Composition and Morphological Characteristics of Extracellular Polymeric Substances of Different Tolerant Bacteria Under Perfluorobutanesulfonic Acid (PFBS) Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Acquisition
2.2. Medium and Chemicals
2.3. Strains Screening and Identification
2.3.1. Strain Screening
2.3.2. Strain Identification
2.4. Effects of PFBS Stress on the Growth Curves and the Electron Transport System Activity (ETSA) of Different Tolerant Bacteria
2.5. Effects of PFBS Stress on Different Strains’ EPS
2.5.1. EPS Extraction
2.5.2. PFBS Extraction
2.6. Analytical Methods
2.6.1. EPS Quantitation
2.6.2. Chemical Analysis
2.6.3. The Fluorescence Spectra Assessment of 3-Dimensional Excitation–Emission Matrix (3D-EEM) and Its Fluorescence Quenching Characterization
2.6.4. Fourier Transform Infrared (FTIR) Analysis
2.7. Statistical Analyses
3. Results and Discussion
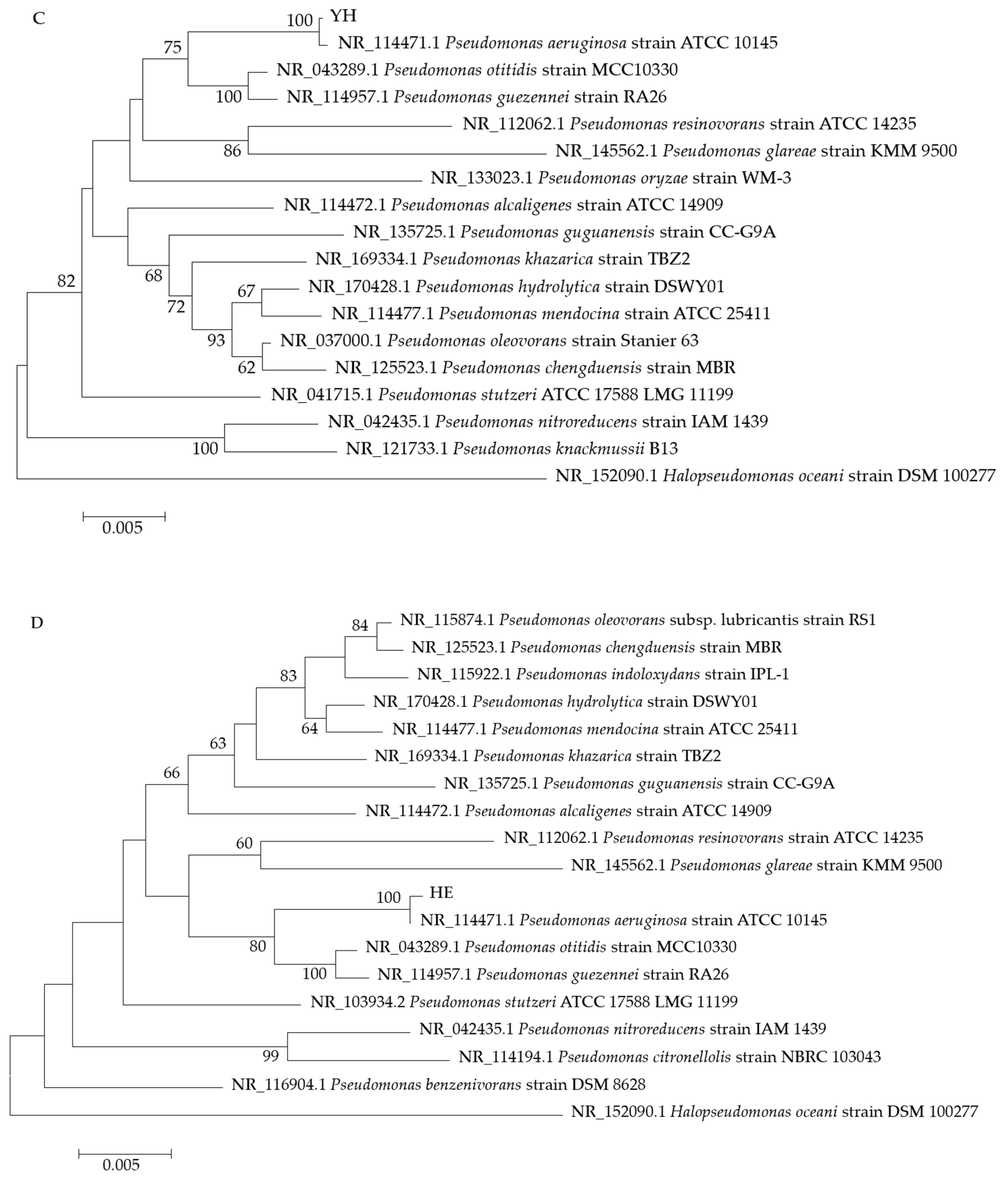
3.1. Strain Screening and Identification
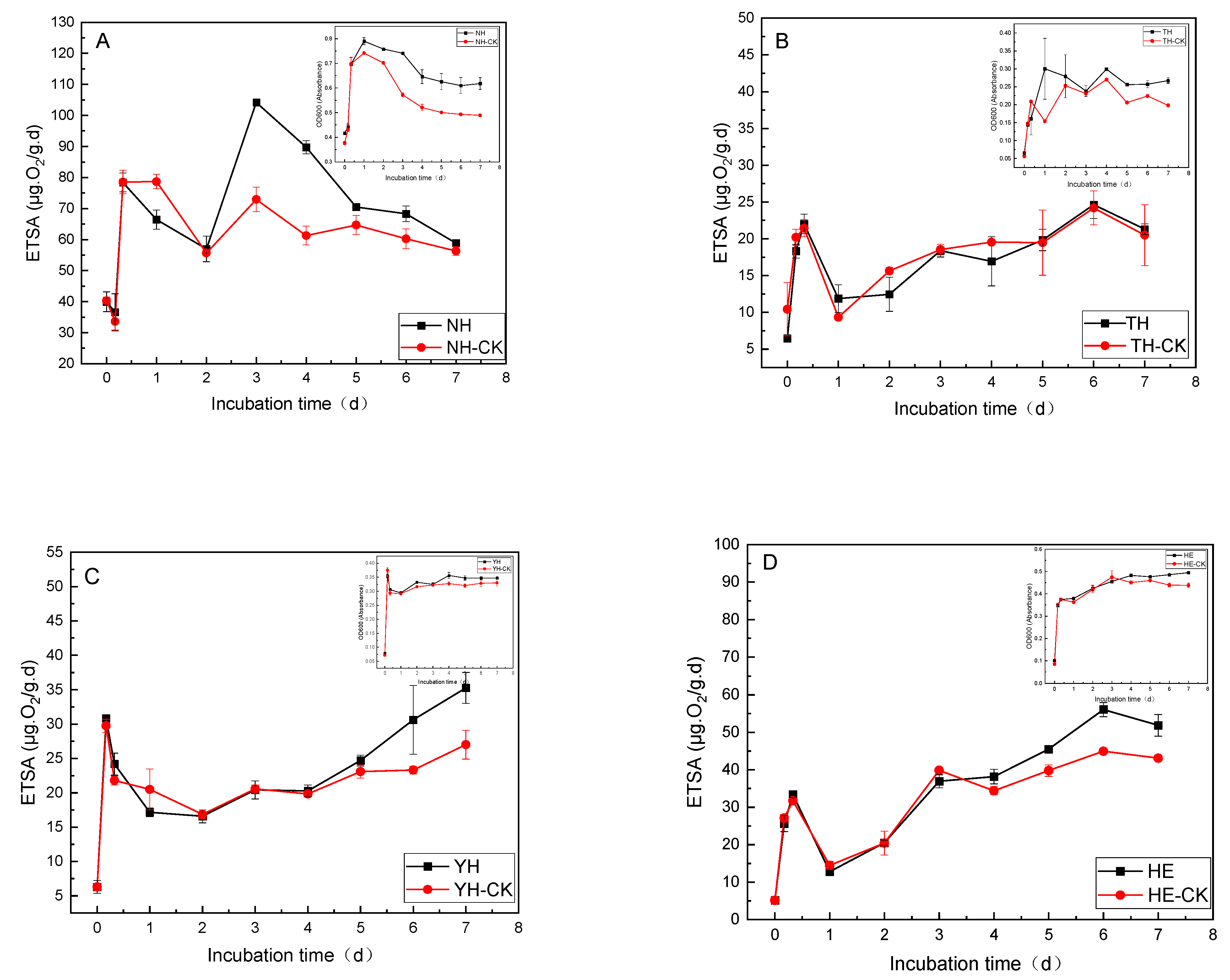
3.2. PFBS Stress Effects on the Growth Curves and ETSA of Different Tolerant Bacteria
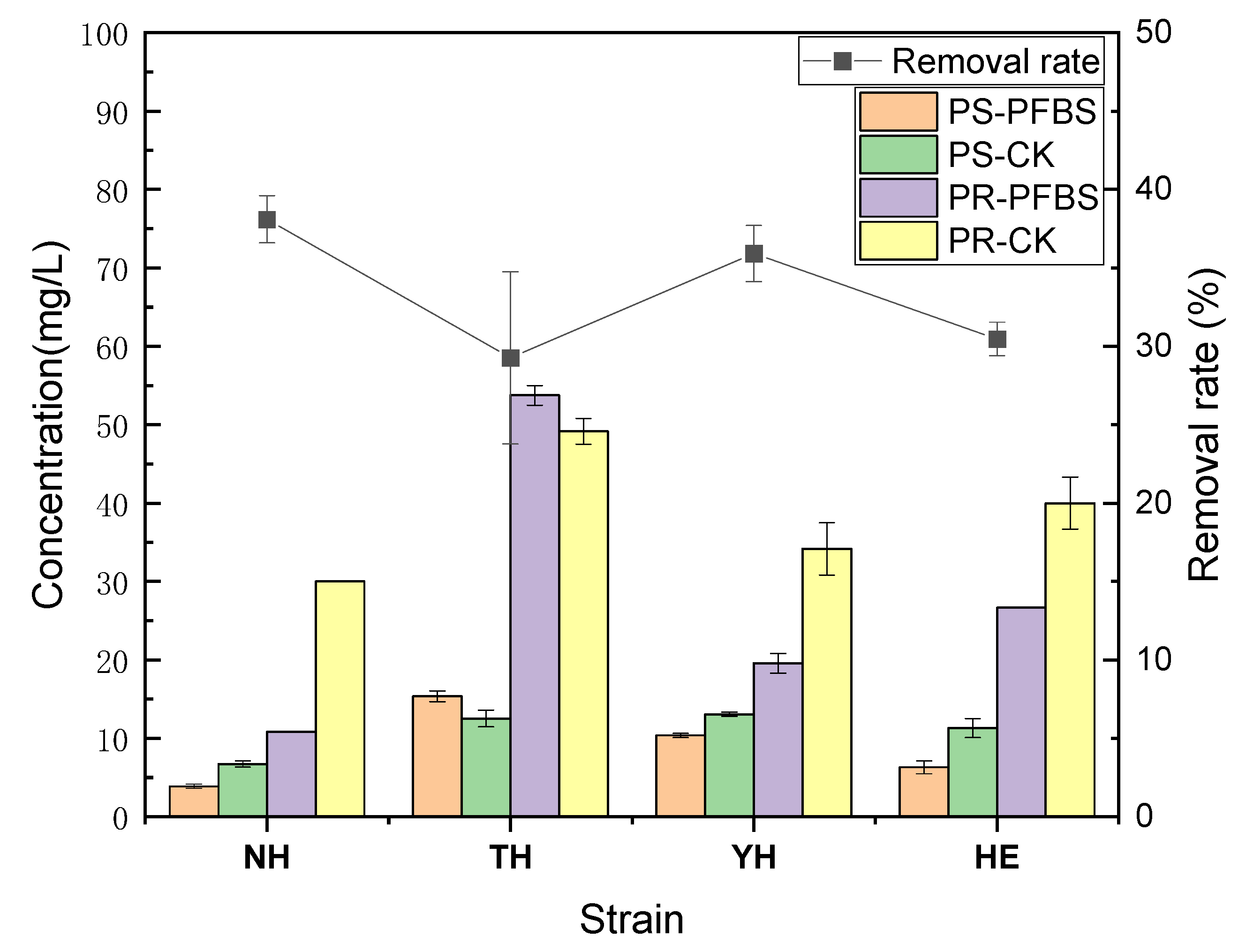
3.3. Effects on the Content of EPS Components and PFBS Removal Rates of Different Strains under the Stress of PFBS
3.4. 3D-EEM of EPS of Different Strains Under PFBS Stress
3.5. FTIR Analysis of EPS of Different Strains Under the Stress of PFBS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, W.; Hu, L.; Chen, Z.; Tang, J.; Pan, Y.; Yan, W.; Chen, X.; Peng, Y.; Chen, L.J.E.; Safety, E. Effects of perfluorinated compounds homologues on chemical property, microbial composition, richness and diversity of urban forest soil. Ecotoxicol. Environ. Saf. 2023, 249, 114458. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Maroli, A.; Tharayil, N.; Karanfil, T. The overlooked short- and ultrashort-chain poly- and perfluorinated substances: A review. Chemosphere 2019, 220, 866–882. [Google Scholar] [CrossRef] [PubMed]
- Huff, D.K.; Morris, L.A.; Sutter, L.; Costanza, J.; Pennell, K.D. Accumulation of six PFAS compounds by woody and herbaceous plants: Potential for phytoextraction. Int. J. Phytoremediat. 2020, 22, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Sarkar, B.; Vithanage, M.; Singh, G.; Tsang, D.C.W.; Mukhopadhyay, R.; Ramadass, K.; Vinu, A.; Sun, Y.; Ramanayaka, S.; et al. Distribution, behaviour, bioavailability and remediation of poly- and per-fluoroalkyl substances (PFAS) in solid biowastes and biowaste-treated soil. Environ. Int. 2021, 155, 106600. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.; Bond, D.; Foley, J. PFAS soil and groundwater contamination via industrial airborne emission and land deposition in SW Vermont and Eastern New York State, USA. Environ. Sci. Process. Impacts 2021, 23, 291–301. [Google Scholar] [CrossRef]
- Li, J.; He, J.; Niu, Z.; Zhang, Y. Legacy per- and polyfluoroalkyl substances (PFASs) and alternatives (short-chain analogues, F-53B, GenX and FC-98) in residential soils of China: Present implications of replacing legacy PFASs. Environ. Int 2020, 135, 105419. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Liu, X.; Wang, X.; Li, B.; Du, Z.; Juhasz, A.; Wang, J.; Wang, J.; Zhu, L. Are PFBS, PFHxS, and 6:2FTSA more friendly to the soil environment compared to PFOS? A new insight based on ecotoxicity study in soil invertebrates (Eisenia fetida). Sci. Total Environ. 2023, 904, 166689. [Google Scholar] [CrossRef]
- Cai, W.; Navarro, D.A.; Du, J.; Ying, G.; Yang, B.; McLaughlin, M.J.; Kookana, R.S. Increasing ionic strength and valency of cations enhance sorption through hydrophobic interactions of PFAS with soil surfaces. Sci. Total Environ. 2022, 817, 152975. [Google Scholar] [CrossRef]
- Xie, Q.; Xu, B.; Rillig, M.C. Litter Decomposition Is Not Affected by Perfluorobutane Sulfonate (PFBS) in Experimental Soil Microcosms. Soil Syst. 2023, 7, 13. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, T.; Meng, J.; Wang, P.; Li, Q.; Lu, Y. Perfluoroalkyl substances in the Daling River with concentrated fluorine industries in China: Seasonal variation, mass flow, and risk assessment. Environ. Sci. Pollut. Res. Int. 2015, 22, 10009–10018. [Google Scholar] [CrossRef]
- Ivantsova, E.; Lu, A.; Martyniuk, C.J. Occurrence and toxicity mechanisms of perfluorobutanoic acid (PFBA) and perfluorobutane sulfonic acid (PFBS) in fish. Chemosphere 2024, 349, 140815. [Google Scholar] [CrossRef] [PubMed]
- Munoz, G.; Liu, J.; Vo Duy, S.; Sauvé, S. Analysis of F-53B, Gen-X, ADONA, and emerging fluoroalkylether substances in environmental and biomonitoring samples: A review. Trends Environ. Anal. Chem. 2019, 23, e00066. [Google Scholar] [CrossRef]
- Lau, C.; Anitole, K.; Hodes, C.; Lai, D.; Pfahles-Hutchens, A.; Seed, J.J.T.S. Perfluoroalkyl Acids: A Review of Monitoring and Toxicological Findings. Toxicol. Sci. 2007, 99, 366–394. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.; Roccaro, P.J.W.R. Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115331–115381. [Google Scholar] [CrossRef]
- Soriano, A.; Schaefer, C.; Urtiaga, A. Enhanced treatment of perfluoroalkyl acids in groundwater by membrane separation and electrochemical oxidation. Chem. Eng. J. Adv. 2020, 4, 100042. [Google Scholar] [CrossRef]
- Bao, Y.; Huang, J.; Cagnetta, G.; Yu, G. Removal of F-53B as PFOS alternative in chrome plating wastewater by UV/Sulfite reduction. Water Res. 2019, 163, 114907. [Google Scholar] [CrossRef]
- Kwon, B.G.; Lim, H.-J.; Na, S.-H.; Choi, B.-I.; Shin, D.-S.; Chung, S.-Y. Biodegradation of perfluorooctanesulfonate (PFOS) as an emerging contaminant. Chemosphere 2014, 109, 221–225. [Google Scholar] [CrossRef]
- Deng, S.; Zhou, Q.; Wang, Y.; Liu, Y.; Xia, K.; Cao, B.; Jiang, Z.; Zhang, Y. Nicosulfuron on the Atrazine-degrading Arthrobacter sp. DNS10 by assays of intracellular accumulation of substrates, Zeta potential, EPS, and stress responses. Int. Biodeterior. Biodegrad. 2023, 181, 105616. [Google Scholar] [CrossRef]
- Wei, Z.; Niu, S.; Wei, Y.; Liu, Y.; Xu, Y.; Yang, Y.; Zhang, P.; Zhou, Q.; Wang, J.J. The role of extracellular polymeric substances (EPS) in chemical-degradation of persistent organic pollutants in soil: A review. Sci. Total Environ. 2024, 912, 168877. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, H.; Buchanan, I.; Mohammed, A.; Liu, Y. Comparison of extracellular polymeric substance (EPS) in nitrification and nitritation bioreactors. Int. Biodeterior. Biodegrad. 2019, 143, 104713. [Google Scholar] [CrossRef]
- Siddharth, T.; Sridhar, P.; Vinila, V.; Tyagi, R.D. Environmental applications of microbial extracellular polymeric substance (EPS): A review. J. Environ. Manag. 2021, 287, 112307. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Li, X.; Allinson, G.; Liu, C.; Gong, Z. Composition and morphology characterization of exopolymeric substances produced by the PAH-degrading fungus of Mucor mucedo. Environ. Sci. Pollut. Res. Int. 2016, 23, 8421–8430. [Google Scholar] [CrossRef] [PubMed]
- Weathers, T.S.; Higgins, C.P.; Sharp, J.O.J.E.S. Enhanced Biofilm Production by a Toluene-Degrading Rhodococcus Observed after Exposure to Perfluoroalkyl Acids. Environ. Sci. Technol. 2015, 49, 5458–5466. [Google Scholar] [CrossRef]
- Yan, W.; Qian, T.; Zhang, L.; Wang, L.; Zhou, Y. Interaction of perfluorooctanoic acid with extracellular polymeric substances-Role of protein. J. Hazard. Mater. 2021, 401, 123381. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.B.; Chai, L.Y.; Xie, Y.; Peng, Q.J.; Peng, Q.Z. Isolation, identification, and degradation performance of a PFOA-degrading strain. Genet. Mol. Res. 2016, 15, 15028043. [Google Scholar] [CrossRef]
- Yuanwei, Q.; Huimin, Y.; Jiyuan, L.; Jinwei, D.; Jingqing, C.; Xiangming, X.J. Impacts of ecological restoration projects on agricultural productivity in China. J. Geogr. Sci. 2013, 23, 404–416. [Google Scholar]
- Zhang, R.C.; Xu, X.J.; Chen, C.; Xing, D.F.; Shao, B.; Liu, W.Z.; Wang, A.J.; Lee, D.J.; Ren, N.Q.J.W.R. Interactions of functional bacteria and their contributions to the performance in integrated autotrophic and heterotrophic denitrification. Water Res. 2018, 143, 355–366. [Google Scholar] [CrossRef]
- He, S.; Ni, Y.; Lu, L.; Chai, Q.; Yu, T.; Shen, Z.; Yang, C. Simultaneous degradation of n-hexane and production of biosurfactants by Pseudomonas sp. strain NEE2 isolated from oil-contaminated soils. Chemosphere 2020, 242, 125237. [Google Scholar] [CrossRef]
- Gui, M.; Chen, Q.; Ni, J. Effect of NaCl on aerobic denitrification by strain Achromobacter sp. GAD-3. Appl. Microbiol. Biotechnol. 2017, 101, 5139–5147. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Keller, J.M.; Kannan, K.; Taniyasu, S.; Yamashita, N.; Day, R.D.; Arendt, M.D.; Segars, A.L.; Kucklick, J.R. Perfluorinated compounds in the plasma of loggerhead and Kemp’s ridley sea turtles from the southeastern coast of the United States. Environ. Sci. Technol. 2005, 39, 9101–9108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, B.; Jin, M.; Gong, T.; Gao, Z. Extraction and analysis of extracellular polymeric substances in membrane fouling in SMBR. Desalination 2007, 227, 286–294. [Google Scholar]
- Tan, C.H.; Koh, K.S.; Xie, C.; Tay, M.; Zhou, Y.; Williams, R.; Ng, W.J.; Rice, S.A.; Kjelleberg, S.J.I.J. The role of quorum sensing signalling in EPS production and the assembly of a sludge community into aerobic granules. ISME J. 2014, 8, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Strotmann, U.; Durand, M.-J.; Thouand, G.; Eberlein, C.; Heipieper, H.J.; Gartiser, S.; Pagga, U. Microbiological toxicity tests using standardized ISO/OECD methods—Current state and outlook. Appl. Microbiol. Biotechnol. 2024, 108, 454. [Google Scholar] [CrossRef] [PubMed]
- Strotmann, U.; Thouand, G.; Pagga, U.; Gartiser, S.; Heipieper, H.J. Toward the future of OECD/ISO biodegradability testing-new approaches and developments. Appl. Microbiol. Biotechnol. 2023, 107, 2073–2095. [Google Scholar] [CrossRef]
- Han, S.; Jin, W.; Tu, R.; Ding, B.; Zhou, X.; Gao, S.; Feng, X.; Yang, Q.; Wang, Q. Screening and mutagenesis of high-efficient degrading bacteria of linear alkylbenzene sulfonates. Chemosphere 2020, 245, 125559. [Google Scholar] [CrossRef]
- Zhou, Q.; Deng, S.; Zhang, Q.; Fan, Q.; Huang, J.; Yu, G. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated sludge. Chemosphere 2010, 81, 453–458. [Google Scholar] [CrossRef]
- Liu, X.; Yin, H.; Peng, H.; Li, H.; Yang, P.; Cao, Y. Effect of Extracellular Polymeric Substance (EPS) on the Adsorption of Perfluorooctane Sulfonate (PFOS) onto Activated Sludge. Environ. Sci. 2017, 38, 3435–3441. [Google Scholar]
- Hudson, N.; Baker, A.; Ward, D.; Reynolds, D.M.; Brunsdon, C.; Carliell-Marquet, C.; Browning, S. Can fluorescence spectrometry be used as a surrogate for the Biochemical Oxygen Demand (BOD) test in water quality assessment? An example from South West England. Sci. Total Environ. 2008, 391, 149–158. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence excitation-emission matrix regional integration to quantify spectra for dissolved organic matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Manuel, P. Functional transformation of Fourier-transform mid-infrared spectrum for the improving of spectral specificity by simple algorithm based on wavelet-like functions. J. Adv. Res. 2018, 14, 53–62. [Google Scholar]
- Wang, X.; Liu, Y.; Liu, Z.; Xing, C.; Yang, Y. Analysis of extracellular polymeric substances changes during the initial stage of aerobic granulation by 3D-EEM and FTIR. Environ. Chem. 2016, 35, 125–132. [Google Scholar]
- Song, J.; Zou, W.; Bian, Y.; Su, F.; Han, R. Adsorption characteristics of methylene blue by peanut husk in batch and column modes. Desalination 2011, 265, 119–125. [Google Scholar] [CrossRef]

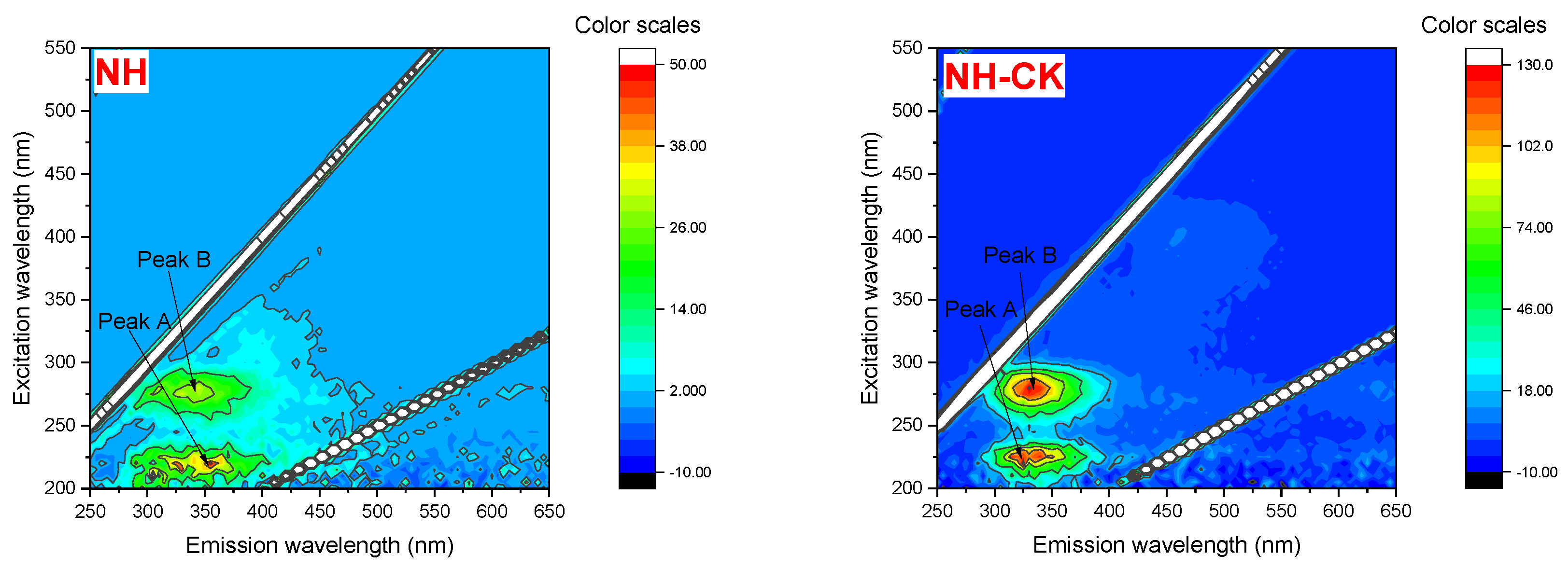
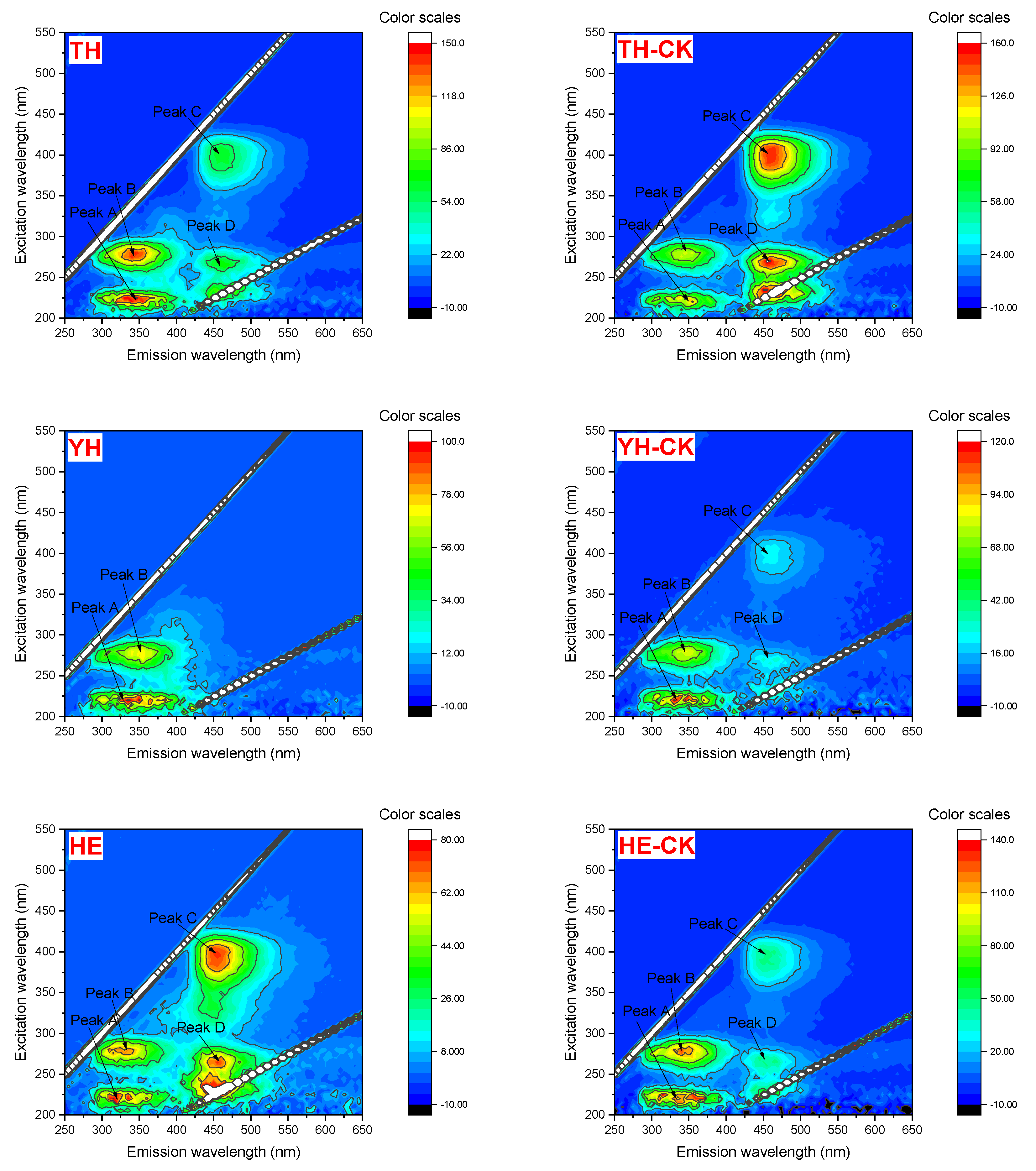

| Strains | PFBS (μg·L−1) | Peak A | Peak B | Peak C | Peak D | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ex/Em (nm) | Int (a.u) | Ex/Em (nm) | Int (a.u) | Ex/Em (nm) | Int (a.u) | Ex/Em (nm) | Int (a.u) | ||
| NH | 0 | 220.0/325.0 | 139.7 | 280.0/330.0 | 132.4 | —— | —— | —— | —— |
| 100 | 220.0/335.0 | 52.4 | 275.0/330.0 | 32.06 | —— | —— | —— | —— | |
| TH | 0 | 225.0/345.0 | 121.7 | 280.0/345.0 | 127.6 | 405.0/460.0 | 185.1 | 270.0/465.0 | 183.7 |
| 100 | 225.0/345.0 | 160.3 | 280.0/345.0 | 145.1 | 400.0/460.0 | 65.47 | 265.0/465.0 | 72.99 | |
| YH | 0 | 220.0/345.0 | 123.3 | 280.0/345.0 | 84.63 | 400.0/460.0 | 25.11 | 265.0/460.0 | 27.33 |
| 100 | 220.0/335.0 | 103.6 | 275.0/350.0 | 74.39 | —— | —— | —— | —— | |
| HE | 0 | 225.0/355.0 | 130.7 | 280.0/340.0 | 129.4 | 400.0/455.0 | 48.47 | 265.0/465.0 | 47.92 |
| 100 | 220.0/315.0 | 80.15 | 280.0/320.0 | 66.54 | 395.0/455.0 | 74.79 | 265.0/455.0 | 75.66 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, R.; Sun, L.; Yu, G.; Xu, J.; Luo, Q.; Wang, X.; Rong, L. Composition and Morphological Characteristics of Extracellular Polymeric Substances of Different Tolerant Bacteria Under Perfluorobutanesulfonic Acid (PFBS) Stress. Toxics 2024, 12, 797. https://doi.org/10.3390/toxics12110797
Tang R, Sun L, Yu G, Xu J, Luo Q, Wang X, Rong L. Composition and Morphological Characteristics of Extracellular Polymeric Substances of Different Tolerant Bacteria Under Perfluorobutanesulfonic Acid (PFBS) Stress. Toxics. 2024; 12(11):797. https://doi.org/10.3390/toxics12110797
Chicago/Turabian StyleTang, Rui, Lina Sun, Guo Yu, Jiayao Xu, Qing Luo, Xiaoxu Wang, and Luge Rong. 2024. "Composition and Morphological Characteristics of Extracellular Polymeric Substances of Different Tolerant Bacteria Under Perfluorobutanesulfonic Acid (PFBS) Stress" Toxics 12, no. 11: 797. https://doi.org/10.3390/toxics12110797
APA StyleTang, R., Sun, L., Yu, G., Xu, J., Luo, Q., Wang, X., & Rong, L. (2024). Composition and Morphological Characteristics of Extracellular Polymeric Substances of Different Tolerant Bacteria Under Perfluorobutanesulfonic Acid (PFBS) Stress. Toxics, 12(11), 797. https://doi.org/10.3390/toxics12110797







