Developmental Toxicity of PEDOT:PSS in Zebrafish: Effects on Morphology, Cardiac Function, and Intestinal Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Husbandry and Zebrafish Egg Production
2.3. Chemicals
2.4. Measurement of Zebrafish Developmental Toxicity and LC50 of PEDOT:PSS
2.5. Measurement of Heart Rate and Other Basic Parameters
2.6. Behavioral Experiments
2.7. Expression Level Analysis of Oxidative Stress-Related Genes
2.8. In Situ Hybridization Experiment
2.9. Histocytological Analysis of Zebrafish Larvae
2.10. Transcriptomic Analysis
2.11. Statistical Analysis
3. Results
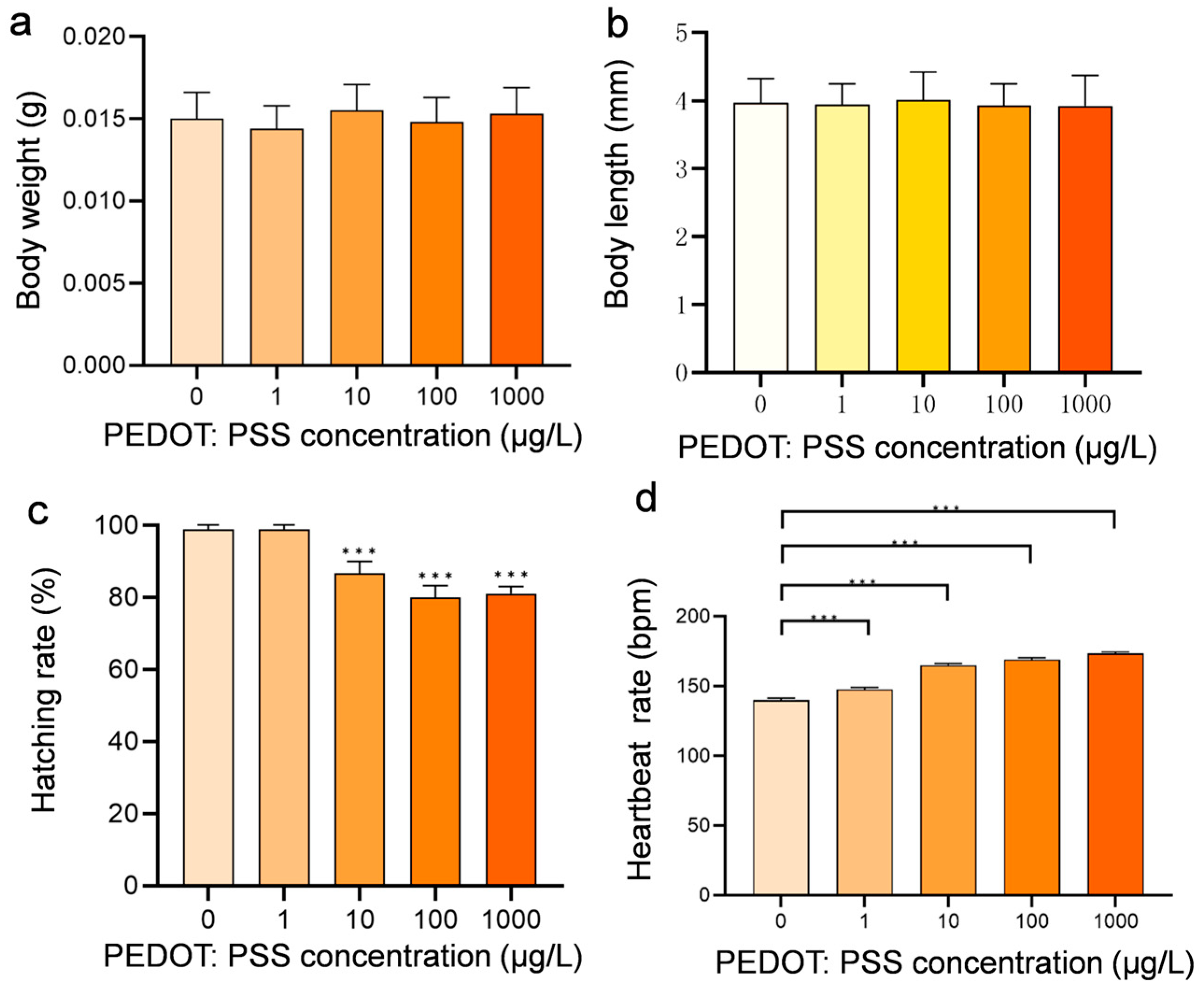
3.1. Developmental Toxicity Effects after Exposure to PEDOT:PSS
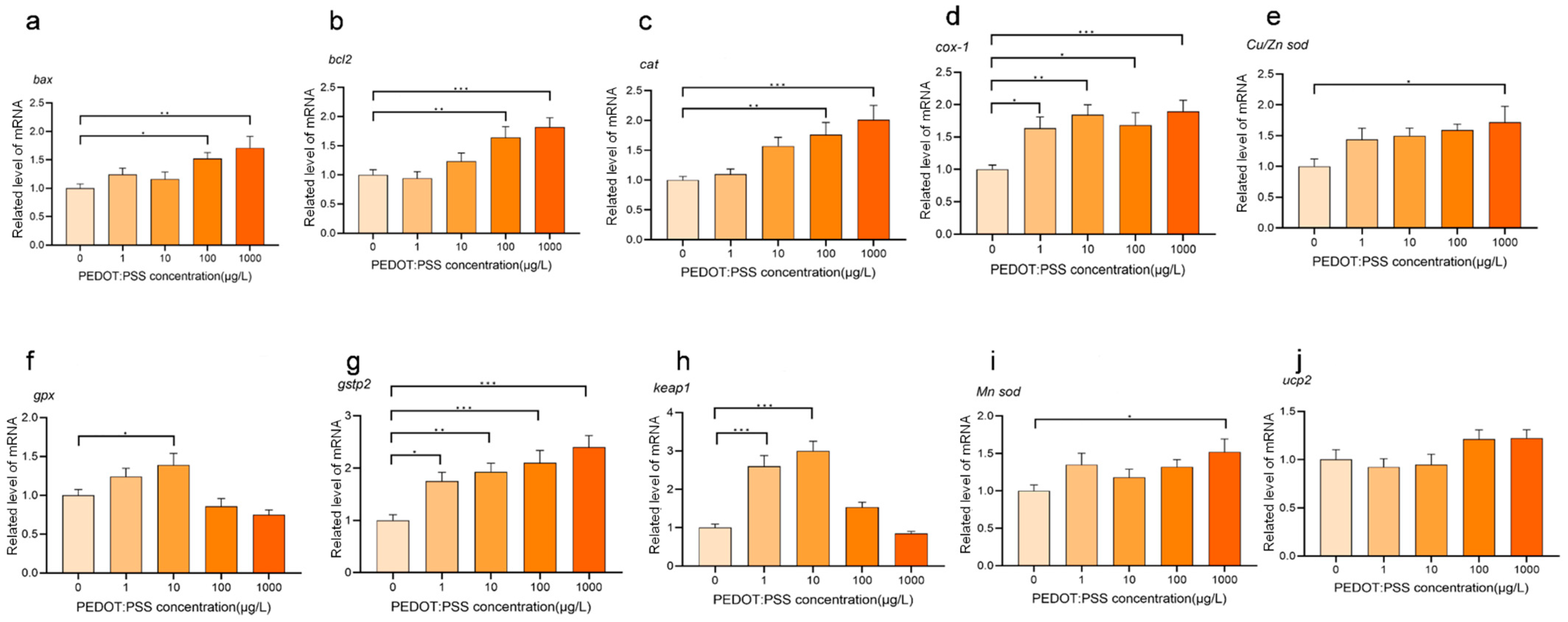
3.2. Effects of PEDOT:PSS on Oxidative Stress-Related Gene Expression
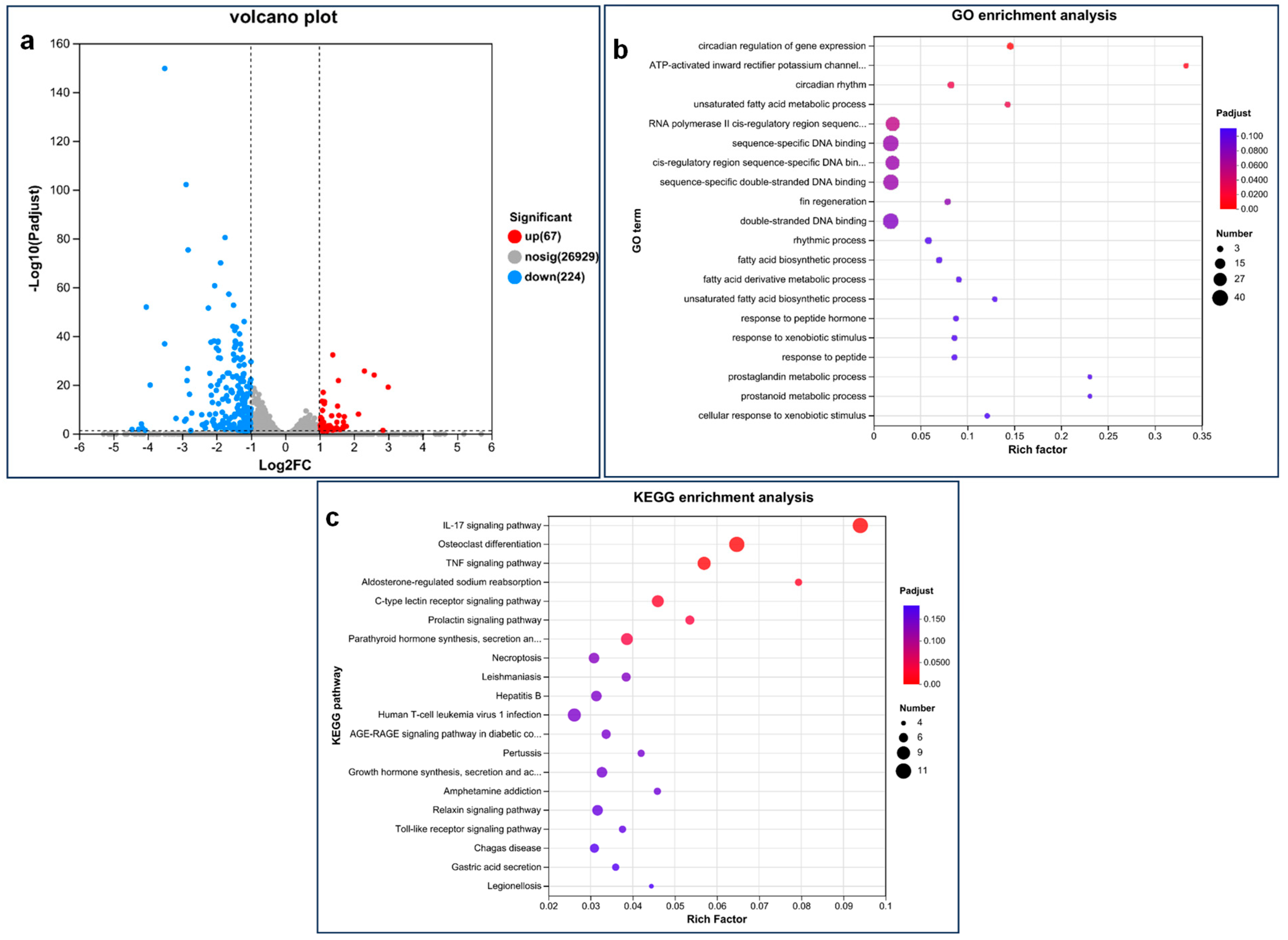
3.3. Transcriptomic Analysis of Zebrafish Larvae after Exposure to PEDOT:PSS
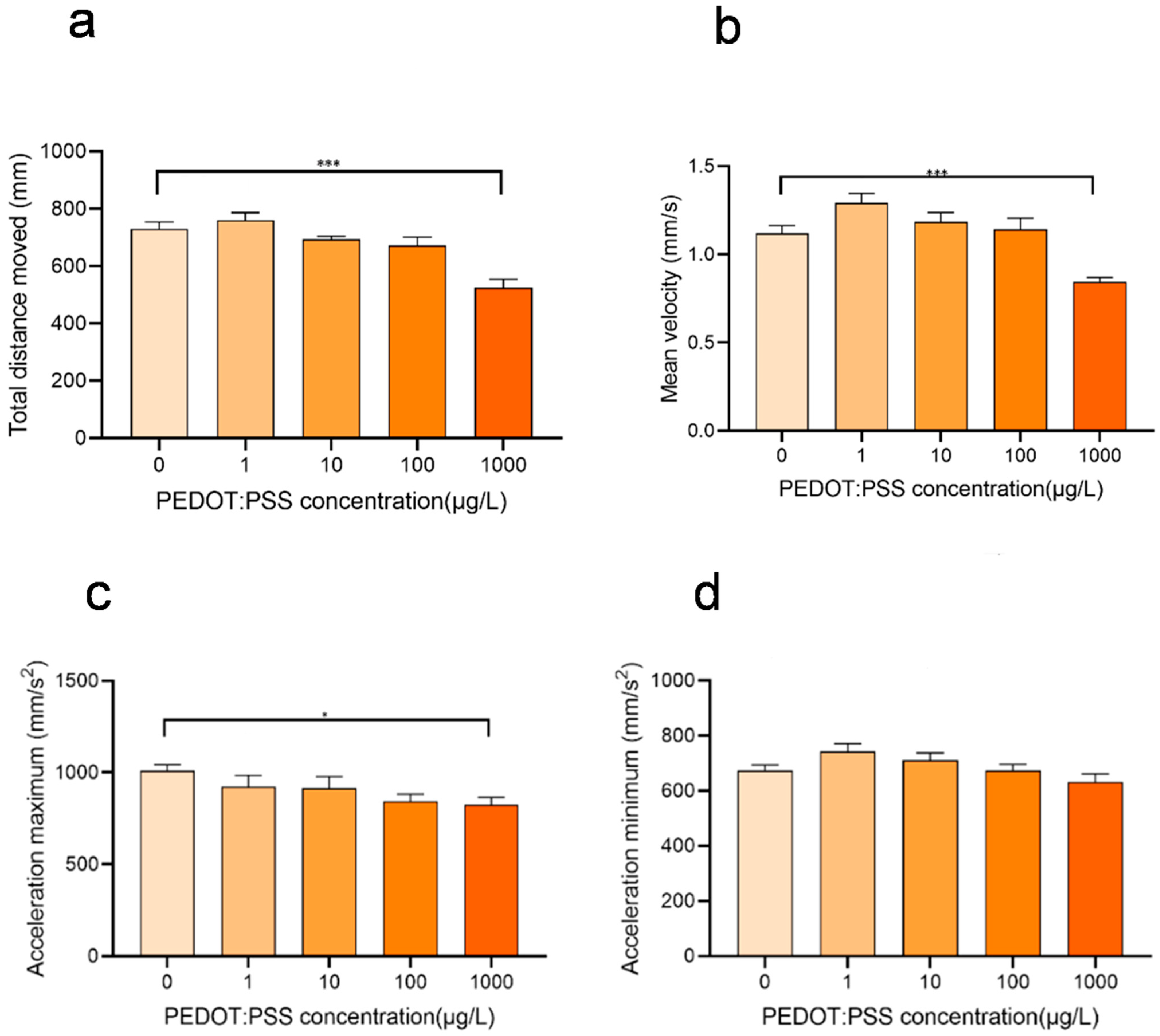
3.4. Concentration-Dependent Effects of PEDOT:PSS on Zebrafish Behavior
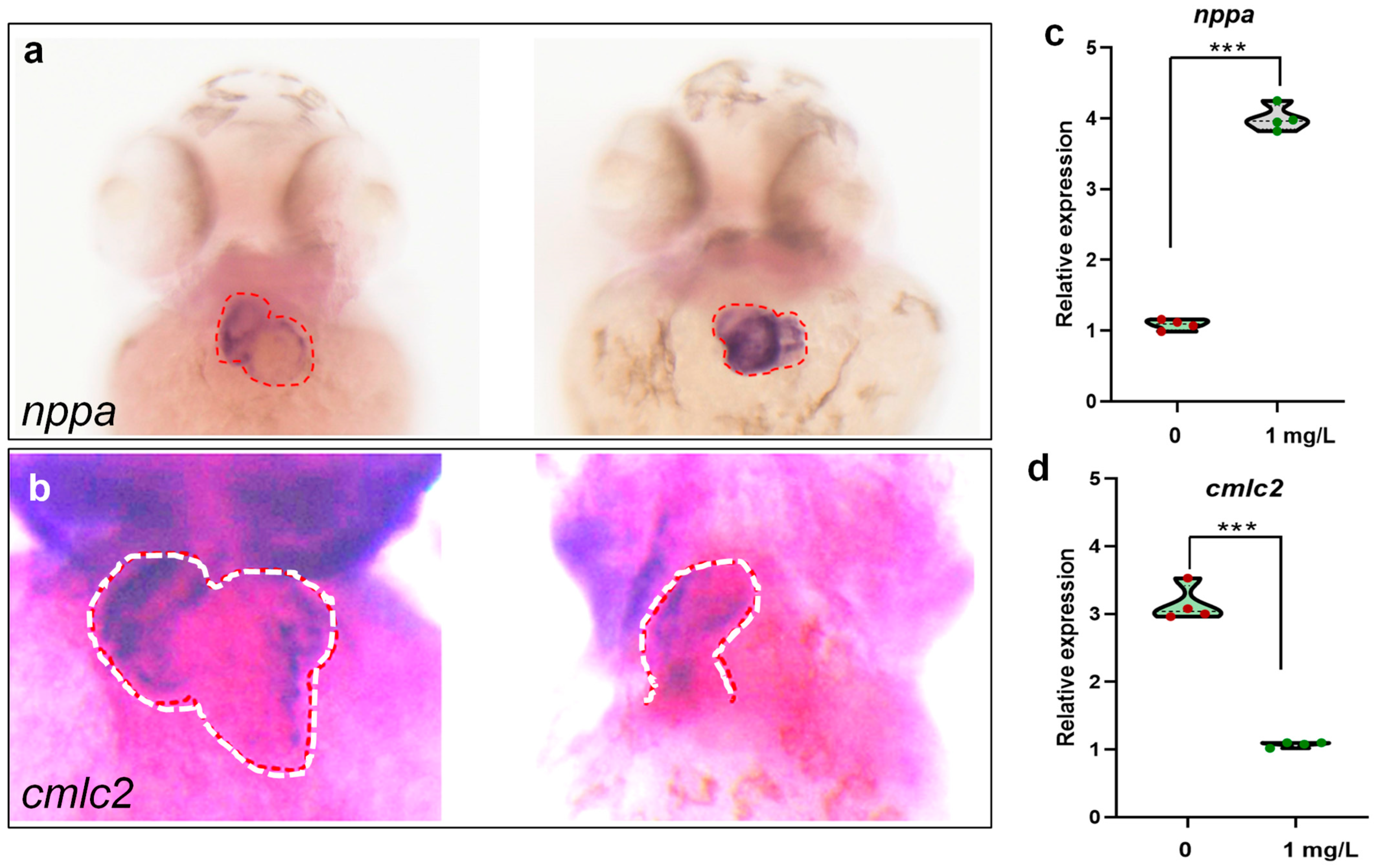
3.5. PEDOT:PSS Exposure Impairs Cardiac Development
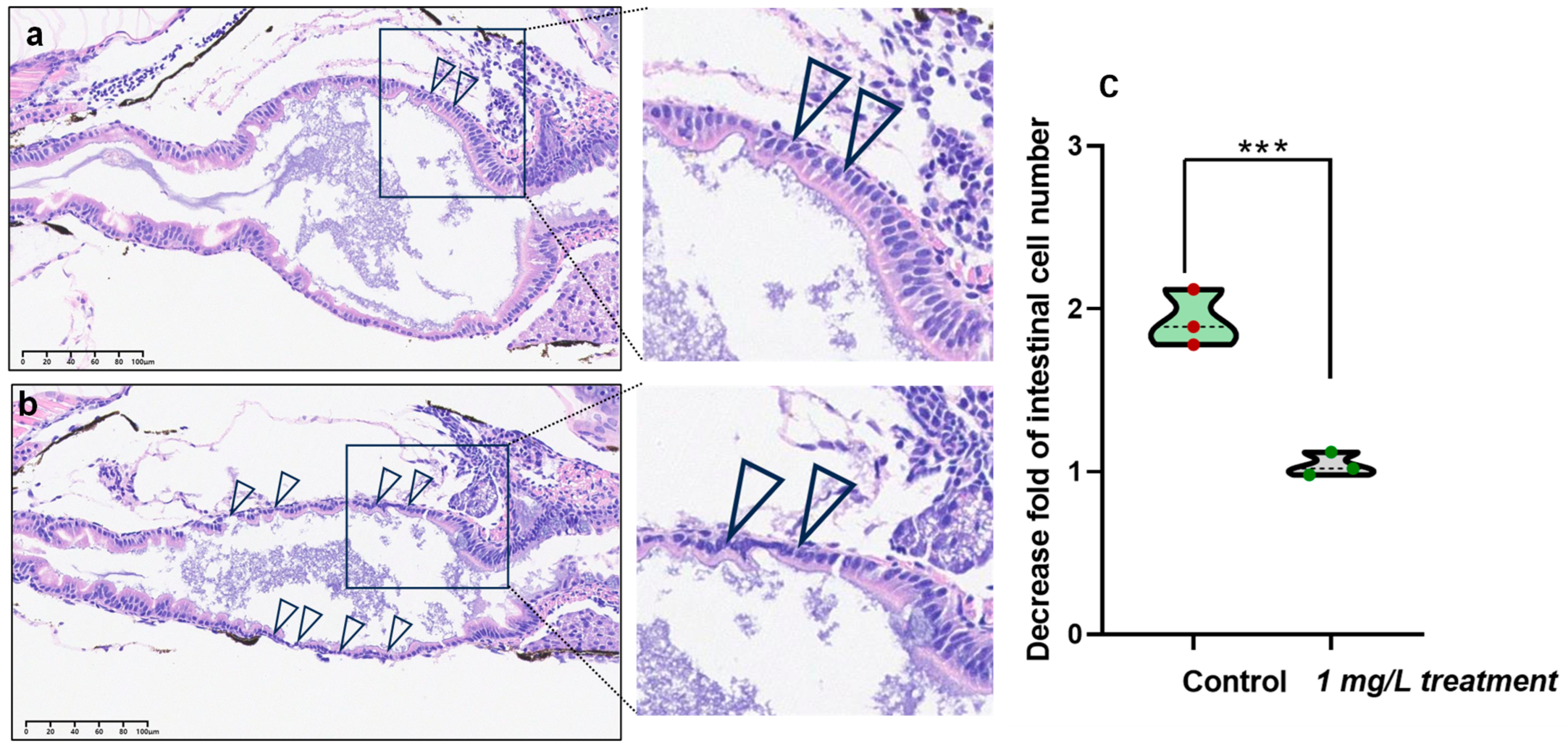
3.6. Detrimental Effects of PEDOT:PSS on Intestinal Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conti, J.; Holtberg, P.; Diefenderfer, J.; Larose, A.; Turnure, J.T.; Westfall, L. International Energy Outlook 2016 with Projections to 2040; USDOE Energy Information Administration (EIA): Washington, DC, USA, 2016.
- Panigrahy, S.; Kandasubramanian, B. Polymeric thermoelectric PEDOT: PSS & composites: Synthesis, progress, and applications. Eur. Polym. J. 2020, 132, 109726. [Google Scholar]
- Abas, A.; Sheng, H.; Ma, Y.; Zhang, X.; Wei, Y.; Su, Q.; Lan, W.; Xie, E. PEDOT:PSS coated CuO nanowire arrays grown on Cu foam for high-performance supercapacitor electrodes. J. Mater. Sci. Mater. Electron. 2019, 30, 10953–10960. [Google Scholar] [CrossRef]
- Gong, C.; Cheng, S.; Meng, X.; Hu, X.; Chen, Y. Recent Advances of PEDOT in Flexible Energy Conversion and Storage Devices. Acta Chim. Sin. 2021, 79, 853–868. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, W.; Zhang, H.; Xie, M.; Duan, X. PEDOT:PSS: From conductive polymers to sensors. Nanotechnol. Precis. Eng. 2021, 4, 045004. [Google Scholar] [CrossRef]
- Gupta, S.; Datt, R.; Mishra, A.; Tsoi, W.C.; Patra, A.; Bober, P. Poly(3,4-ethylenedioxythiophene):Poly(styrene sulfonate) in antibacterial, tissue engineering and biosensors applications: Progress, challenges and perspectives. J. Appl. Polym. Sci. 2022, 139, e52663. [Google Scholar] [CrossRef]
- Liang, Y.; Offenhausser, A.; Ingebrandt, S.; Mayer, D. PEDOT:PSS-Based Bioelectronic Devices for Recording and Modulation of Electrophysiological and Biochemical Cell Signals. Adv. Healthc. Mater. 2021, 10, 2100061. [Google Scholar] [CrossRef]
- Zhang, W.; Su, Z.; Zhang, X.; Wang, W.; Li, Z. Recent progress on PEDOT-based wearable bioelectronics. View 2022, 3, 20220030. [Google Scholar] [CrossRef]
- Fan, Z.; Ouyang, J. Thermoelectric Properties of PEDOT:PSS. Adv. Electron. Mater. 2019, 5, 1800769. [Google Scholar] [CrossRef]
- Hung, J.-N.; Vo, D.N.K.; Ho, H.P.T.; Tsai, M.-H. PEDOT:PSS in Solution Form Exhibits Strong Potential in Inhibiting SARS-CoV-2 Infection of the Host Cells by Targeting Viruses and Also the Host Cells. Biomacromolecules 2022, 23, 3535–3548. [Google Scholar] [CrossRef]
- Zhu, J.; Omura, T.; Wakisaka, M. Biological response of protists Haematococcus lacustris and Euglena gracilis to conductive polymer poly (3,4-ethylenedioxythiophene) polystyrene sulfonate. Lett. Appl. Microbiol. 2021, 72, 619–625. [Google Scholar] [CrossRef]
- Wixon, J. Danio rerio, the zebrafish. Yeast 2000, 17, 225–231. [Google Scholar] [CrossRef]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Adhish, M.; Manjubala, I. Effectiveness of zebrafish models in understanding human diseases—A review of models. Heliyon 2023, 9, e14557. [Google Scholar] [CrossRef]
- Bowley, G.; Kugler, E.; Wilkinson, R.; Lawrie, A.; van Eeden, F.; Chico, T.J.A.; Evans, P.C.; Noël, E.S.; Serbanovic-Canic, J. Zebrafish as a tractable model of human cardiovascular disease. Br. J. Pharmacol. 2022, 179, 900–917. [Google Scholar] [CrossRef]
- Kolvenbach, C.M.; Dworschak, G.C.; Rieke, J.M.; Woolf, A.S.; Reutter, H.; Odermatt, B.; Hilger, A.C. Modelling human lower urinary tract malformations in zebrafish. Mol. Cell. Pediatr. 2023, 10, 2. [Google Scholar] [CrossRef]
- MacArthur Clark, J.A.; Sun, D. Guidelines for the ethical review of laboratory animal welfare People’s Republic of China National Standard GB/T 35892-2018 [Issued 6 February 2018 Effective from 1 September 2018]. Anim. Models Exp. Med. 2020, 3, 103–113. [Google Scholar] [CrossRef]
- Yang, H.; Liang, X.; Zhao, Y.; Gu, X.; Mao, Z.; Zeng, Q.; Chen, H.; Martyniuk, C.J. Molecular and behavioral responses of zebrafish embryos/larvae after sertraline exposure. Ecotoxicol. Environ. Saf. 2020, 208, 111700. [Google Scholar] [CrossRef]
- Jia, P.-P.; Chandrajith, R.; Junaid, M.; Li, T.-Y.; Li, Y.-Z.; Wei, X.-Y.; Liu, L.; Pei, D.-S. Elucidating environmental factors and their combined effects on CKDu in Sri Lanka using zebrafish. Environ. Pollut. 2023, 332, 121967. [Google Scholar] [CrossRef]
- Katayama, S. Oxidative stress marker. Nihon rinsho. Jpn. J. Clin. Med. 2006, 64 (Suppl. S6), 142–146. [Google Scholar]
- Rudnicka, E.; Duszewska, A.M.; Kucharski, M.; Tyczyński, P.; Smolarczyk, R. Oxidative stress and reproductive function: Oxidative stress in polycystic ovary syndrome. Reproduction 2022, 164, F145. [Google Scholar] [CrossRef]
- Scavuzzi, B.M.; Holoshitz, J. Endoplasmic Reticulum Stress, Oxidative Stress, and Rheumatic Diseases. Antioxidants 2022, 11, 1306. [Google Scholar] [CrossRef]
- Pei, D.-S.; Sun, Y.-H.; Chen, C.-H.; Chen, S.-P.; Wang, Y.-P.; Hu, W.; Zhu, Z.-Y. Identification and characterization of a novel gene differentially expressed in zebrafish cross-subfamily cloned embryos. BMC Dev. Biol. 2008, 8, 29. [Google Scholar] [CrossRef]
- Garbinato, C.; Schneider, S.E.; Sachett, A.; Decui, L.; Conterato, G.M.; Müller, L.G.; Siebel, A.M. Exposure to ractopamine hydrochloride induces changes in heart rate and behavior in zebrafish embryos and larvae. Environ. Sci. Pollut. Res. Int. 2020, 27, 21468–21475. [Google Scholar] [CrossRef]
- Wiprich, M.T.; Zanandrea, R.; Altenhofen, S.; Bonan, C.D. Influence of 3-nitropropionic acid on physiological and behavioral responses in zebrafish larvae and adults. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 234, 108772. [Google Scholar] [CrossRef]
- Patel, M.Y.; Stovall, K.; Franklin, J.L. The intrinsic apoptotic pathway lies upstream of oxidative stress in multiple organs. Free Radic. Biol. Med. 2020, 158, 13–19. [Google Scholar] [CrossRef]
- Carlsson, G.; Blomberg, M.; Pohl, J.; Örn, S. Swimming activity in zebrafish larvae exposed to veterinary antiparasitic pharmaceuticals. Environ. Toxicol. Pharmacol. 2018, 63, 74–77. [Google Scholar] [CrossRef]
- Burnett, J.C., Jr. Atrial Natriuretic Peptide, Heart Failure and the Heart as an Endocrine Organ. Clin. Chem. 2019, 65, 1602–1603. [Google Scholar] [CrossRef]
- Man, J.; Barnett, P.; Christoffels, V.M. Structure and function of the Nppa–Nppb cluster locus during heart development and disease. Cell. Mol. Life Sci. 2018, 75, 1435–1444. [Google Scholar] [CrossRef]
- Park, J.J.; Lee, H.; Kim, K.H.; Yoo, B.; Kang, S.; Baek, S.H.; Jeon, E.; Kim, J.; Cho, M.; Chae, S.C.; et al. Heart failure and atrial fibrillation: Tachycardia-mediated acute decompensation. ESC Hear. Fail. 2021, 8, 2816–2825. [Google Scholar] [CrossRef]
- Hong, T.; Park, H.; An, G.; Park, J.; Song, G.; Lim, W. Fluchloralin induces developmental toxicity in heart, liver, and nervous system during early zebrafish embryogenesis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 271, 109679. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.; Meng, Y.; Wei, Y.; Xu, Z.; Ma, J.; Zhong, K.; Cao, Z.; Liao, X.; Lu, H. Famoxadone-cymoxanil induced cardiotoxicity in zebrafish embryos. Ecotoxicol. Environ. Saf. 2020, 205, 111339. [Google Scholar] [CrossRef]
- Park, H.; Song, G.; Hong, T.; An, G.; Park, S.; Lim, W. Exposure to the herbicide fluridone induces cardiovascular toxicity in early developmental stages of zebrafish. Sci. Total Environ. 2023, 867, 161535. [Google Scholar] [CrossRef]
- Zhang, Q.; He, X.; Yao, S.; Lin, T.; Zhang, L.; Chen, D.; Chen, C.; Yang, Q.; Li, F.; Zhu, Y.-M.; et al. Ablation of Mto1 in zebrafish exhibited hypertrophic cardiomyopathy manifested by mitochondrion RNA maturation deficiency. Nucleic Acids Res. 2021, 49, 4689–4704. [Google Scholar] [CrossRef]
- Imperatore, R.; Orso, G.; Facchiano, S.; Scarano, P.; Hoseinifar, S.H.; Ashouri, G.; Guarino, C.; Paolucci, M. Anti-inflammatory and immunostimulant effect of different timing-related administration of dietary polyphenols on intestinal inflammation in zebrafish, Danio rerio. Aquaculture 2023, 563, 738878. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, C.; Han, Z.; Chen, K.; Wu, X.; Qiu, X. Combined exposure to microplastics and amitriptyline caused intestinal damage, oxidative stress and gut microbiota dysbiosis in zebrafish (Danio rerio). Aquat. Toxicol. 2023, 260, 106589. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Liang, H.; Ma, C.; Cui, N.; Cao, H.; Wei, W.; Liu, Y. Single and combined effects of polyethylene microplastics and acetochlor on accumulation and intestinal toxicity of zebrafish (Danio rerio). Environ. Pollut. 2023, 333, 122089. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Ruiz de Morales, J.M.G.; Puig, L.; Daudén, E.; Cañete, J.D.; Pablos, J.L.; Martín, A.O.; Juanatey, C.G.; Adán, A.; Montalbán, X.; Borruel, N.; et al. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef]
- Varfolomeev, E.; Vucic, D. Intracellular regulation of TNF activity in health and disease. Cytokine 2018, 101, 26–32. [Google Scholar] [CrossRef]
- Yao, Z.; Getting, S.J.; Locke, I.C. Regulation of TNF-Induced Osteoclast Differentiation. Cells 2021, 11, 132. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Gou, D.; Bu, L.-K.; Wei, X.-Y.; Hu, H.; Huo, W.-B.; Sultan, M.; Pei, D.-S. Developmental Toxicity of PEDOT:PSS in Zebrafish: Effects on Morphology, Cardiac Function, and Intestinal Health. Toxics 2024, 12, 150. https://doi.org/10.3390/toxics12020150
Yang G, Gou D, Bu L-K, Wei X-Y, Hu H, Huo W-B, Sultan M, Pei D-S. Developmental Toxicity of PEDOT:PSS in Zebrafish: Effects on Morphology, Cardiac Function, and Intestinal Health. Toxics. 2024; 12(2):150. https://doi.org/10.3390/toxics12020150
Chicago/Turabian StyleYang, Guan, Dongzhi Gou, Ling-Kang Bu, Xing-Yi Wei, Huan Hu, Wen-Bo Huo, Marriya Sultan, and De-Sheng Pei. 2024. "Developmental Toxicity of PEDOT:PSS in Zebrafish: Effects on Morphology, Cardiac Function, and Intestinal Health" Toxics 12, no. 2: 150. https://doi.org/10.3390/toxics12020150
APA StyleYang, G., Gou, D., Bu, L.-K., Wei, X.-Y., Hu, H., Huo, W.-B., Sultan, M., & Pei, D.-S. (2024). Developmental Toxicity of PEDOT:PSS in Zebrafish: Effects on Morphology, Cardiac Function, and Intestinal Health. Toxics, 12(2), 150. https://doi.org/10.3390/toxics12020150






