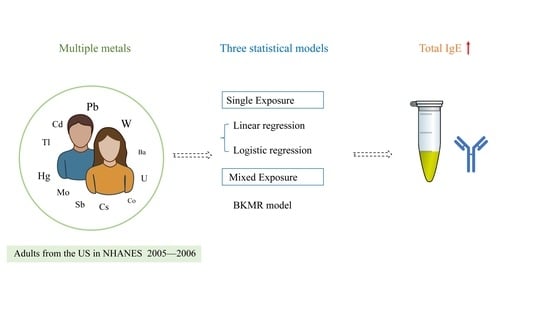
The Associations between Exposure to Multiple Heavy Metals and Total Immunoglobulin E in U.S. Adults
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Urinary Heavy Metals Measurements
2.3. Serum Total IgE Measurements
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
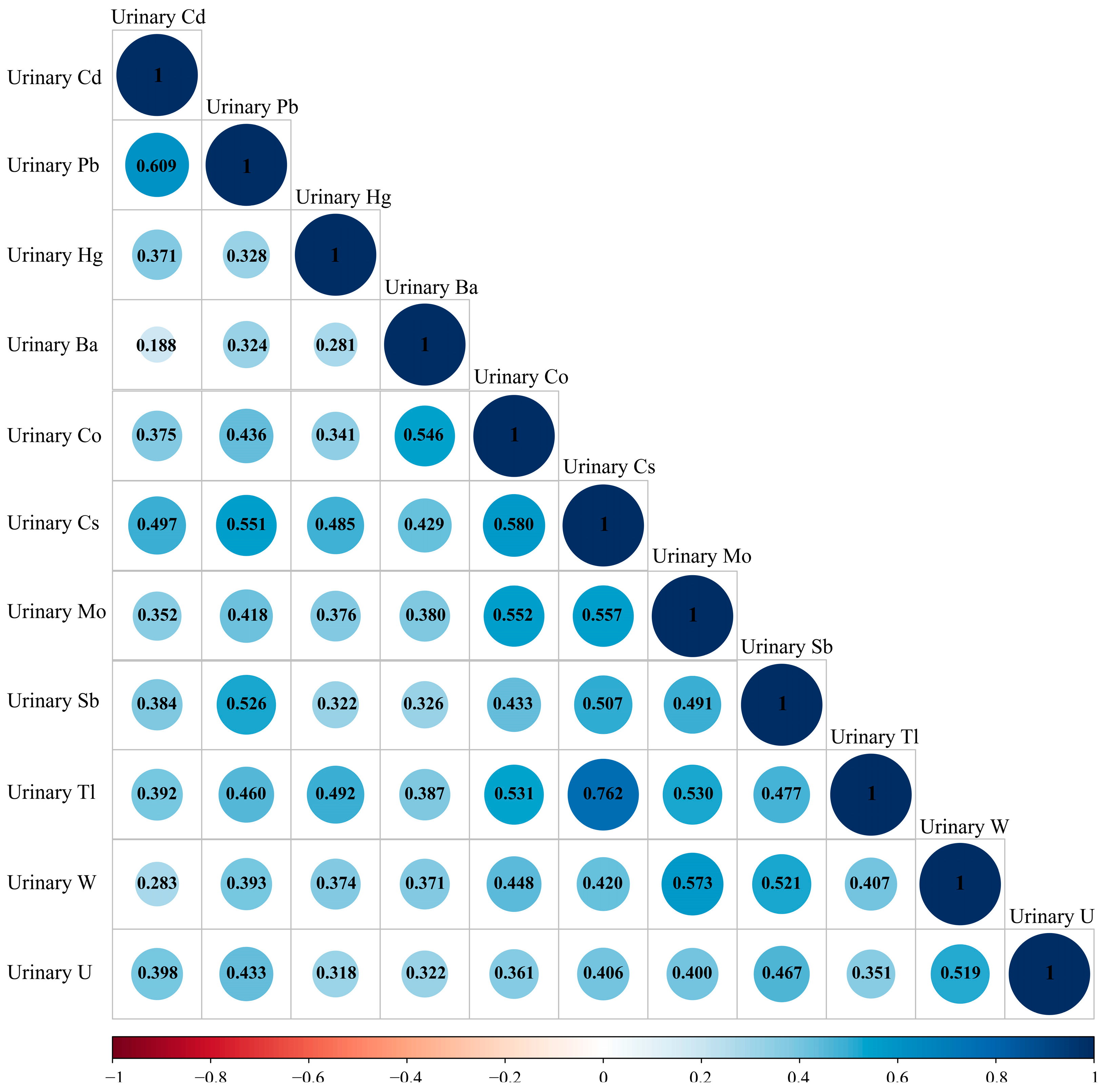
3.2. Urine Metal Concentration Distribution and Correlation
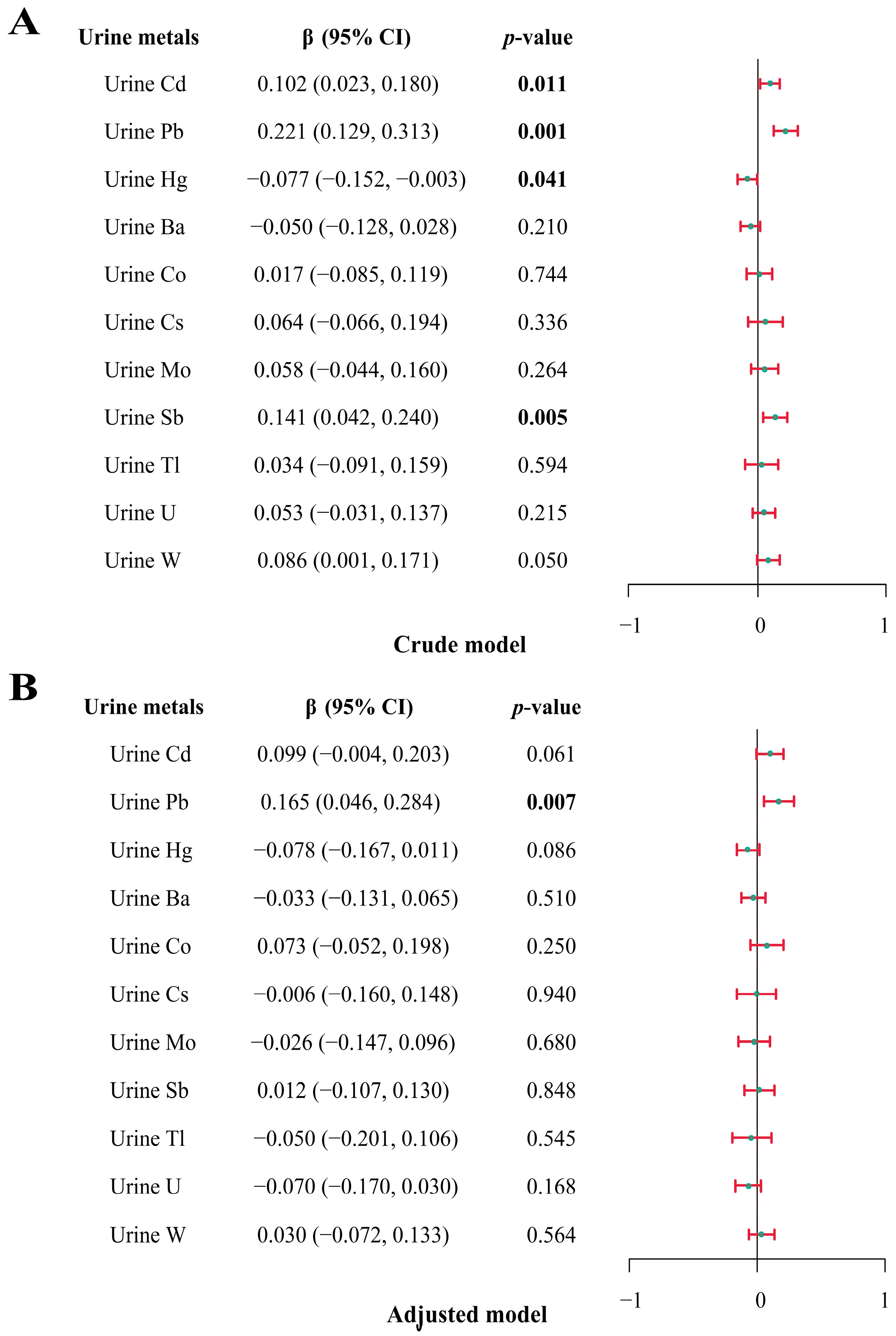
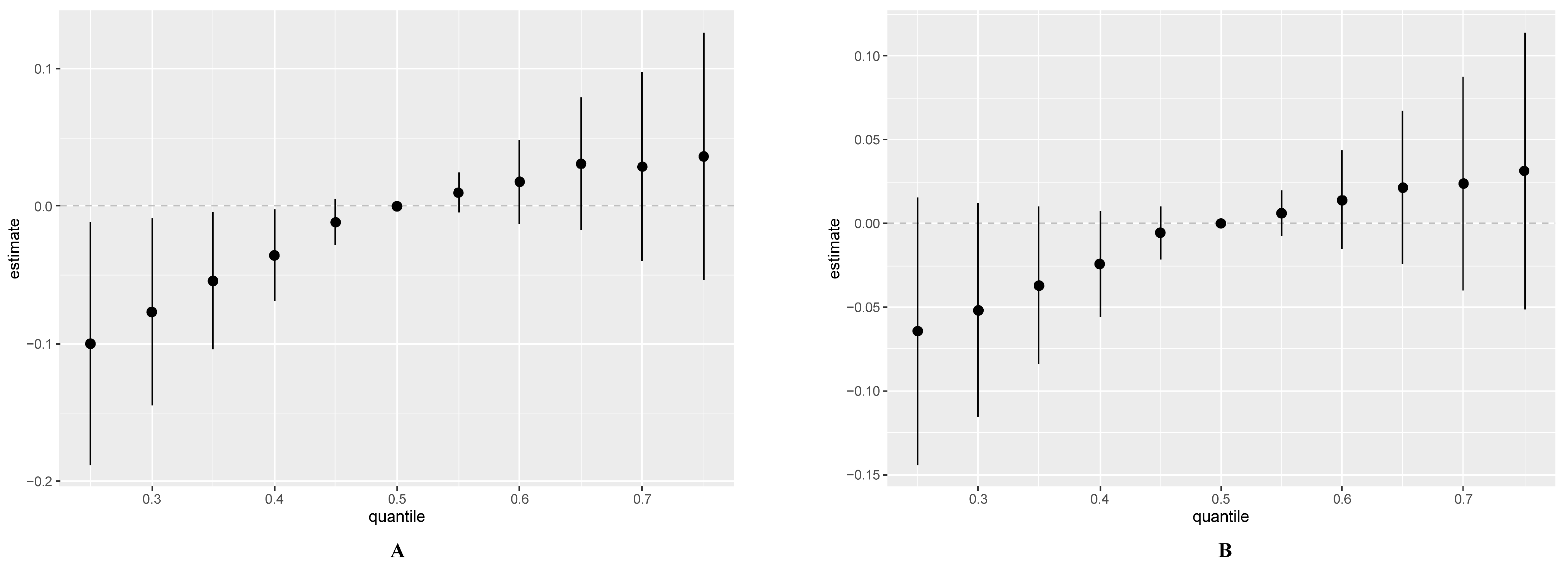
3.3. Association between Heavy-Metal Exposure and Total IgE Using Multiple Linear Regression
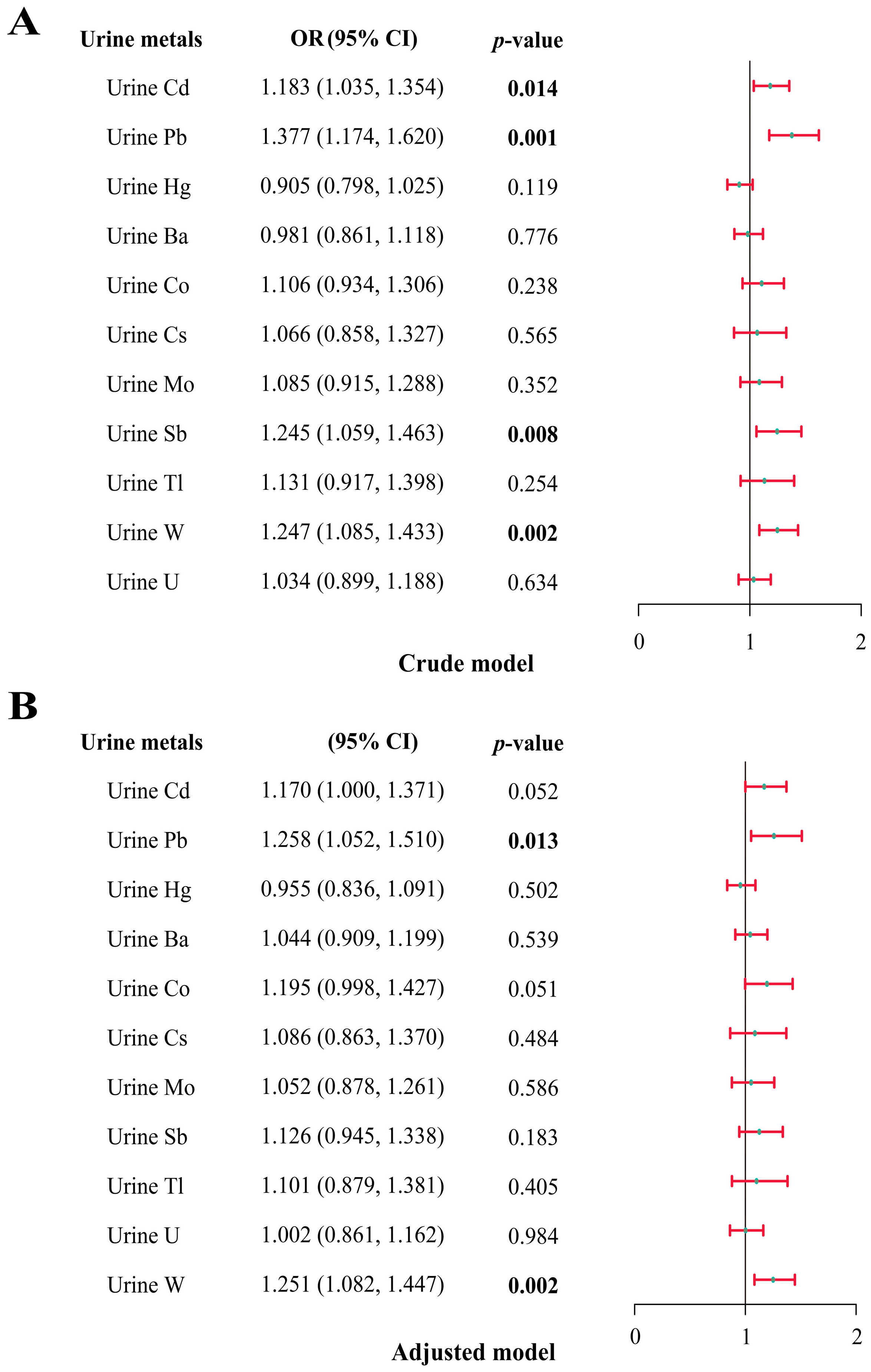
3.4. Multiple Logistic Regression Analysis of the Relationship between Multiple Metals and Total IgE
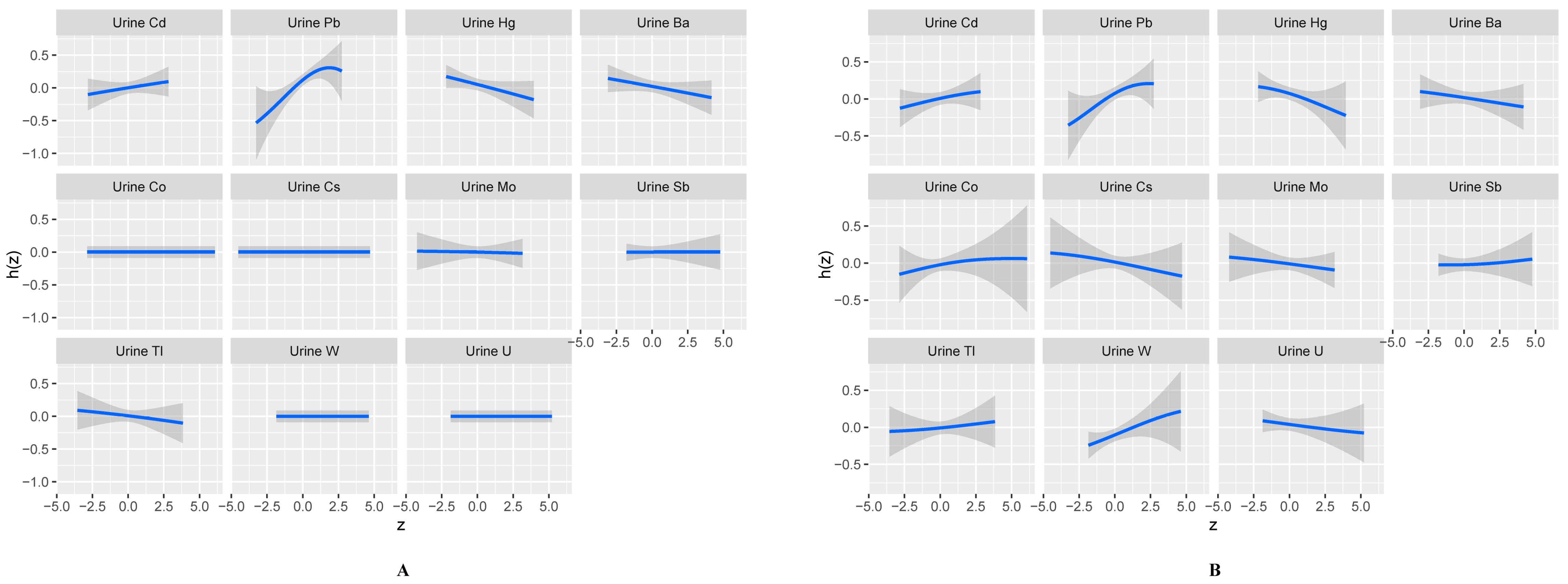
3.5. Effects of Exposure to Multiple Metals on Total IgE Using the BKMR Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lippi, G.; Cervellin, G.; Sanchis-Gomar, F. Immunoglobulin E (IgE) and ischemic heart disease. Which came first, the chicken or the egg? Ann. Med. 2014, 46, 456–463. [Google Scholar] [CrossRef]
- Mahachie John, J.M.; Baurecht, H.; Rodríguez, E.; Naumann, A.; Wagenpfeil, S.; Klopp, N.; Mempel, M.; Novak, N.; Bieber, T.; Wichmann, H.E.; et al. Analysis of the high affinity IgE receptor genes reveals epistatic effects of FCER1A variants on eczema risk. Allergy 2010, 65, 875–882. [Google Scholar] [CrossRef]
- Lee, S. IgE-mediated food allergies in children: Prevalence, triggers, and management. Korean J. Pediatr. 2017, 60, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Katelaris, C.H.; Lee, B.W.; Potter, P.C.; Maspero, J.F.; Cingi, C.; Lopatin, A.; Saffer, M.; Xu, G.; Walters, R.D. Prevalence and diversity of allergic rhinitis in regions of the world beyond Europe and North America. Clin. Exp. Allergy 2012, 42, 186–207. [Google Scholar] [CrossRef]
- Wang, H.T.; Warren, C.M.; Gupta, R.S.; Davis, C.M. Prevalence and Characteristics of Shellfish Allergy in the Pediatric Population of the United States. J. Allergy Clin. Immunol. Pract. 2020, 8, 1359–1370. [Google Scholar] [CrossRef]
- Stern, J.; Pier, J.; Litonjua, A.A. Asthma epidemiology and risk factors. Semin. Immunopathol. 2020, 42, 5–15. [Google Scholar] [CrossRef]
- Ridd, M.J.; King, A.J.L.; Le Roux, E.; Waldecker, A.; Huntley, A.L. Systematic review of self-management interventions for people with eczema. Br. J. Dermatol. 2017, 177, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Novak, N.; Bieber, T. Allergic and nonallergic forms of atopic diseases. J. Allergy Clin. Immunol. 2003, 112, 252–262. [Google Scholar] [CrossRef]
- Kemter, A.M.; Nagler, C.R. Influences on allergic mechanisms through gut, lung, and skin microbiome exposures. J. Clin. Invest. 2019, 129, 1483–1492. [Google Scholar] [CrossRef]
- Hamzah, N.A.; Mohd Tamrin, S.B.; Ismail, N.H. Metal dust exposure and lung function deterioration among steel workers: An exposure-response relationship. Int. J. Occup. Environ. Health 2016, 22, 224–232. [Google Scholar] [CrossRef]
- Hashem, M.A.; Hasan, M.A.; Nayan, A.H.; Payel, S.; Hasan, M.; Sahen, M.S. The environmental impacts of heavy metals in soil, certain plants and wastewater near industrial area of Brahmanbaria, Bangladesh. Environ. Monit. Assess. 2021, 193, 688. [Google Scholar] [CrossRef]
- Schubert, S.; Brans, R.; Reich, A.; Hansen, A.; Buhl, T.; Skudlik, C.; Mempel, M.; Schön, M.P.; John, S.M.; Geier, J. Assessment of occupational exposure and spectrum of contact sensitization in metalworkers with occupational dermatitis: Results of a cohort study within the OCCUDERM project. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1536–1544. [Google Scholar] [CrossRef]
- Quirce, S.; Vandenplas, O.; Campo, P.; Cruz, M.J.; de Blay, F.; Koschel, D.; Moscato, G.; Pala, G.; Raulf, M.; Sastre, J.; et al. Occupational hypersensitivity pneumonitis: An EAACI position paper. Allergy 2016, 71, 765–779. [Google Scholar] [CrossRef]
- Huang, X.; Xie, J.; Cui, X.; Zhou, Y.; Wu, X.; Lu, W.; Shen, Y.; Yuan, J.; Chen, W. Association between Concentrations of Metals in Urine and Adult Asthma: A Case-Control Study in Wuhan, China. PLoS ONE 2016, 11, e0155818. [Google Scholar] [CrossRef]
- Rosenman, K.D.; Beckett, W.S. Web based listing of agents associated with new onset work-related asthma. Respir. Med. 2015, 109, 625–631. [Google Scholar] [CrossRef]
- Crewe, J.; Carey, R.; Glass, D.; Peters, S.; Abramson, M.J.; Benke, G.; Reid, A.; Driscoll, T.; Fritschi, L. A comprehensive list of asthmagens to inform health interventions in the Australian workplace. Aust. N. Z. J. Public. Health 2016, 40, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Jung, C.R.; Lin, C.Y.; Hwang, B.F. Combined exposure to heavy metals in PM2.5 and pediatric asthma. J. Allergy Clin. Immunol. 2021, 147, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Lutz, P.M.; Wilson, T.J.; Ireland, J.; Jones, A.L.; Gorman, J.S.; Gale, N.L.; Johnson, J.C.; Hewett, J.E. Elevated immunoglobulin E (IgE) levels in children with exposure to environmental lead. Toxicology 1999, 134, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.J.; Karmaus, W.J.J.; Yang, C.C. Lead exposure, IgE, and the risk of asthma in children. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Ran, Z.; Wang, B.; Zhang, S.Y. Associations of exposure to metals with total and allergen-specific IgE: An NHANES analysis (2005-2006). Sci. Total Environ. 2024, 906, 167385. [Google Scholar] [CrossRef]
- Braun, J.M.; Gennings, C.; Hauser, R.; Webster, T.F. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environ. Health Perspect. 2016, 124, A6–A9. [Google Scholar] [CrossRef]
- Carlin, D.J.; Rider, C.V.; Woychik, R.; Birnbaum, L.S. Unraveling the health effects of environmental mixtures: An NIEHS priority. Environ. Health Perspect. 2013, 121, A6–A8. [Google Scholar] [CrossRef]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. Int. 2016, 23, 8244–8259. [Google Scholar] [CrossRef] [PubMed]
- Stafoggia, M.; Breitner, S.; Hampel, R.; Basagaña, X. Statistical Approaches to Address Multi-Pollutant Mixtures and Multiple Exposures: The State of the Science. Curr. Environ. Health Rep. 2017, 4, 481–490. [Google Scholar] [CrossRef]
- Wu, L.; Cui, F.; Ma, J.; Huang, Z.; Zhang, S.; Xiao, Z.; Li, J.; Ding, X.; Niu, P. Associations of multiple metals with lung function in welders by four statistical models. Chemosphere 2022, 298, 134202. [Google Scholar] [CrossRef] [PubMed]
- Valeri, L.; Mazumdar, M.M.; Bobb, J.F.; Claus Henn, B.; Rodrigues, E.; Sharif, O.I.A.; Kile, M.L.; Quamruzzaman, Q.; Afroz, S.; Golam, M.; et al. The Joint Effect of Prenatal Exposure to Metal Mixtures on Neurodevelopmental Outcomes at 20–40 Months of Age: Evidence from Rural Bangladesh. Environ. Health Perspect. 2017, 125, 067015. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, N.; Wang, H.; Su, W.; Song, Q.; Liang, Q.; Liang, M.; Sun, C.; Li, Y.; Lowe, S.; et al. Combined exposure to multiple metals on cardiovascular disease in NHANES under five statistical models. Environ. Res. 2022, 215, 114435. [Google Scholar] [CrossRef]
- Ge, X.; Yang, A.; Huang, S.; Luo, X.; Hou, Q.; Huang, L.; Zhou, Y.; Li, D.; Lv, Y.; Li, L.; et al. Sex-specific associations of plasma metals and metal mixtures with glucose metabolism: An occupational population-based study in China. Sci. Total Environ. 2021, 760, 143906. [Google Scholar] [CrossRef]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef]
- Satarug, S.; Gobe, G.C.; Vesey, D.A.; Phelps, K.R. Cadmium and Lead Exposure, Nephrotoxicity, and Mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef]
- Min, K.B.; Min, J.Y. Environmental lead exposure and increased risk for total and allergen-specific IgE in US adults. J. Allergy Clin. Immunol. 2015, 135, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Hassanvand, M.S.; Jaafarzadeh, N.; Ghadiri, A.; Shamsipour, M.; Dehcheshmeh, M.G. Increased allergic and asthmatic risks in children residing in industrial areas by surveying the pre-inflammatory (IgE, IL-4 and IL-13) biomarkers. J. Environ. Health Sci. Eng. 2022, 20, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Hassanvand, M.S.; Jaafarzadeh, N.; Ghadiri, A.; Shamsipour, M.; Dehcheshmeh, M.G. Effect of ambient air PM2.5-bound heavy metals on blood metal(loid)s and children’s asthma and allergy pro-inflammatory (IgE, IL-4 and IL-13) biomarkers. J. Trace Elem. Med. Biol. 2021, 68, 126826. [Google Scholar] [CrossRef]
- Yang, S.N.; Hsieh, C.C.; Kuo, H.F.; Lee, M.S.; Huang, M.Y.; Kuo, C.H.; Hung, C.H. The effects of environmental toxins on allergic inflammation. Allergy Asthma Immunol. Res. 2014, 6, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Annesi-Maesano, I.; Pollitt, R.; King, G.; Bousquet, J.; Hellier, G.; Sahuquillo, J.; Huel, G. In utero exposure to lead and cord blood total IgE. Is there a connection? Allergy 2003, 58, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Bolt, A.M.; Mann, K.K. Tungsten: An Emerging Toxicant, Alone or in Combination. Curr. Environ. Health Rep. 2016, 3, 405–415. [Google Scholar] [CrossRef]
- Keith, L.S.; Wohlers, D.W.; Moffett, D.B.; Rosemond, Z.A. ATSDR evaluation of potential for human exposure to tungsten. Toxicol. Ind. Health 2007, 23, 309–345. [Google Scholar] [CrossRef]
- Scammell, M.K.; Sennett, C.; Laws, R.L.; Rubin, R.L.; Brooks, D.R.; Amador, J.J.; López-Pilarte, D.; Ramirez-Rubio, O.; Friedman, D.J.; McClean, M.D.; et al. Urinary Metals Concentrations and Biomarkers of Autoimmunity among Navajo and Nicaraguan Men. Int. J. Environ. Res. Public. Health 2020, 17, 5263. [Google Scholar] [CrossRef]
- Strenzke, N.; Grabbe, J.; Plath, K.E.; Rohwer, J.; Wolff, H.H.; Gibbs, B.F. Mercuric chloride enhances immunoglobulin E-dependent mediator release from human basophils. Toxicol. Appl. Pharmacol. 2001, 174, 257–263. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, T.; Hu, W.; Wang, X.; Xu, B.; Lin, Z.; Hofer, T.; Stefanoff, P.; Chen, Y.; Wang, X.; et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int. 2019, 123, 325–336. [Google Scholar] [CrossRef] [PubMed]


| Variable | Catalogue | N (%) or Median [IQR] |
|---|---|---|
| Age (years old) | 20–45 years old | 674 (47.2) |
| 46–65 years old | 429 (30.0) | |
| >65 years old | 326 (22.8) | |
| Gender | Male | 691 (48.4) |
| Female | 738 (51.6) | |
| Race/ethnicity | Mexican American | 277 (19.4) |
| Other Hispanic | 43 (3.0) | |
| Non-Hispanic White | 742 (51.9) | |
| Non-Hispanic Black | 320 (22.4) | |
| Other Race | 47 (3.3) | |
| Body mass index (kg/m2) | Normal | 445 (31.1) |
| Overweight | 500 (35.0) | |
| Obesity | 484 (33.9) | |
| Educational level | Below than high school | 402 (28.1) |
| High school | 325 (22.7) | |
| Above than high school | 702 (49.1) | |
| Income (USD) | <20,000 | 331 (23.2) |
| ≥20,000 | 1098 (76.8) | |
| Smoke | No | 746 (52.2) |
| Yes | 683 (47.8) | |
| Alcohol | No | 458 (32.1) |
| Yes | 971 (67.9) | |
| Total Immunoglobulin E (kU/L) (median [IQR]) | 43.7 [17.3, 126.0] a | |
| Creatinine (mg/dL) (median [IQR]) | 119.0 [70.0, 174.0] |
| Metal | Detection Frequency | Percentile | ||||
|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | ||
| Cd | 88.77 | 0.02 | 0.14 | 0.28 | 0.53 | 1.24 |
| Pb | 97.57 | 0.12 | 0.36 | 0.66 | 1.14 | 2.53 |
| Hg | 92.00 | 0.06 | 0.22 | 0.46 | 1.01 | 3.00 |
| Ba | 98.86 | 0.22 | 0.68 | 1.31 | 2.57 | 6.67 |
| Co | 99.61 | 0.10 | 0.23 | 0.37 | 0.58 | 1.45 |
| Cs | 100.00 | 1.27 | 3.07 | 5.06 | 7.55 | 12.40 |
| Mo | 100.00 | 8.68 | 25.70 | 46.60 | 74.95 | 148.15 |
| Sb | 84.64 | 0.02 | 0.04 | 0.07 | 0.12 | 0.32 |
| Tl | 99.88 | 0.04 | 0.10 | 0.17 | 0.25 | 0.41 |
| W | 91.86 | 0.02 | 0.04 | 0.08 | 0.14 | 0.43 |
| U | 92.31 | 0.00 | 0.00 | 0.01 | 0.01 | 0.03 |
| Metal a | PIPs | Metal b | PIPs |
|---|---|---|---|
| Cd | 0.352 | Cd | 0.008 |
| Pb | 0.854 | Pb | 0.837 |
| Hg | 0.453 | Hg | 0.006 |
| Ba | 0.230 | Ba | 0.003 |
| Co | 0.287 | Co | 0.000 |
| Cs | 0.271 | Cs | 0.000 |
| Mo | 0.178 | Mo | 0.003 |
| Sb | 0.209 | Sb | 0.002 |
| Tl | 0.154 | Tl | 0.010 |
| U | 0.159 | U | 0.000 |
| W | 0.486 | W | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Ding, X.; Niu, P.; Chen, T.; Yan, T. The Associations between Exposure to Multiple Heavy Metals and Total Immunoglobulin E in U.S. Adults. Toxics 2024, 12, 116. https://doi.org/10.3390/toxics12020116
Song X, Ding X, Niu P, Chen T, Yan T. The Associations between Exposure to Multiple Heavy Metals and Total Immunoglobulin E in U.S. Adults. Toxics. 2024; 12(2):116. https://doi.org/10.3390/toxics12020116
Chicago/Turabian StyleSong, Xin, Xiaowen Ding, Piye Niu, Tian Chen, and Tenglong Yan. 2024. "The Associations between Exposure to Multiple Heavy Metals and Total Immunoglobulin E in U.S. Adults" Toxics 12, no. 2: 116. https://doi.org/10.3390/toxics12020116
APA StyleSong, X., Ding, X., Niu, P., Chen, T., & Yan, T. (2024). The Associations between Exposure to Multiple Heavy Metals and Total Immunoglobulin E in U.S. Adults. Toxics, 12(2), 116. https://doi.org/10.3390/toxics12020116