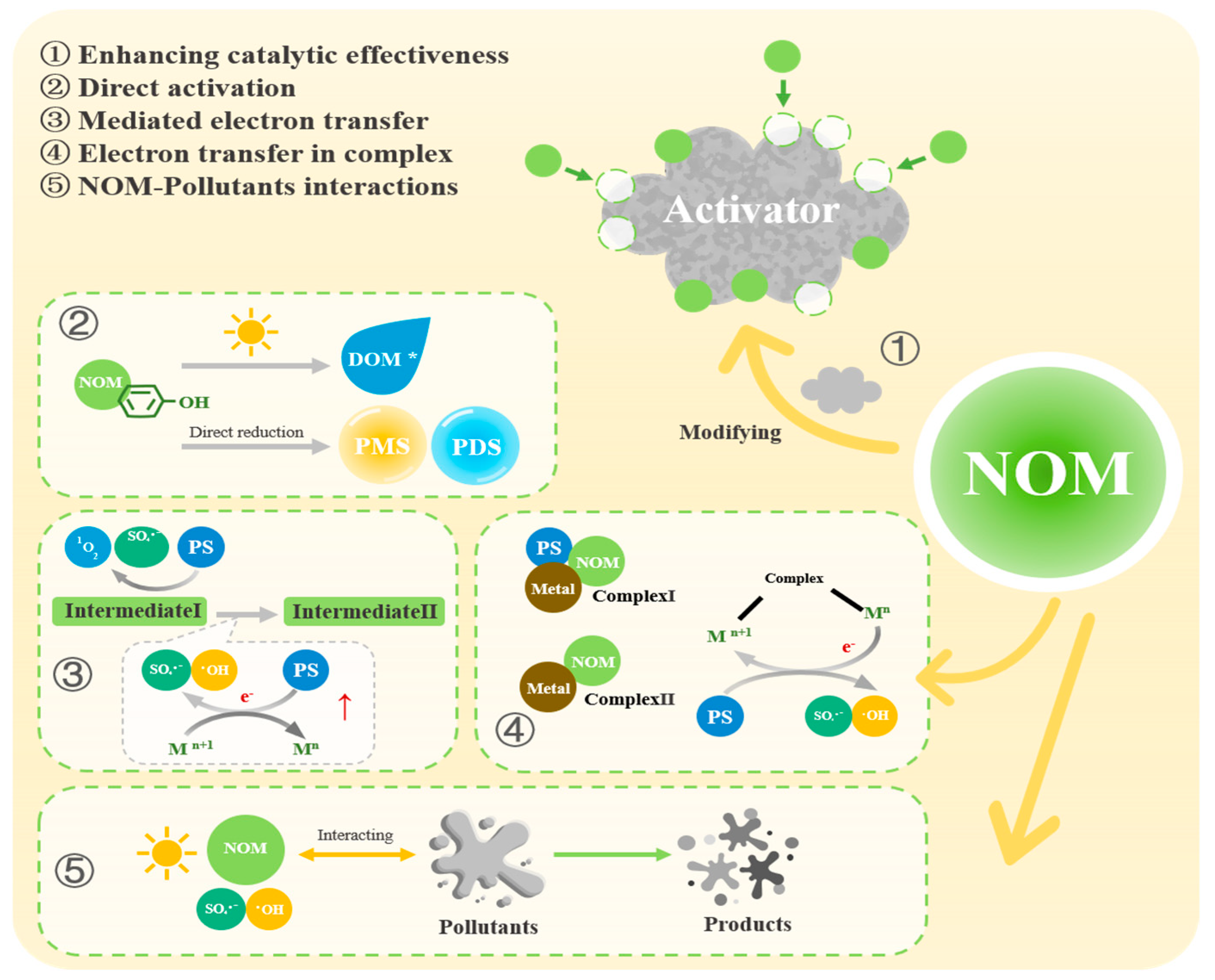
3. Promoting Effects and Mechanisms of NOM on OP Removal by PS-AOPs
3.1. NOM Enhances the Catalytic Effectiveness of Activators
3.2. NOM Contributes to the Generation of ROS
3.2.1. NOM Directly Generates ROS
3.2.2. NOM Generates Intermediates and Complexes for the Production of ROS
3.3. The Interaction Between NOM and Pollutants
4. Conclusions and Prospects
- (1)
- The underlying reaction mechanisms of NOM in PS-AOPs. NOM contains antioxidant functional groups that scavenge free radicals, as well as electron-rich functional groups that enhance the efficiency of PS-AOPs. NOM can play various roles in reactions, including as a reactant, activator, or intermediate. However, the impact of the NOM source, type, and specific functional group composition in complex PS-AOPs is not well understood and requires further investigation. Additionally, the influence of NOM on ROS generation during PS-AOPs in various environments, particularly soil, is still unclear. The effects of traditional probes and quenchers on secondary reaction intermediates and coexisting active substances are not fully understood, highlighting the importance of considering the types and amounts of probes and quenchers used. It may also be beneficial to explore in situ characterization methods for ROS at soil interfaces to obtain more precise information [112,113].
- (2)
- Exploiting suitable methods to characterize the effects of actual NOM. The complicated structure of NOM components and the lack of corresponding characterization methods hinder the study of NOM effects in PS-AOPs. Consequently, model compounds as NOM representatives are usually utilized to simplify the study and obtain clearer and more explicable results [29,47,83]. However, these results may differ from those obtained using actual NOM. Therefore, suitable characterization methods need to be developed to enable a more comprehensive understanding of NOM reactions.
- (3)
- Developing practical applications of PS-AOPs with NOM. Current research is primarily focused on laboratory simulations using batch-reactor systems. However, the oxidation system can be influenced by various factors such as utilization methods, reaction conditions, and environmental composition [14,109]. Therefore, it is essential to comprehensively consider these variable factors based on different situations (e.g., groundwater environments) to maximize the promoting effects of NOM and enhance the efficiency of PS-AOPs. In future studies, it may be beneficial to establish model parameters for the removal of OPs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassani, A.; Scaria, J.; Ghanbari, F.; Nidheesh, P.V. Sulfate radicals-based advanced oxidation processes for the degradation of pharmaceuticals and personal care products: A review on relevant activation mechanisms, performance, and perspectives. Environ. Res. 2023, 217, 114789. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yin, M.; Yang, W.; Li, H.; Zhong, Y.; Mo, L.; Liang, Y.; Ma, X.; Sun, X. Emerging pollutants in water environment: Occurrence, monitoring, fate, and risk assessment. Water Environ. Res. 2019, 91, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-P.; Lin, Y.-L.; Zhang, T.-Y.; Cao, T.-C.; Xu, B.; Pan, Y.; Zhang, X.-T.; Gao, N.-Y. Modelling of iohexol degradation in a Fe(II)-activated persulfate system. Chem. Eng. J. 2019, 367, 86–93. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Lu, S.; Wang, Z.; Wang, Y.; Zhang, G.; Guo, X.; Guo, W.; Zhang, T.; Xi, B. Degradation difference of ofloxacin and levofloxacin by UV/H2O2 and UV/PS (persulfate): Efficiency, factors and mechanism. Chem. Eng. J. 2020, 385, 123987. [Google Scholar] [CrossRef]
- Ren, L.; Zhong, Y.; Xu, J.; Chen, J.; Zou, T.; Liao, X.-L.; Chen, Z.-F.; Yu, L. Nano Fe3-xCuxO4 as the heterogeneous catalyst in an advanced oxidation process for excellent peroxymonosulfate activation toward climbazole degradation. Chem. Eng. J. 2022, 439, 135553. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Liu, D.; Yi, M.; Chang, F.; Li, H.; Du, Y. A Review of Sulfate Radical-Based and Singlet Oxygen-Based Advanced Oxidation Technologies: Recent Advances and Prospects. Catalysts 2022, 12, 1092. [Google Scholar] [CrossRef]
- Lian, L.; Yao, B.; Hou, S.; Fang, J.; Yan, S.; Song, W. Kinetic Study of Hydroxyl and Sulfate Radical-Mediated Oxidation of Pharmaceuticals in Wastewater Effluents. Environ. Sci. Technol. 2017, 51, 2954–2962. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Gendy, E.A.; Ifthikar, J.; Chen, Z. Heterogeneous activation of persulfate by metal and non-metal catalyst for the degradation of sulfamethoxazole: A review. Chem. Eng. J. 2022, 437, 135277. [Google Scholar] [CrossRef]
- Chen, N.; Lee, D.; Kang, H.; Cha, D.; Lee, J.; Lee, C. Catalytic persulfate activation for oxidation of organic pollutants: A critical review on mechanisms and controversies. J. Environ. Chem. Eng. 2022, 10, 107654. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Sun, K.; Lin, C.; Ma, J.; He, M.; Ouyang, W. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review. Chem. Eng. J. 2019, 372, 836–851. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, Z.; Duan, X.; Long, M.; Spinney, R.; Dionysiou, D.D.; Xiao, R.; Alvarez, P.J.J. Merits and Limitations of Radical vs. Nonradical Pathways in Persulfate-Based Advanced Oxidation Processes. Environ. Sci. Technol. 2023, 57, 12153–12179. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zou, J.; Lin, J.; Li, Q.; Li, J.; Huang, Y.; Yang, H.; Yuan, B.; Ma, J. Elimination of acetaminophen in sodium carbonate-enhanced thermal/peroxymonosulfate process: Performances, influencing factors and mechanism. Chem. Eng. J. 2022, 449, 137765. [Google Scholar] [CrossRef]
- Ao, X.; Li, Z.; Zhang, H. A comprehensive insight into a rapid degradation of sulfamethoxazole by peroxymonosulfate enhanced UV-A LED/g-C3N4 photocatalysis. J. Clean. Prod. 2022, 356, 131822. [Google Scholar] [CrossRef]
- Dan, J.; Wang, Q.; Rao, P.; Dong, L.; Zhang, M.; Zhang, X.; He, Z.; Gao, N.; Deng, J. Bimetallic oxides with package structure for enhanced degradation of bisphenol a through peroxymonosulfate activation. Chem. Eng. J. 2022, 429, 132189. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, S.; Zhong, H.; Yan, M.; Yang, X. Activation of persulfates by carbonaceous materials: A review. Chem. Eng. J. 2021, 418, 129297. [Google Scholar] [CrossRef]
- Lee, J.; von Gunten, U.; Kim, J.-H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Lian, Q.; Roy, A.; Kizilkaya, O.; Gang, D.D.; Holmes, W.; Zappi, M.E.; Zhang, X.; Yao, H. Uniform Mesoporous Amorphous Cobalt-Inherent Silicon Oxide as a Highly Active Heterogeneous Catalyst in the Activation of Peroxymonosulfate for Rapid Oxidation of 2,4-Dichlorophenol: The Important Role of Inherent Cobalt in the Catalytic Mechanism. ACS Appl. Mater. Interfaces 2020, 12, 57190–57206. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Y.; Zhu, K.; Zhang, H. Understanding oxygen-deficient La2CuO4-δ perovskite activated peroxymonosulfate for bisphenol A degradation: The role of localized electron within oxygen vacancy. Appl. Catal. B Environ. 2021, 284, 119732. [Google Scholar] [CrossRef]
- You, J.; Li, J.; Zhang, H.; Luo, M.; Xing, B.; Ren, Y.; Liu, Y.; Xiong, Z.; He, C.; Lai, B. Removal of Bisphenol A via peroxymonosulfate activation over graphite carbon nitride supported NiCx nanoclusters catalyst: Synergistic oxidation of high-valent nickel-oxo species and singlet oxygen. J. Hazard. Mater. 2023, 445, 130440. [Google Scholar] [CrossRef]
- Sharma, V.K.; Feng, M.; Dionysiou, D.D.; Zhou, H.-C.; Jinadatha, C.; Manoli, K.; Smith, M.F.; Luque, R.; Ma, X.; Huang, C.-H. Reactive High-Valent Iron Intermediates in Enhancing Treatment of Water by Ferrate. Environ. Sci. Technol. 2022, 56, 30–47. [Google Scholar] [CrossRef]
- Ren, W.; Cheng, C.; Shao, P.; Luo, X.; Zhang, H.; Wang, S.; Duan, X. Origins of Electron-Transfer Regime in Persulfate-Based Nonradical Oxidation Processes. Environ. Sci. Technol. 2022, 56, 78–97. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Li, H.; Qian, J.; Lv, L.; Pan, B. Degradation of phosphonates in Co(II)/peroxymonosulfate process: Performance and mechanism. Water Res. 2021, 202, 117397. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Qi, Y.; Feng, S.; Peng, X.; Wang, W.; Yue, Y.; Shang, Y.; Li, Y.; Gao, B.; Xu, X. Enhanced degradation of clothianidin in peroxymonosulfate/catalyst system via core-shell FeMn @ N-C and phosphate surrounding. Appl. Catal. B Environ. 2020, 267, 118717. [Google Scholar] [CrossRef]
- Sillanpaa, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manag. 2018, 208, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yao, B.; Luo, Z.; Li, W.; Li, C.; Ye, Z.; Gong, X.; Yang, J.; Zhou, Y. Applications and influencing factors of the biochar-persulfate based advanced oxidation processes for the remediation of groundwater and soil contaminated with organic compounds. Sci. Total Environ. 2022, 836, 155421. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, Z.; Xu, Y.; Tian, J.; Qi, H.; Lin, W.; Cui, F. Oxidation of sulfamethoxazole (SMX) by chlorine, ozone and permanganate—A comparative study. J. Hazard. Mater. 2014, 274, 258–269. [Google Scholar] [CrossRef]
- Shi, Z.; Jin, C.; Bai, R.; Gao, Z.; Zhang, J.; Zhu, L.; Zhao, Z.; Strathmann, T.J. Enhanced Transformation of Emerging Contaminants by Permanganate in the Presence of Redox Mediators. Environ. Sci. Technol. 2020, 54, 1909–1919. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, Y.; Lei, Y.; Pan, Y.; Cheng, S.; Ouyang, G.; Yang, X. Redox-Active Moieties in Dissolved Organic Matter Accelerate the Degradation of Nitroimidazoles in SO4•−-Based Oxidation. Environ. Sci. Technol. 2021, 55, 14844–14853. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Wu, H.; Zhu, Z.; Zhou, J.; Guo, H. Trade-off effect of dissolved organic matter on degradation and transformation of micropollutants: A review in water decontamination. J. Hazard. Mater. 2023, 450, 130996. [Google Scholar] [CrossRef]
- Guo, K.; Wei, W.; Wu, S.; Song, W.; Fang, J. Abatement of Structurally Diverse Micropollutants by the UV/Permanganate Process: Roles of Hydroxyl Radicals and Reactive Manganese Species. ACS EST Water 2022, 2, 593–603. [Google Scholar] [CrossRef]
- Wu, Q.-Y.; Yang, Z.-W.; Du, Y.; Ouyang, W.-Y.; Wang, W.-L. The promotions on radical formation and micropollutant degradation by the synergies between ozone and chemical reagents (synergistic ozonation): A review. J. Hazard. Mater. 2021, 418, 126327. [Google Scholar] [CrossRef] [PubMed]
- Westerhoff, P.; Aiken, G.; Amy, G.; Debroux, J. Relationships between the structure of natural organic matter and its reactivity towards molecular ozone and hydroxyl radicals. Water Res. 1999, 33, 2265–2276. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, Y.; Pan, Y.; Yu, J.; Lei, Y.; Lei, X.; Ouyang, G.; Yang, X. Role of Antioxidant Moieties in the Quenching of a Purine Radical by Dissolved Organic Matter. Environ. Sci. Technol. 2022, 56, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhu, X.; Wang, Z.; Zhu, D.; Yang, S.; Liu, H.; Li, B.; Tian, S. Insight into utilisation of ilex pur-purea waste extract as an eco-friendly promoter in Fe activated persulfate oxidation for remediation of organic contaminated soil. Chem. Eng. J. 2022, 431, 134222. [Google Scholar] [CrossRef]
- Xu, M.; Deng, J.; Cai, A.; Ma, X.; Li, J.; Li, Q.; Li, X. Comparison of UVC and UVC/persulfate processes for tetracycline removal in water. Chem. Eng. J. 2020, 384, 123320. [Google Scholar] [CrossRef]
- Chow, A.T. Natural organic matter under human-influenced environments: Implications for future environmental quality research. J. Environ. Qual. 2021, 50, 1347–1350. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Kunhikrishnan, A.; James, T.; McDowell, R.; Senesi, N. Dissolved organic matter: Biogeochemistry, dynamics, and environmental significance in soils. In Advances in Agronomy; Sparks, D.L., Ed.; ScienceDirect: Amsterdam, The Netherlands, 2011; Volume 110, pp. 1–75. [Google Scholar]
- Xu, W.; Walpen, N.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Pyrogenic Dissolved Organic Matter (pyDOM) from Biomass-Derived Chars. Environ. Sci. Technol. 2021, 55, 11434–11444. [Google Scholar] [CrossRef]
- Yang, X.; Rosario-Ortiz, F.L.; Lei, Y.; Pan, Y.; Lei, X.; Westerho, P. Multiple Roles of Dissolved Organic Matter in Advanced Oxidation Processes. Environ. Sci. Technol. 2022, 56, 11111–11131. [Google Scholar] [CrossRef]
- Dong, X.; Yang, X.; Hua, S.; Wang, Z.; Cai, T.; Jiang, C. Unraveling the mechanisms for persulfate-based remediation of triphenyl phosphate-contaminated soils: Complicated soil constituent effects on the formation and propagation of reactive oxygen species. Chem. Eng. J. 2021, 426, 130662. [Google Scholar] [CrossRef]
- Cuypers, C.; Grotenhuis, T.; Nierop, K.G.J.; Franco, E.M.; de Jager, A.; Rulkens, W. Amorphous and condensed organic matter domains: The effect of persulfate oxidation on the composition of soil/sediment organic matter. Chemosphere 2002, 48, 919–931. [Google Scholar] [CrossRef]
- Jia, J.; Liu, D.; Tian, J.; Wang, W.; Ni, J.; Wang, X. Visible-light-excited humic acid for peroxymonosulfate activation to degrade bisphenol A. Chem. Eng. J. 2020, 400, 125853. [Google Scholar] [CrossRef]
- Jia, H.; Li, L.; Fan, X.; Liu, M.; Deng, W.; Wang, C. Visible light photodegradation of phenanthrene catalyzed by Fe(III)-smectite: Role of soil organic matter. J. Hazard. Mater. 2013, 256–257, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chu, L.; Wang, X.; Fang, G.; Liu, C.; Chen, H.; Gu, C.; Gao, J. Roles of Natural Phenolic Compounds in Polycyclic Aromatic Hydrocarbons Abiotic Attenuation at Soil–Air Interfaces through Oxidative Coupling Reactions. Environ. Sci. Technol. 2023, 57, 11967–11976. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Chen, L.; Yan, L.; Cheng, T.; Wang, X.; Zhang, Y. Singlet oxygen-dominated transformation of oxytetracycline by peroxymonosulfate with CoAl-LDH modified hierarchical porous ceramics: Toxicity assessment. Chem. Eng. J. 2022, 436, 135199. [Google Scholar] [CrossRef]
- Li, W.; Guo, H.; Wang, C.; Zhang, Y.; Cheng, X.; Wang, J.; Yang, B.; Du, E. ROS reevaluation for degradation of 4-chloro-3,5-dimethylphenol (PCMX) by UV and UV/persulfate processes in the water: Kinetics, mechanism, DFT studies and toxicity evolution. Chem. Eng. J. 2020, 390, 124610. [Google Scholar] [CrossRef]
- Liu, Y.; Qiu, T.; Wu, Y.; Wang, S.; Liu, M.; Dong, W. Remediation of soil contaminated with ibuprofen by persulfate activated with gallic acid and ferric iron. Chem. Eng. J. 2021, 426, 127653. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, T.; Chen, X.; Duan, X.; Xu, G.; Bu, L.; Zhou, S.; Shi, Z. Unveiling the interaction of epigallocatechin-3-gallate with peroxymonosulfate for degradation of bisphenol S: Two-stage kinetics and identification of reactive species. Sep. Purif. Technol. 2021, 274, 119040. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Wang, B.; Wang, S.; Liu, M.; Wu, Y.; Lu, L.; Ren, H.; Li, H.; Dong, W.; et al. Degradation of ibuprofen in soil systems by persulfate activated with pyrophosphate chelated Fe(II). Chem. Eng. J. 2020, 379, 122145. [Google Scholar] [CrossRef]
- Guan, C.; Jiang, J.; Pang, S.; Ma, J.; Chen, X.; Lim, T.-T. Nonradical transformation of sulfamethoxazole by carbon nanotube activated peroxydisulfate: Kinetics, mechanism and product toxicity. Chem. Eng. J. 2019, 378, 122147. [Google Scholar] [CrossRef]
- Guan, C.; Jiang, J.; Pang, S.; Luo, C.; Ma, J.; Zhou, Y.; Yang, Y. Oxidation Kinetics of Bromophenols by Nonradical Activation of Peroxydisulfate in the Presence of Carbon Nanotube and Formation of Brominated Polymeric Products. Environ. Sci. Technol. 2017, 51, 10718–10728. [Google Scholar] [CrossRef]
- Guan, C.; Jiang, J.; Luo, C.; Pang, S.; Jiang, C.; Ma, J.; Jin, Y.; Li, J. Transformation of Iodide by Carbon Nanotube Activated Peroxydisulfate and Formation of Iodoorganic Compounds in the Presence of Natural Organic Matter. Environ. Sci. Technol. 2017, 51, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xu, R.; Chen, Z.; Gao, Z.; Zheng, S.; Song, H. Kinetics and mechanisms of diniconazole degradation by α-MnO2 activated peroxymonosulfate. Sep. Purif. Technol. 2022, 281, 119850. [Google Scholar] [CrossRef]
- Wang, M.-M.; Liu, L.-J.; Xi, J.-R.; Ding, Y.; Liu, P.-X.; Mao, L.; Ni, B.-J.; Wang, W.-K.; Xu, J. Lattice doping of Zn boosts oxygen vacancies in Co3O4 Nanocages: Improving persulfate activation via forming Surface-Activated complex. Chem. Eng. J. 2023, 451, 138605. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Yoshimura, C. Photocatalytic degradation of organic micropollutants: Inhibition mechanisms by different fractions of natural organic matter. Water Res. 2020, 174, 115643. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Bruning, H.; Liu, W.R.; Rijnaarts, H.; Yntema, D. Effect of dissolved natural organic matter on the photocatalytic micropollutant removal performance of TiO2 nanotube array. J. Photochem. Photobiol. A Chem. 2019, 371, 216–222. [Google Scholar] [CrossRef]
- Lutze, H.V.; Bircher, S.; Rapp, I.; Kerlin, N.; Bakkour, R.; Geisler, M.; von Sonntag, C.; Schmidt, T.C. Degradation of Chlorotriazine Pesticides by Sulfate Radicals and the Influence of Organic Matter. Environ. Sci. Technol. 2015, 49, 1673–1680. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Xiao, Y.; Chang, V.W.C.; Lim, T.-T. Kinetic and mechanistic investigation of azathioprine degradation in water by UV, UV/H2O2 and UV/persulfate. Chem. Eng. J. 2016, 302, 526–534. [Google Scholar] [CrossRef]
- Ling, L.; Zhang, D.; Fang, J.; Fan, C.; Shang, C. A novel Fe(II)/citrate/UV/peroxymonosulfate process for micropollutant degradation: Optimization by response surface methodology and effects of water matrices. Chemosphere 2017, 184, 417–428. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Khan, Z.U.H.; Ali, H.S.; Murtaza, B.; Khan, H.M.; Imran, M.; Muhammad, N. Hydroxyl and sulfate radical mediated degradation of ciprofloxacin using nano zerovalent manganese catalyzed S2O82-. Chem. Eng. J. 2019, 356, 199–209. [Google Scholar] [CrossRef]
- Guan, C.; Jiang, J.; Pang, S.; Chen, X.; Webster, R.D.; Lim, T.-T. Facile synthesis of pure g-C3N4 materials for peroxymonosulfate activation to degrade bisphenol A: Effects of precursors and annealing ambience on catalytic oxidation. Chem. Eng. J. 2020, 387, 123726. [Google Scholar] [CrossRef]
- Xie, P.; Ma, J.; Liu, W.; Zou, J.; Yue, S.; Li, X.; Wiesner, M.R.; Fang, J. Removal of 2-MIB and geosmin using UV/persulfate: Contributions of hydroxyl and sulfate radicals. Water Res. 2015, 69, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zheng, Y.; Shang, C.; Yin, R. Concentration-dependent chloride effect on radical distribution and micropollutant degradation in the sulfate radical-based AOPs. J. Hazard. Mater. 2022, 430, 128450. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Hua, Z.; Wu, Z.; Chen, C.; Hou, S.; Huang, B.; Wang, Y.; Wang, D.; Li, X.; Li, C.; et al. Insights into the effects of bromide at fresh water levels on the radical chemistry in the UV/peroxydisulfate process. Water Res. 2021, 197, 117042. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Zhou, S.; Shi, Z.; Bi, C.; Zhu, S.; Gao, N. Iron electrode as efficient persulfate activator for oxcarbazepine degradation: Performance, mechanism, and kinetic modeling. Sep. Purif. Technol. 2017, 178, 66–74. [Google Scholar] [CrossRef]
- Canonica, S.; Schonenberger, U. Inhibitory Effect of Dissolved Organic Matter on the Transformation of Selected Anilines and Sulfonamide Antibiotics Induced by the Sulfate Radical. Environ. Sci. Technol. 2019, 53, 11783–11791. [Google Scholar] [CrossRef]
- Lei, X.; Lei, Y.; Guan, J.; Westerhoff, P.; Yang, X. Kinetics and Transformations of Diverse Dissolved Organic Matter Fractions with Sulfate Radicals. Environ. Sci. Technol. 2022, 56, 4457–4466. [Google Scholar] [CrossRef]
- Kim, C.; Chin, Y.-P.; Son, H.; Hwang, I. Activation of persulfate by humic substances: Stoichiometry and changes in the optical properties of the humic substances. Water Res. 2022, 212, 118107. [Google Scholar] [CrossRef]
- Zhou, X.; Jawad, A.; Luo, M.; Luo, C.; Zhang, T.; Wang, H.; Wang, J.; Wang, S.; Chen, Z.; Chen, Z. Regulating activation pathway of Cu/persulfate through the incorporation of unreducible metal oxides: Pivotal role of surface oxygen vacancies. Appl. Catal. B Environ. 2021, 286, 119914. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, H.; Shi, Z.; Xing, Q.; Pi, Y. Activation of peroxymonosulfate by sulfonated cobalt (II) phthalocyanine for the degradation of organic pollutants: The role of high-valent cobalt-oxo species. Chem. Eng. J. 2023, 455, 140671. [Google Scholar] [CrossRef]
- Liao, G.; Qing, X.; Xu, P.; Li, L.; Lu, P.; Chen, W.; Xia, D. Synthesis of single atom cobalt dispersed on 2D carbon nanoplate and degradation of acetaminophen by peroxymonosulfate activation. Chem. Eng. J. 2022, 427, 132027. [Google Scholar] [CrossRef]
- Canonica, S.; Laubscher, H.U. Inhibitory effect of dissolved organic matter on triplet-induced oxidation of aquatic contaminants. Photochem. Photobiol. Sci. 2008, 7, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.P.; Sander, M. Antioxidant Properties of Humic Substances. Environ. Sci. Technol. 2012, 46, 4916–4925. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q. Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process. J. Hazard. Mater. 2015, 300, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhao, Y.; Pan, Y.; Lei, Y.; Zhou, Y.; Li, C.; Zhang, X.; Yang, X. Quantification of the diverse inhibitory effects of dissolved organic matter on transformation of micropollutants in UV/persulfate treatment. Water Res. 2022, 223, 118967. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Bu, L.; Wu, Y.; Zhou, S.; Shi, Z. Accelerated degradation of bisphenol A induced by the interaction of EGCG and Cu(II) in Cu(II)/EGCG/peroxymonosulfate process. Chem. Eng. J. 2020, 395, 125134. [Google Scholar] [CrossRef]
- Bu, L.; Bi, C.; Shi, Z.; Zhou, S. Significant enhancement on ferrous/persulfate oxidation with epigallocatechin-3-gallate: Simultaneous chelating and reducing. Chem. Eng. J. 2017, 321, 642–650. [Google Scholar] [CrossRef]
- Fang, Z.; Zhao, J.; Li, Y.; Wang, Y.; Qiu, T.; Wu, Y.; Dong, W.; Mailhot, G. Improving Fenton-like system with Catechin, an environmental-friendly polyphenol: Effects and mechanism. Chem. Eng. J. 2021, 426, 127946. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, J.; Wang, Y.; Wu, Y.; Dong, W.; Nie, M.; Wang, X. Enhancement of peroxymonosulfate activation by sinapic acid accelerating Fe(III)/Fe(II) cycle. Chem. Eng. J. 2022, 446, 137177. [Google Scholar] [CrossRef]
- Zhang, L.; Qian, Z.; Wang, L.; Jin, P.; Yang, S. Gallic acid enhanced bisphenol A degradation through Fe3+/peroxymonosulfate process. Water Supply 2022, 22, 4852–4863. [Google Scholar] [CrossRef]
- Li, M.; Luo, R.; Wang, C.; Zhang, M.; Zhang, W.; Klu, P.K.; Yan, Y.; Qi, J.; Sun, X.; Wang, L.; et al. Iron-tannic modified cotton derived Fe0/graphitized carbon with enhanced catalytic activity for bisphenol A degradation. Chem. Eng. J. 2019, 372, 774–784. [Google Scholar] [CrossRef]
- Wang, X.; Cai, W.; Ye, D.; Zhu, Y.; Cui, M.; Xi, J.; Liu, J.; Xing, W. Bio-based polyphenol tannic acid as universal linker between metal oxide nanoparticles and thermoplastic polyurethane to enhance flame retardancy and mechanical properties. Compos. Part B Eng. 2021, 224, 109206. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Dong, W. Trace catechin enhanced degradation of organic pollutants with activated peroxymonosulfate: Comprehensive identification of working oxidizing species. Chem. Eng. J. 2022, 429, 132408. [Google Scholar] [CrossRef]
- Yan, J.; Hu, L.; Gao, W.; Yang, L.; Qian, L.; Han, L.; Chen, M. Remediation of 1,2-dichlorobenzene contaminated soil by activated persulfate using green synthesized nanoscale zero valent iron: Activation mechanism and degradation pathways. J. Soils Sediments 2022, 22, 1135–1144. [Google Scholar] [CrossRef]
- Tang, S.; Liu, H.; Zhu, E.; Zhao, T.; Wang, Z.; Jiao, T.; Zhang, Q.; Yuan, D. Boosting peroxydisulfate Fenton-like reaction by protocatechuic acid chelated-Fe2+ with broad pH range. Sep. Purif. Technol. 2022, 301, 122056. [Google Scholar] [CrossRef]
- Meng, Y.; Li, Z.; Tan, J.; Li, J.; Wu, J.; Zhang, T.; Wang, X. Oxygen-doped porous graphitic carbon nitride in photocatalytic peroxymonosulfate activation for enhanced carbamazepine removal: Performance, influence factors and mechanisms. Chem. Eng. J. 2022, 429, 130860. [Google Scholar] [CrossRef]
- Li, Z.X.; Lowry, G.V.; Fan, J.; Liu, F.; Chen, J.W. High molecular weight components of natural organic matter preferentially adsorb onto nanoscale zero valent iron and magnetite. Sci. Total Environ. 2018, 628–629, 177–185. [Google Scholar] [CrossRef]
- Hu, J.D.; Zevi, Y.; Kou, X.M.; Xiao, J.; Wang, X.J.; Jin, Y. Effect of dissolved organic matter on the stability of magnetite nanoparticles under different pH and ionic strength conditions. Sci. Total Environ. 2010, 408, 3477–3489. [Google Scholar] [CrossRef]
- Rong, X.; Xie, M.; Kong, L.; Natarajan, V.; Ma, L.; Zhan, J. The magnetic biochar derived from banana peels as a persulfate activator for organic contaminants degradation. Chem. Eng. J. 2019, 372, 294–303. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Zhang, R.; Ding, Y.; Ren, Z.; Fu, M.; Cao, X.; Zeng, G. Singlet oxygen-dominated activation of peroxymonosulfate by passion fruit shell derived biochar for catalytic degradation of tetracycline through a non-radical oxidation pathway. J. Hazard. Mater. 2021, 419, 126495. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.-N.; Wang, L. Metal-free activation of persulfates by corn stalk biochar for the degradation of antibiotic norfloxacin: Activation factors and degradation mechanism. Chemosphere 2019, 237, 124454. [Google Scholar] [CrossRef]
- Wu, J.; Xu, Y.; Wu, P.; Chen, M.; Rehman, S.; Ye, Q.; Zhu, N. Effects of different dissolved organic matter on peroxymonosulfate activation over Co-Fe binary metal: Experiments and density functional theory. Chem. Eng. J. 2022, 450, 137770. [Google Scholar] [CrossRef]
- Zheng, M.; Daniels, K.D.; Park, M.; Nienhauser, A.B.; Clevenger, E.C.; Li, Y.; Snyder, S.A. Attenuation of pharmaceutically active compounds in aqueous solution by UV/CaO2 process: Influencing factors, degradation mechanism and pathways. Water Res. 2019, 164, 114922. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Li, Y.M.; Ping, Q.; Wang, L. MP-UV/CaO2 as a pretreatment method for the removal of carbamazepine and primidone in waste activated sludge and improving the solubilization of sludge. Water Res. 2019, 151, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Cui, Z.; Bai, Y.; Su, R. Indirect photodegradation of sulfathiazole and sulfamerazine: Influence of the CDOM components and seawater factors (salinity, pH, nitrate and bicarbonate). Sci. Total Environ. 2021, 750, 141762. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Teel, A.L.; Watts, R.J. Persulfate activation by subsurface minerals. J. Contam. Hydrol. 2010, 115, 34–45. [Google Scholar] [CrossRef]
- Ahmad, M.; Teel, A.L.; Watts, R.J. Mechanism of Persulfate Activation by Phenols. Environ. Sci. Technol. 2013, 47, 5864–5871. [Google Scholar] [CrossRef]
- Fang, G.; Gao, J.; Dionysiou, D.D.; Liu, C.; Zhou, D. Activation of Persulfate by Quinones: Free Radical Reactions and Implication for the Degradation of PCBs. Environ. Sci. Technol. 2013, 47, 4605–4611. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, T.; Yu, Y.; Wu, Y.; Pan, Y.; Yang, X. A novel peroxymonosulfate (PMS)-enhanced iron coagulation process for simultaneous removal of trace organic pollutants in water. Water Res. 2020, 185, 116136. [Google Scholar] [CrossRef]
- Fang, G.; Chen, X.; Wu, W.; Liu, C.; Dionysiou, D.D.; Fan, T.; Wang, Y.; Zhu, C.; Zhou, D. Mechanisms of Interaction between Persulfate and Soil Constituents: Activation, Free Radical Formation, Conversion, and Identification. Environ. Sci. Technol. 2018, 52, 14352–14361. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, J.; Gao, Y.; Pang, S.-Y.; Yang, Y.; Ma, J.; Gu, J.; Li, J.; Wang, Z.; Wang, L.-H.; et al. Activation of peroxymonosulfate by phenols: Important role of quinone intermediates and involvement of singlet oxygen. Water Res. 2017, 125, 209–218. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, J.; Gao, Y.; Ma, J.; Pang, S.-Y.; Li, J.; Lu, X.-T.; Yuan, L.-P. Activation of Peroxymonosulfate by Benzoquinone: A Novel Nonradical Oxidation Process. Environ. Sci. Technol. 2015, 49, 12941–12950. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Sleiman, M.; Ferronato, C.; Chovelon, J.-M.; Richard, C. Reactivity of sulfate radicals with natural organic matters. Environ. Chem. Lett. 2017, 15, 733–737. [Google Scholar] [CrossRef]
- Pan, T.; Wang, Y.; Yang, X.; Huang, X.-f.; Qiu, R.-l. Gallic acid accelerated BDE47 degradation in PMS/Fe(III) system: Oxidation intermediates autocatalyzed redox cycling of iron. Chem. Eng. J. 2020, 384, 123248. [Google Scholar] [CrossRef]
- Karim, A.V.; Jiao, Y.; Zhou, M.; Nidheesh, P.V. Iron-based persulfate activation process for environmental decontamination in water and soil. Chemosphere 2021, 265, 129057. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shao, P.; Gao, S.; Bai, Z.; Tian, J. Benzoquinone-assisted heterogeneous activation of PMS on Fe3S4 via formation of active complexes to mediate electron transfer towards enhanced bisphenol A degradation. Water Res. 2022, 226, 119218. [Google Scholar] [CrossRef]
- Dong, Z.-Y.; Lin, Y.-L.; Zhang, T.Y.; Hu, C.-Y.; Pan, Y.; Pan, R.; Tang, Y.-L.; Xu, B.; Gao, N.-Y. Enhanced coagulation and oxidation by the Mn(VII)-Fe(III)/peroxymonosulfate process: Performance and mechanisms. Water Res. 2022, 226, 119200. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Huang, D.; Wu, Y.; Dong, W. Enhanced activation of persulfate by Fe(III) and catechin without light: Reaction kinetics, parameters and mechanism. J. Hazard. Mater. 2021, 413, 125420. [Google Scholar] [CrossRef]
- Dong, X.; Feng, R.; Yang, X.; Jiang, Y.; Chen, L.; Chen, L.; Jiang, C.; Cai, T. Complexation and reduction of soil iron minerals by natural polyphenols enhance persulfate activation for the remediation of triphenyl phosphate (TPHP)-contaminated soil. Chem. Eng. J. 2022, 435, 134610. [Google Scholar] [CrossRef]
- Ruiz, S.H.; Wickramasekara, S.; Abrell, L.; Gao, X.D.; Chefetz, B.; Chorover, J. Complexation of trace organic contaminants with fractionated dissolved organic matter: Implications for mass spectrometric quantification. Chemosphere 2013, 91, 344–350. [Google Scholar] [CrossRef]
- Yang, P.; Jiang, T.; Cong, Z.; Liu, G.; Guo, Y.; Liu, Y.; Shi, J.; Hu, L.; Yin, Y.; Cai, Y.; et al. Loss and Increase of the Electron Exchange Capacity of NaturalOrganic Matter during Its Reduction and Reoxidation: The Role ofQuinone and Nonquinone Moieties. Environ. Sci. Technol. 2022, 56, 6744–6753. [Google Scholar] [CrossRef]
- Dai, H.; Wu, B.; Chen, B.; Ma, B.; Chu, C. Diel Fluctuation of Extracellular Reactive Oxygen Species Production in the Rhizosphere of Rice. Environ. Sci. Technol. 2022, 56, 9075–9082. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yu, Y.; Lei, X.; Liang, X.; Cheng, S.; Ouyang, G.; Yang, X. Assessing the Use of Probes and Quenchers for Understanding the Reactive Species in Advanced Oxidation Processes. Environ. Sci. Technol. 2023, 57, 5433–5444. [Google Scholar] [CrossRef] [PubMed]

| References | Effects of NOM | Type of NOM | Type of PS-AOPs | Target Pollutant |
|---|---|---|---|---|
| [83] | Fe(III)-CAT complexes, quinone intermediates, and CAT radicals are involved in ROS generation by generating intermediates and electron transfer. | Catechin (CAT)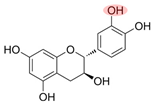 | CAT/Fe(III)/PMS | Ofloxacin |
| [81] | An Fe-TA structure is formed. TA can bind strongly to the carbon substrate through hydrogen bonding, promoting material recombination and improving catalyst performance. | Tannic acid (TA) | Fe-TCs 1/PMS | Bisphenol A |
| [84] | Fe(II) is reduced to Fe0, preventing the aggregation of nanoparticles and promoting dispersion. | Extracted tea polyphenol (TP)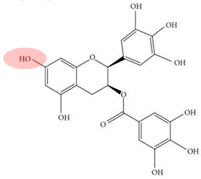 | nZVI/PDS | 1,2-Dichlorobenzene |
| [85] | PCA complexes Fe(III) and reduces it to Fe(II). Semiquinones and ortho-quinones generated by PCA conversion promote the conversion of Fe(III)/Fe(II). | Protocatechuic acid (PCA) | PCA/Fe(II)/PDS | Methyl orange |
| [79] | Formation of quinone compounds by SA during electron transfer and oxidation promotes the Fe(III)/Fe(II) cycle. | Sinapic acid (SA) | SA/Fe(III)/PMS | Methylparaben |
| [86] | HA interacts with pollutants and co-adsorbs or accumulates on the catalyst to promote the degradation of carbamazepine. HA activates PMS to some extent. | Humic acid (HA) | HA/OCN 2/PMS | Carbamazepine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Lin, H.; Li, X.; Wang, Y.; Ye, L.; Mai, Y.; Wu, P.; Ni, Z.; Lin, Q.; Qiu, R. The Dual Role of Natural Organic Matter in the Degradation of Organic Pollutants by Persulfate-Based Advanced Oxidation Processes: A Mini-Review. Toxics 2024, 12, 770. https://doi.org/10.3390/toxics12110770
Luo D, Lin H, Li X, Wang Y, Ye L, Mai Y, Wu P, Ni Z, Lin Q, Qiu R. The Dual Role of Natural Organic Matter in the Degradation of Organic Pollutants by Persulfate-Based Advanced Oxidation Processes: A Mini-Review. Toxics. 2024; 12(11):770. https://doi.org/10.3390/toxics12110770
Chicago/Turabian StyleLuo, Dan, Hansen Lin, Xingzhen Li, Yu Wang, Long Ye, Yuebang Mai, Peihao Wu, Zhuobiao Ni, Qingqi Lin, and Rongliang Qiu. 2024. "The Dual Role of Natural Organic Matter in the Degradation of Organic Pollutants by Persulfate-Based Advanced Oxidation Processes: A Mini-Review" Toxics 12, no. 11: 770. https://doi.org/10.3390/toxics12110770
APA StyleLuo, D., Lin, H., Li, X., Wang, Y., Ye, L., Mai, Y., Wu, P., Ni, Z., Lin, Q., & Qiu, R. (2024). The Dual Role of Natural Organic Matter in the Degradation of Organic Pollutants by Persulfate-Based Advanced Oxidation Processes: A Mini-Review. Toxics, 12(11), 770. https://doi.org/10.3390/toxics12110770







