Chemical Assessment of Drinking Water Quality and Associated Human Health Risk of Heavy Metals in Gutai Mountains, Romania
Abstract
:1. Introduction
2. Materials and Methods
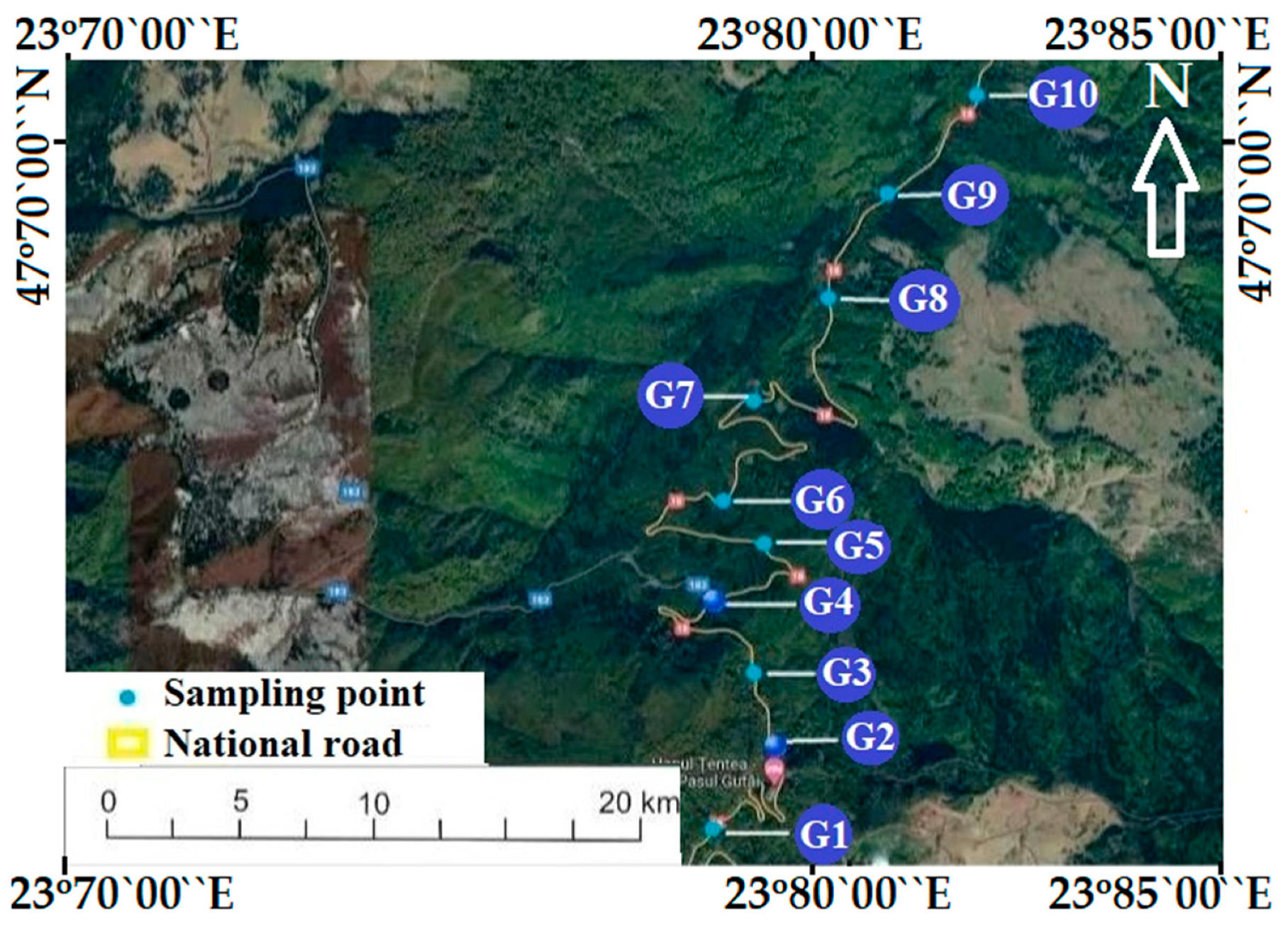
2.1. Study Area Location and Sampling
2.2. Chemical Analysis
2.3. Data Analysis
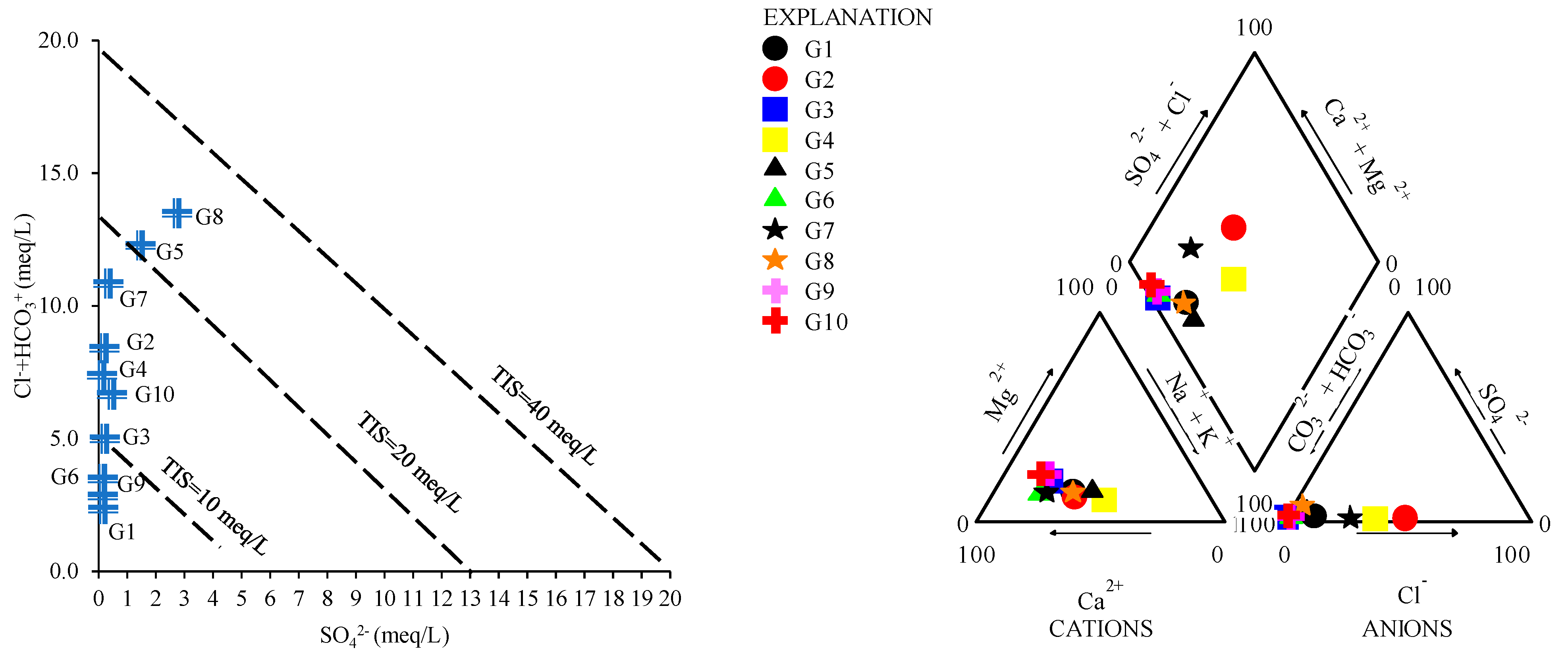
2.3.1. Water Typology
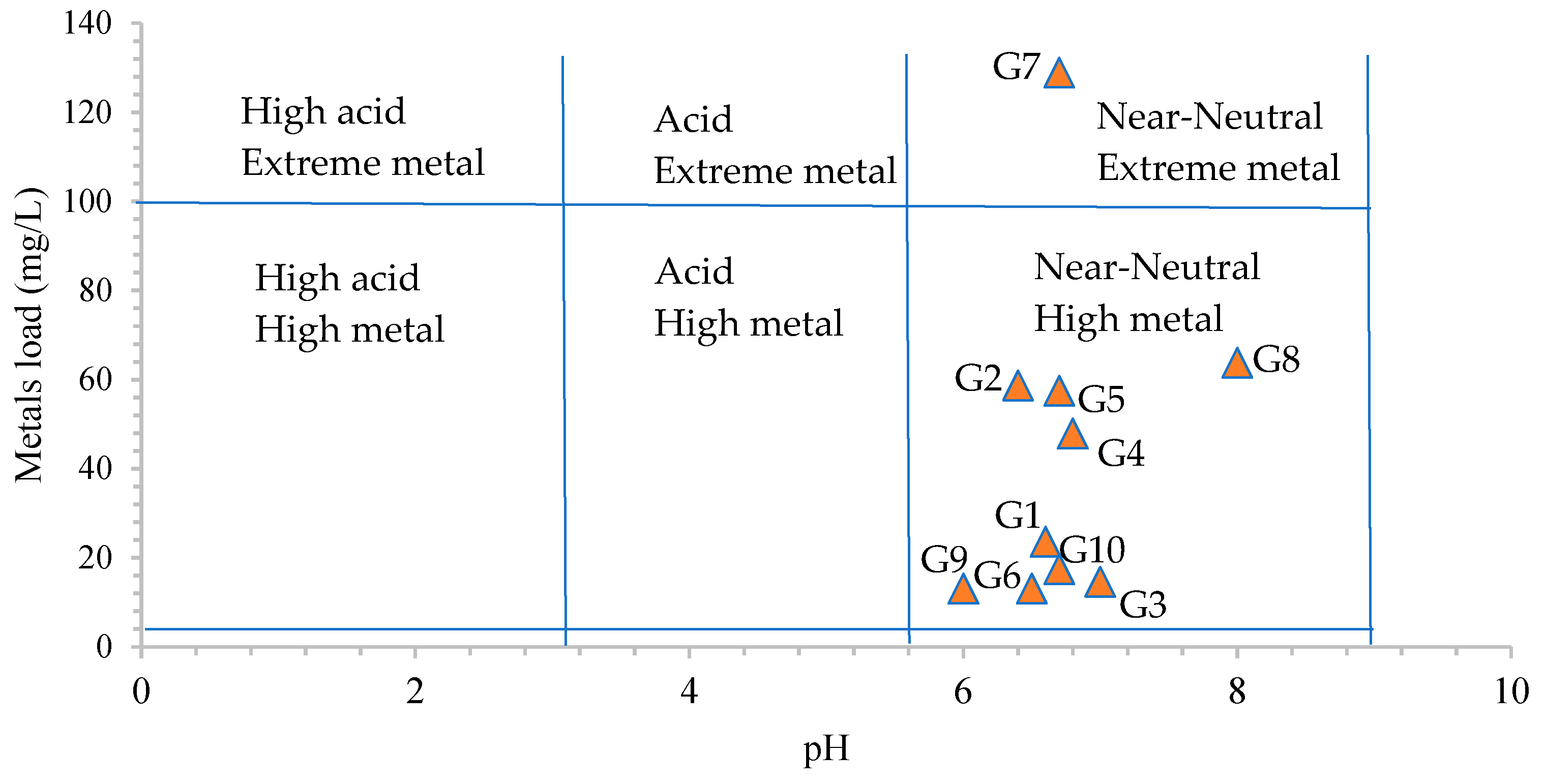
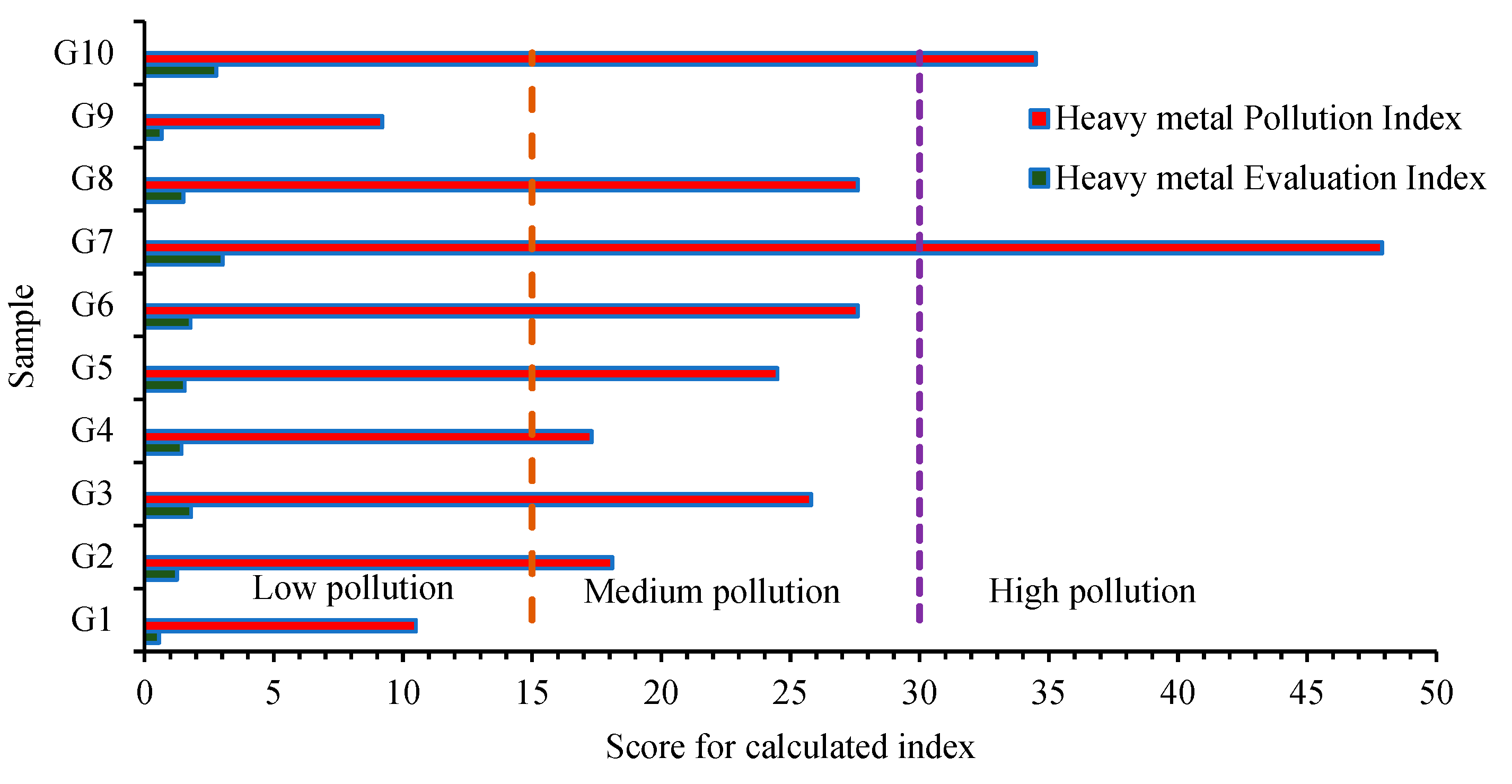
2.3.2. Heavy Metal Pollution Evaluation
2.3.3. Human Health Risk Evaluation
3. Results and Discussions
3.1. Physico-Chemical Assessment of Studied Samples
3.2. Trend of Metal Composition in the Studied Samples
3.3. Heavy Metal Pollution and Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masoud, A.M.; Mohamed, H.A. Coupled multivariate statistical analysis and WQI approaches for groundwater quality assessment in Wadi El-Assiuty downstream area, Eastern Desert, Egypt. J. Afr. Earth Sci. 2020, 172, 103982. [Google Scholar] [CrossRef]
- Zakaria, N.; Anornu, G.; Adomako, D.; Owusu-Nimo, F.; Gibrilla, A. Evolution of groundwater hydrogeochemistry and assessment of groundwater quality in the Anayari catchment. Groundw. Sustain. Dev. 2020, 12, 100489. [Google Scholar] [CrossRef]
- Jain, N.; Yrvatikar, R.; Raxamwar, T.S. Comparative study of psyhico-chemical parameters and water quality index of river. Mater. Today Proc. 2022, 60, 859–867. [Google Scholar] [CrossRef]
- Adeyemi, A.A.; Ojekunle, Z.O. Concentrations and health risk assessment of industrial heavy metals pollutions in groundwater in Ogun State. Sci. Afr. 2021, 11, e00666. [Google Scholar] [CrossRef]
- Laha, F.; Gashi, F.; Cadraku, H. Variations in the physico-chemical parameters of Grinaja catchment groundwater. IFAC-Pap. Online 2019, 49, 200–205. [Google Scholar]
- Dimri, D.; Daverey, A.; Kuman, A.; Sharma, A. Monitoring water quality of River Ganga using multivariate techniques and WQI (Water Quality Index) in Western Himalayan region of Uttarakhand, India. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100375. [Google Scholar] [CrossRef]
- El Fadili, H.; Ben Ali, M.; El Mahi, M.; Cooray, A.T.; Lofi, E.M. A comprehensive health risk assessment and groundwater quality for irrigation and drinking purposes around municipal solid waste sanitary landfill: A case study in Morocco. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100698. [Google Scholar] [CrossRef]
- Sousa, J.M.C.; Peron, A.; Silva, F.C.C.; Dantas, E.B.S.; Lima, A.M.V.; Oliveira, V.A.; Matos, L.A.; Paz, M.F.C.J.; Alencar, M.V.O.B.; Islam, M.T.; et al. Psyhico-chemical and genotoxicity analysis of Guaribas river water in the Notheast Brazil. Chemosphere 2017, 177, 334–338. [Google Scholar] [CrossRef]
- Burr, B.M.; Adams, G.L.; Krejca, J.K.; Paul, R.J.; Warren, M.L. Troglomorphic sculpins of the Cottus carolinae species group in Perry County, Missouri: Distribution, external morphology, and conservation status. Environ. Biol. Fish. 2001, 62, 279–296. [Google Scholar] [CrossRef]
- Yani, R.W.E.; Palupi, R.; Bramantoro, T.; Setijanto, D. Analysis of calcium levels in groundwater and dental caries in the coastal population of an archipelago country. Maced. J. Med. Sci. 2019, 7, 134. [Google Scholar] [CrossRef]
- Varol, M.; Tokatli, C. Seasonal variations of toxic metal(loid)s in groundwater collected from an intensive agricultural area in northwestern Turkey and associated health risk assessment. Environ. Res. 2022, 204, 111922. [Google Scholar] [CrossRef]
- Khosravi, K.; Barzegar, R.; Golkarian, A.; Busico, G.; Cuoco, E.; Mastrocicco, M.; Colombani, N.; Tedesco, D.; Ntona, M.M.; Kazakis, N. Predictive modeling of selected trace elements in groundwater using hybrid algorithms of iterative classifier optimizer. J. Contam. Hydrol. 2021, 242, 103849. [Google Scholar] [CrossRef]
- Affum, A.O.; Osae, S.D.; Kwaansa-Ansah, E.E.; Miyittah, M.K. Quality assessment and potential health risk of heavy metals in leafy and non-leafy vegetables irrigated with groundwater and municopal-waste-dominated stream in the Western Region, Ghana. Heliyon 2020, 6, 05829. [Google Scholar] [CrossRef]
- Habib, M.A.; Islam, A.R.M.; Bodrud-Doza, M.; Mukta, F.A.; Khan, R.; Siddique, M.A.B.; Phoungthong, K.; Techato, K. Simultaneous appraisals of pathway and probable health risk associated with trace metals contamination in groundwater from Barapukuria coal basin, Bangladesh. Chemosphere 2020, 242, 125183. [Google Scholar] [CrossRef]
- Cadar, O.; Miclean, M.; Cadar, S.; Tanaselia, C.; Senila, L.; Senila, M. Assessment of heavy metals in cows milk in Rodnei mountains area, Romania. Environ. Eng. Manag. J. 2015, 14, 2523–2528. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Nyambura, C.; Hashim, N.O.; Chege, M.W.; Tokonami, S.; Omonya, F.W. Cancer and non-cancer health risks from carcinogenic heavy metal exposures in underground water from Kilimambogo, Kenya. Groundw. Sustain. Dev. 2020, 10, 100315. [Google Scholar] [CrossRef]
- Saha, S.; Reza, A.H.M.; Roy, M.K. Hydrochemical evaluation of groundwater quality of the Tista floodplain, Rangpur, Bangladesh. Appl. Water Sci. 2019, 9, 198. [Google Scholar] [CrossRef]
- Liang, C.; Wang, W.; Ke, X.; Ou, A.; Wang, D. Hydrochemical Characteristics and Formation Mechanism of Strontium-Rich Groundwater in Tianjiazhai, Fugu, China. Water 2022, 14, 1874. [Google Scholar] [CrossRef]
- Mahey, S.; Kumar, R.; Sharma, M.; Kumar, V.; Bhardwaj, R. A critical review on toxicity of cobalt and its bioremediation strategies. SN Appl. Sci. 2020, 2, 1279. [Google Scholar] [CrossRef]
- Guerra, D.L.; Batista, A.C.; Viana, R.R.; Airoldi, C. Adsorption of rubidium on raw and MTZ-and MBI-imogolite hybrid surfaces: An evidence of the chelate effect. Desalination 2011, 275, 107–117. [Google Scholar] [CrossRef]
- Edet, A.E.; Offiong, O.E. Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal 2002, 57, 295–304. [Google Scholar] [CrossRef]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bharwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef] [PubMed]
- Dippong, T.; Mihali, C.; Marian, M.; Mare Rosca, O.; Resz, M.A. Correlations between chemical, hydrological and biotic factors in rivers from the protected area of Tisa Superioara, Romania. Process Saf. Environ. Prot. 2023, 176, 40–55. [Google Scholar] [CrossRef]
- SR EN ISO 5667-3:2018; Calitatea apei. Prelevare. Partea 3: Conservarea ¸si Manevrarea Probelor de Apa. ISO: Geneva, Switzerland, 2018. (In Romanian)
- SR EN ISO 10523:2012; Calitatea apei. Determinarea pH-ului. ISO: Geneva, Switzerland, 2012. (In Romanian)
- ISO 7888: 1985; Water Quality—Determination of Electrical Conductivity. ISO: Geneva, Switzerland, 1985.
- SR EN ISO 5814: 2013; Calitatea apei. Determinarea Continutului de Oxigen Dizolvat. Metoda Electrochimica cu Sonda. ISO: Geneva, Switzerland, 2013. (In Romanian)
- SR EN ISO 7027:2001; Calitatea Apei. Determinarea Turbidita¸tii. ISO: Geneva, Switzerland, 2001. (In Romanian)
- SR ISO 9297:2001; Calitatea Apei. Determinarea Con¸tinutului de Cloruri. Titrare cu Azotat de Argint utilizând Cromatul ca Indicator (Metoda Mohr). ISO: Geneva, Switzerland, 2001. (In Romanian)
- SR ISO 6058:2008; Calitatea apei. Determinarea Calciului. Metoda Titrimetrica cu EDTA. ISO: Geneva, Switzerland, 2008. (In Romanian)
- Loucif, K.; Neffar, S.; Menasria, T.; Mazzi, M.C.; Houhamdi, M.; Chenchouni, H. Physico-chemical and bacteriological quality assessment of surface water at Lake Tonga in Algeria. Environ. Nanotechnol. Monit. Management. 2020, 13, 100284. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Marandi, A.; Shand, P. Groundwater chemistry and Gibbs diagram. Appl. Geochem. 2018, 97, 209–212. [Google Scholar] [CrossRef]
- Piper, A.M. A Graphic Procedure in the Geochemical Interpretation of Water Analysis; U.S. Geological Survey: Washington, DC, USA, 1953.
- Dauda, M.; Habib, G.A. Graphical techniques of hydro-chemical data. J. Environ. Earth Sci. 2015, 5, 65–76. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Ogarekpe, N.M.; Nnaji, C.C.; Oyebode, O.J.; Ekpenyong, M.G.; Ofem, O.I.; Tenebe, I.T.; Asitok, A.D. Groundwater quality index and potential human health risk assessment of heavy metals in water: A case study of Calabar metropolis, Nigeria. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100780. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (EPA). Dermal Exposure Assessment: A Summary of EPA Approaches; EPA: Washington, DC, USA, 2007.
- Harter, T.; Davis, H.; Mathews, M.C.; Meyer, R.D. Shallow groundwater quality on dairy farms with irrigated forage crops. J. Contam. Hydrol. 2022, 55, 287–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Al-Maktoumi, A.; Al-Mamari, H.; Izady, A.; Nikoo, M.R.; Al-Busaidi, H. Oxygenation of aquifers with fluctuating water table: A laboratory and modeling study. J. Hydrol. 2020, 590, 125261. [Google Scholar] [CrossRef]
- Lu, B.T.; Luo, J.L.; Norton, P.R. Environmentally assisted cracking mechanism of pipeline steel in near-neutral pH groundwater. Corros. Sci. 2010, 52, 1787–1795. [Google Scholar] [CrossRef]
- Wilkin, R.T.; DiGiulio, D.C. Geochemical impacts to groundwater from geologic carbon sequestration: Controls on pH and inorganic carbon concentrations from reaction path and kinetic modelling. Environ. Sci. Technol. 2010, 44, 4821–4827. [Google Scholar] [CrossRef] [PubMed]
- Serban, G.; Sabău, D.; Bătinaș, R.; Brețcan, P.; Ignat, E.; Nacu, S. Water resources from Romanian Upper Tisa Basin. In Water Resources Management in Romania; Springer: Berlin/Heidelberg, Germany, 2020; pp. 393–434. [Google Scholar]
- Mohammed, I.; Al-Khalaf, S.K.H.; Alwan, H.H.; Naje, A.S. Environmental assessment of Karbala water treatment plant using water quality index (WQI). Mater. Today Proc. 2022, 60, 1554–1560. [Google Scholar] [CrossRef]
- Rivett, M.O.; Buss, S.R.; Morgan, P.; Smith, J.W.; Bemment, C.D. Nitrate attenuation in groundwater: A review of biogeochemical controlling processes. Water Res. 2008, 42, 4215–4232. [Google Scholar] [CrossRef]
- Adesakin, T.A.; Oyewale, A.T.; Bayero, U.; Mohammed, A.N.; Aduwo, I.A.; Ahmed, P.Z.; Abubakar, N.D.; Barje, I.B. Assessment of bacteriological quality and physico-chemical parameters of domestic water sources in Samaru community, Zaria, Northwest Nigeria. Heliyon 2020, 6, E04773. [Google Scholar] [CrossRef]
- El-Said, G.F.; El-Sadaawy, M.M.; Moneer, A.A.; Shaltout, N.A. The effect of fluoride on the distribution of some minerals in the surface water of an Egyptian lagoon at the Mediterranean Sea. Egypt. J. Aquat. Res. 2015, 4, 31–39. [Google Scholar] [CrossRef]
- Rupias, O.J.B.; Pereira, S.Y.; de Abreu, A.E.S. Hydrogeochemistry and groundwater quality assessment using the water quality index and heavy-metal pollution index in the alluvial plain of Atibaia river-Campinas/SP, Brazil. Groundw. Sustain. Dev. 2021, 15, 100661. [Google Scholar] [CrossRef]
- Huang, G.; Hou, Q.; Han, D.; Liu, R.; Song, J. Large scale occurrence of aluminium-rich shallow groundwater in the Pearl River Delta after the rapid urbanization: Co-effects of anthropogenic and geogenic factors. J. Contam. Hydrol. 2023, 254, 104130. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, J.A.; Bhosle, A.B. Analysis of chloride, sodium and potassium in groundwater samples of Nanded City in Mahabharata, India. Eur. J. Experim Biol. 2011, 1, 74–82. [Google Scholar]
- Adimalla, N.; Qian, H. Groundwater quality evaluation using water quality index (WQI) for drinking purposes and human health risk (HHR) assessment in an agricultural region of Nanganur, south India. Ecotoxicol. Environ. Saf. 2019, 176, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Rapant, S.; Cvečková, V.; Fajčíková, K.; Sedláková, D.; Stehlíková, B. Impact of calcium and magnesium in groundwater and drinking water on the health of inhabitants of the Slovak Republic. Int. J. Environ. Res. Public. Health. 2017, 14, 278. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Yao, F.; Xiong, S.; Wu, Z.; Niu, G.; Lu, T. Strontium in public drinking water and associated public health risks in Chinese cities. Environ. Sci. Pollut. Res. 2021, 28, 23048–23059. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, S.L.; Zheng, W. Manganese toxicity upon overexposure: A decade in review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Krisham, G.; Taloor, A.K.; Sudarsan, N.; Bhattacharya, P.; Kumar, S.; Ghosh, N.C.; Singh, S.; Sharma, A.; Rao, M.S.; Mittal, S.; et al. Occurrences of potentially toxic trace metals in groundwater of the state of Punjab in northern India. Groundw. Sustain. Dev. 2021, 15, 100655. [Google Scholar] [CrossRef]
- Weisner, M.L.; Harris, M.S.; Mitsova, D.; Liu, W. Drinking water disparities and aluminum concentrations: Assessing socio-spatial dimensions across an urban landscape. Social. Sci. Human. Open. 2023, 8, 100536. [Google Scholar] [CrossRef]
- Lall, U.; Josset, L.; Russo, T. A snapshot of the world’s groundwater challenges. Annual Rev. Environ. Res. 2020, 45, 171–194. [Google Scholar] [CrossRef]
- Nasher, N.R. Groundwater quality of arsenic contaminated aquifers in the Munshiganj district, Bangladesh using water quality index. HydroResearch 2022, 5, 71–84. [Google Scholar] [CrossRef]
- Malecki, J.J.; Kadzikiewicz-Schoeneich, M.; Szostakiewicz-Hołownia, M. Concentration and mobility of copper and zinc in the hypergenic zone of a highly urbanized area. Environ. Earth Sci. 2016, 75, 24. [Google Scholar] [CrossRef]
- Choudhury, T.R.; Ferdous, J.; Haque, M.M.; Rahman, M.M.; Quraishi, S.B.; Rahman, M.S. Assessment of heavy metals and radionuclides in groundwater and associated human health risk appraisal in the vicinity of Rooppur nuclear power plant, Bangladesh. J. Contam. Hydrol. 2022, 251, 104072. [Google Scholar] [CrossRef]
- Jafarzadeh, N.; Heidari, K.; Meshkinian, A.; Kamani, H.; Mohammadi, A.A.; Conti, G.O. Non-carcinogenic risk assessment of exposure to heavy metals in underground water resources in Saraven, Iran: Spatial distribution, monte-carlo simultation, sensitive analysis. Environ. Res. 2022, 204, 112002. [Google Scholar] [CrossRef]
- Krok, B.; Mohammadian, S.; Noll, H.M.; Surau, C.; Markwort, S.; Fritzsche, A.; Nachev, M.; Sures, B.; Meckenstock, R.U. Remediation of zinc-contaminated groundwater by iron oxide in situ adsorption barriers—From lab to the field. Sci. Total Environ. 2022, 897, 151066. [Google Scholar] [CrossRef]
- Rashid, A.; Ayub, M.; Javed, A.; Khan, S.; Gao, X.; Li, C.; Ullah, Z.; Sardar, T.; Muhammad, J.; Nazneen, S. Potentially harmful metals, and health risk evaluatoin in groundwater of Mardan, Pakistan: Application of geostatistical approach and geographic information system. Geosci. Front. 2021, 12, 101128. [Google Scholar] [CrossRef]
- Eslami, H.; Esmaeili, A.; Razaeian, M.; Salari, M.; Hosseini, A.N.; Mobini, M.; Barani, A. Potentially toxic metal concentration, spatial distribution, and health risk assessment in drinking groundwater resources of southeast Iran. Geosci. Front. 2022, 13, 101276. [Google Scholar] [CrossRef]
- Lyu, P.; Li, L.; Huang, X.; Xie, J.; Ye, J.; Tian, Y.; Huang, J.; Zhu, C. Ternary Ca-Mg-Al layered-hydroxides for synergistic remediation of As, Cd, and Pb from both contaminated soil and groundwater: Characteristics, effectiveness, and immobilization mechanisms. J. Hazard. Mater. 2023, 442, 130030. [Google Scholar] [CrossRef] [PubMed]
- Abbaszade, G.; Tserendorj, D.; Salazar-Yanez, N.; Zachary, D.; Volgyesi, P.; Toth, E. Lead and stable lead isotopes as tracers of soil pollution and human health risk assessment in former industrail citisen of Hungary. Appl. Geochem. 2022, 145, 105397. [Google Scholar] [CrossRef]
- Li, L.; Sun, F.; Liu, Q.; Zhao, X.; Song, K. Development of regional water quality criteria of lead for protecting aquatic organism in Taihu Lake, China. Ecotoxicol. Environ. Saf. 2021, 222, 112479. [Google Scholar] [CrossRef] [PubMed]
- Rathor, G.; Chopra, N.; Adhikari, T. Nickel as a Pollutant and its Management. Int. Res. J. Environ. Sci. 2014, 3, 94–98. [Google Scholar]
- Zeng, J.; Tabelin, C.B.; Gao, W.; Tang, L.; Luo, X.; Ke, W.; Jiang, J.; Xue, S. Heterogeneous distributions of heavy metals in the soil-groundwater system empowers the knowledge of the pollution migration at a smelting site. Chem. Eng. J. 2023, 454, 140307. [Google Scholar] [CrossRef]
- Qu, Y.; Ji, S.; Sun, Q.; Zhao, F.; Li, Z.; Zhang, M.; Li, Y.; Zheng, L.; Song, H.; Zhang, W.; et al. Association of urinary nickel levels with diabetes and fasting blood glucose levels: A nationwide Chine population-based study. Ecotoxicol. Environ. Saf. 2023, 252, 114601. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S. Interpretation of groundwater chemistry using piper and Chadha’s diagrams: A comparative study from Perambalur Taluk. Elixir Geosci. 2013, 54, 12208–12211. [Google Scholar]
- Zhou, M.F.; Li, M.Y.H.; Wang, Z.; Li, X.C.; Liu, J. The genesis of regolith-hosted rare earth element and scandium deposits: Current understanding and outlook to future prospecting. Chin. Sci. Bull. 2020, 65, 3809–3824. [Google Scholar] [CrossRef]
- Frengstad, B.J.; Lax, K.; Tarvainen, T.; Jæger, Ø.; Wigum, B.J. The chemistry of bottled mineral and spring waters from Norway, Sweden, Finland and Iceland. J. Geochem. Explor. 2010, 107, 350–361. [Google Scholar] [CrossRef]
- Resz, M.A.; Roman, C.; Senila, M.; Török, A.I.; Kovacs, E. A Comprehensive Approach to the Chemistry, Pollution Impact and Risk Assessment of Drinking Water Sources in a Former Industrialized Area of Romania. Water 2023, 15, 1180. [Google Scholar] [CrossRef]
- Dippong, T.; Resz, M.H. Quality and Health Risk Assessment of Groundwaters in the Protected Area of Tisa River Basin. Int. J. Environ. Res. Public. Health 2022, 19, 14898. [Google Scholar] [CrossRef]
- Moldovan, A.; Hoaghia, M.A.; Kovacs, E.; Mirea, I.C.; Kenesz, M.; Arghir, R.A.; Petculescu, A.; Levei, E.A.; Moldovan, O.T. Quality and Health Risk Assessment Associated with Water Consumption—A Case Study on Karstic Springs. Water 2020, 12, 3510. [Google Scholar] [CrossRef]

| Samples | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | G9 | G10 | Mean | MAC * | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | |||||||||||||
| EC (μS/cm) | 150 ± 15 | 663 ± 50 | 77 ± 8 | 454 ± 50 | 327 ± 30 | 332 ± 33 | 68 ± 7 | 82 ± 8 | 72 ± 7 | 125 ± 12 | 232 | 2500 | |
| DO (mg/L) | 8.3 ± 0.8 | 8.2 ± 0.8 | 8.6 ± 0.9 | 8.0 ± 0.8 | 8.4 ± 0.8 | 8.3 ± 0.8 | 7.7 ± 0.8 | 8.5 ± 0.9 | 8.9 ± 0.8 | 6.9 ± 0.7 | 8.18 | - | |
| pH | 6.6 ± 0.5 | 6.4 ± 0.5 | 7.0 ± 0.5 | 6.8 ± 0.5 | 6.7 ± 0.5 | 6.5 ± 0.5 | 6.7 ± 0.5 | 8.0 ± 0.5 | 6.5 ± 0.5 | 6.7 ± 0.5 | 6.79 | 6.5–9.5 | |
| ORP (mV) | 208 ± 21 | 188 ± 20 | 196 ± 20 | 223 ± 22 | 212 ± 21 | 221 ± 22 | 179 ± 18 | 192 ± 20 | 234 ± 23 | 199 ± 20 | 205 | - | |
| T (NTU) | 4.4 ± 0.4 | 4.8 ± 0.5 | 5.2 ± 0.5 | 4.4 ± 0.4 | 5.6 ± 0.5 | 5.4 ± 0.5 | 4.3 ± 0.4 | 3.2 ± 0.3 | 2.5 ± 0.2 | 3.8 ± 0.4 | 4.4 | <5 | |
| TDS (mg/L) | 110 ± 11 | 185 ± 19 | 166 ± 17 | 203 ± 20 | 158 ± 16 | 177 ± 18 | 149 ± 15 | 225 ± 23 | 141 ± 14 | 266 ± 27 | 178 | - | |
| NH4+ (mg/L) | 0.82 ± 0.08 | 0.84 ± 0.08 | 0.40 ± 0.04 | 0.65 ± 0.07 | 0.52 ± 0.05 | 0.60 ± 0.06 | 1.20 ± 0.01 | 0.28 ± 0.03 | 0.11 ± 0.01 | 0.17 ± 0.02 | 0.56 | 0.5 | |
| NO3− (mg/L) | 23.1 ± 2.3 | 23.7 ± 2.4 | 20.5 ± 2.0 | 21.3 ± 2.1 | 13.9 ± 1.4 | 14.6 ± 1.5 | 22.5 ± 2.3 | 18.1 ± 1.8 | 4.1 ± 0.4 | 4.0 ± 0.4 | 16.6 | 50 | |
| NO2− (mg/L) | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.12 ± 0.01 | 0.21 ± 0.02 | 0.07 ± 0.01 | 0.44 ± 0.04 | 0.25 ± 0.03 | 0.28 ± 0.03 | 0.45 ± 0.05 | 0.38 ± 0.04 | 0.23 | 3 | |
| PO43− (mg/L) | 0.33 ± 0.03 | 0.05 ± 0.01 | 0.12 ± 0.01 | 0.21 ± 0.02 | 0.09 ± 0.01 | 0.40 ± 0.05 | 0.15 ± 0.02 | 0.09 ± 0.01 | 0.05 ± 0.01 | 0.33 ± 0.03 | 0.18 | 0.2 | |
| SO42− (mg/L) | 7.8 ± 0.8 | 9.5 ± 0.9 | 10.7 ± 1.1 | 5.8 ± 0.6 | 70.3 ± 7.0 | 6.9 ± 0.7 | 16.8 ± 1.7 | 132 ± 12 | 7.3 ± 0.7 | 22.6 ± 2.3 | 29.0 | 250 | |
| CO32− (mg/L) | 85 ± 9 | 106 ± 11 | 186 ± 18 | 121 ± 12 | 455 ± 45 | 123 ± 12 | 223 ± 22 | 556 ± 60 | 97 ± 10 | 257 ± 25 | 221 | - | |
| HCO3− (mg/L) | 109 ± 10 | 156 ± 15 | 303 ± 30 | 192 ± 20 | 628 ± 63 | 205 ± 21 | 365 ± 36 | 737 ± 70 | 167 ± 17 | 403 ± 40 | 327 | - | |
| F− (mg/L) | 0.15 ± 0.02 | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.17 ± 0.02 | 0.15 ± 0.02 | 0.10 ± 0.01 | 0.19 ± 0.02 | 0.16 ± 0.02 | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.14 | 1.5 | |
| Cl− (mg/L) | 20 ± 2 | 207 ± 20 | 1.2 ± 0.1 | 150 ± 15 | 70 ± 7 | 4.3 ± 0.4 | 172 ± 17 | 50 ± 5 | 3.6 ± 0.4 | 1.9 ± 0.2 | 68 | 250 | |
| Br− (mg/L) | 0.7 ± 0.1 | 3.5 ± 0.3 | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.3 ± 0.1 | 1.0 ± 0.1 | 65 ± 7 | 1.8 ± 0.2 | 2.4 ± 0.2 | 1.0 ± 0.1 | 7.8 | - | |
| Samples Parameter | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | G9 | G10 | Mean | MAC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca (mg/L) | 12.5 ± 1.2 | 31.5 ± 3.1 | 8.8 ± 0.9 | 19.9 ± 2.0 | 25.8 ± 2.6 | 8.7 ± 0.9 | 83.2 ± 8.3 | 34.2 ± 3.5 | 8.1 ± 0.8 | 11.3 ± 1.1 | 24.4 | 100 |
| Mg (mg/L) | 2.0 ± 0.2 | 4.1 ± 0.4 | 1.7 ± 2.0 | 3.0 ± 0.3 | 4.9 ± 0.5 | 1.0 ± 0.1 | 10.8 ± 1.1 | 5.3 ± 0.5 | 1.9 ± 0.2 | 2.5 ± 0.3 | 3.7 | 50 |
| Na (mg/L) | 7.9 ± 0.8 | 20.9 ± 2.0 | 2.4 ± 0.2 | 23.1 ± 2.3 | 24.1 ± 2.4 | 2.2 ± 0.2 | 27.5 ± 2.8 | 21.5 ± 2.2 | 2.5 ± 0.3 | 2.4 ± 0.2 | 13.5 | 200 |
| K (mg/L) | 1.1 ± 0.1 | 2.0 ± 0.2 | 1.5 ± 0.1 | 1.7 ± 0.2 | 2.5 ± 0.3 | 1.0 ± 0.1 | 6.9 ± 0.7 | 2.6 ± 0.3 | 0.5 ± 0.1 | 0.9 ± 0.1 | 2.1 | 10 |
| Al (μg/L) | 2.4 ± 0.2 | 1.7 ± 0.1 | 2.1 ± 0.2 | 1.5 ± 0.1 | 1.9 ± 0.2 | 1.3 ± 0.1 | 3.1 ± 0.3 | 3.6 ± 0.4 | 3.3 ± 0.3 | 5.1 ± 0.5 | 2.6 | 200 |
| As (μg/L) | 1.6 ± 0.2 | 1.5 ± 0.2 | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.8 ± 0.2 | 3.3 ± 0.3 | 5.2 ± 0.5 | 3.1 ± 0.3 | 1.5 ± 0.1 | 2.3 ± 0.2 | 2.3 | 10 |
| Ba (μg/L) | 12.6 ± 1.3 | 36.1 ± 3.6 | 22.4 ± 2.2 | 16.2 ± 1.6 | 15.9 ± 1.6 | 11.6 ± 1.2 | 59.3 ± 6.0 | 7.8 ± 0.8 | 4.9 ± 0.5 | 67.9 ± 0.7 | 25.5 | 700 |
| Cd (μg/L) | 0.5 ± 0.1 | 0.8 ± 0.1 | 1.6 ± 0.2 | 0.4 ± 0.1 | 1.0 ± 0.1 | 1.8 ± 0.2 | 2.3 ± 0.2 | 1.6 ± 0.1 | 0.3 ± 0.1 | 1.5 ± 0.2 | 1.2 | 5 |
| Co (μg/L) | 1.1 ± 0.1 | 3.3 ± 0.3 | 1.6 ± 0.2 | 1.9 ± 0.2 | 2.9 ± 0.3 | 4.8 ± 0.5 | 5.9 ± 0.6 | 4.5 ± 0.5 | 1.5 ± 0.2 | 5.2 ± 0.5 | 3.3 | - |
| Cr (μg/L) | 3.4 ± 0.3 | 4.5 ± 0.5 | 2.5 ± 0.3 | 3.3 ± 0.3 | 6.2 ± 0.6 | 4.3 ± 0.4 | 3.3 ± 0.3 | 2.9 ± 0.3 | 4.6 ± 0.5 | 3.5 ± 0.4 | 3.9 | 50 |
| Cu (μg/L) | 1.8 ± 0.2 | 2.2 ± 0.2 | 14.8 ± 1.5 | 12.2 ± 1.2 | 19 ± 0.2 | 16.1 ± 1.6 | 9.9 ± 1.0 | 1.5 ± 0.2 | 2.6 ± 0.3 | 14.3 ± 1.4 | 9.4 | 100 |
| Fe (μg/L) | 2.1 ± 0.2 | 5.8 ± 0.6 | 45.5 ± 4.6 | 38.5 ± 3.9 | 18.5 ± 1.9 | 33.4 ± 3.3 | 55.6 ± 5.6 | 23.2 ± 2.3 | 8.9 ± 0.9 | 58.7 ± 5.9 | 29.0 | 200 |
| Li (μg/L) | 9.8 ± 1.0 | 18.8 ± 1.9 | 13.2 ± 1.3 | 15.5 ± 1.6 | 27.2 ± 2.7 | 9.3 ± 0.9 | 53.2 ± 5.3 | 33.1 ± 3.3 | 5.4 ± 0.5 | 10.6 ± 1.1 | 19.6 | 50 |
| Mn (μg/L) | 1.7 ± 0.2 | 21.3 ± 2.1 | 22.3 ± 2.2 | 15.2 ± 1.5 | 9.1 ± 0.9 | 20.4 ± 2.0 | 35.3 ± 3.5 | 11.9 ± 1.2 | 5.5 ± 0.5 | 48.3 ± 5.0 | 19.1 | 50 |
| Ni (μg/L) | 0.8 ± 0.1 | 1.9 ± 0.2 | 2.4 ± 0.2 | 1.6 ± 0.2 | 0.9 ± 0.1 | 1.7 ± 0.2 | 3.3 ± 0.3 | 2.4 ± 0.2 | 1.2 ± 0.1 | 3.9 ± 0.4 | 2.0 | 20 |
| Sc (μg/L) | 3.4 ± 0.3 | 1.7 ± 0.2 | 2.7 ± 0.3 | 1.2 ± 0.1 | 1.9 ± 0.2 | 1.1 ± 0.1 | 1.3 ± 0.1 | 2.1 ± 0.2 | 1.4 ± 0.1 | 1.8 ± 0.2 | 1.9 | - |
| Se (μg/L) | 2.3 ± 0.2 | 0.8 ± 0.1 | 4.1 ± 0.4 | 3.3 ± 0.3 | 5.1 ± 0.5 | 1.9 ± 0.2 | 1.2 ± 0.1 | 5.6 ± 0.6 | 3.8 ± 0.4 | 6.2 ± 0.6 | 3.4 | 10 |
| Sr (μg/L) | 25.5 ± 2.6 | 72.1 ± 7.0 | 13.3 ± 1.3 | 37.2 ± 3.7 | 44.5 ± 4.5 | 14.6 ± 1.5 | 226 ± 22 | 55.2 ± 5.5 | 11.3 ± 1.1 | 18.2 ± 1.8 | 51.8 | 200 |
| Pb(μg/L) | 1.3 ± 0.1 | 2.9 ± 0.3 | 3.5 ± 0.4 | 4.4 ± 0.4 | 5.5 ± 0.5 | 1.8 ± 0.2 | 7.2 ± 0.7 | 3.2 ± 0.3 | 1.2 ± 0.1 | 5.8 ± 0.5 | 3.7 | 10 |
| Rb (μg/L) | 1.4 ± 0.2 | 1.9 ± 0.2 | 2.5 ± 0.3 | 1.6 ± 0.2 | 2.4 ± 0.2 | 1.8 ± 0.2 | 3.6 ± 0.4 | 2.5 ± 0.3 | 4.4 ± 0.4 | 4.8 ± 0.5 | 4.3 | - |
| Zn (μg/L) | 1.8 ± 0.2 | 2.8 ± 0.3 | 37.5 ± 3.7 | 2.7 ± 0.3 | 1.2 ± 0.1 | 7.3 ± 0.7 | 8.9 ± 0.9 | 10.5 ± 0.1 | 4.6 ± 0.5 | 5.4 ± 0.5 | 8.3 | 5000 |
| CDI | ||||||||||
| mg kg−1 /day | G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | G9 | G10 |
| Cd | 1.4 × 10−5 | 2.3 × 10−5 | 4.6 × 10−5 | 1.1 × 10−5 | 2.9 × 10−5 | 5.1 × 10−5 | 6.6 × 10−5 | 4.6 × 10−5 | 9.0 × 10−5 | 4.3 × 10−5 |
| As | 4.6 × 10−5 | 3.7 × 10−5 | 3.7 × 10−5 | 4.0 × 10−5 | 5.1 × 10−5 | 9.4 × 10−5 | 1.5 × 10−4 | 8.9 × 10−5 | 4.3 × 10−5 | 6.6 × 10−5 |
| Mn | 4.9 × 10−5 | 6.4 × 10−4 | 6.4 × 10−4 | 4.3 × 10−4 | 2.6 × 10−4 | 5.8 × 10−4 | 1.0 × 10−3 | 3.4 × 10−4 | 1.6 × 10−4 | 1.4 × 10−3 |
| Cu | 5.1 × 10−5 | 4.2 × 10−4 | 4.2 × 10-−4 | 3.5 × 10−4 | 5.4 × 10−4 | 4.6 × 10−4 | 2.8 × 10−4 | 4.3 × 10−5 | 7.4 × 10−5 | 4.1. × 10−4 |
| HQ | ||||||||||
| G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | G9 | G10 | |
| Cd | 2.0 × 10−5 | 6.5 × 10−5 | 6.5 × 10−5 | 1.6 × 10−5 | 4.1 × 10−5 | 7.3 × 10−5 | 9.4 × 10−5 | 6.5 × 10−5 | 1.2 × 10−5 | 6.1 × 10−5 |
| As | 0.152 | 0.124 | 0.124 | 0.133 | 0.171 | 0.314 | 0.495 | 0.295 | 0.143 | 0.219 |
| Mn | 3.5 × 10−4 | 4.6 × 10−3 | 4.6 × 10−3 | 3.1 × 10−3 | 1.9 × 10−3 | 4.2 × 10−3 | 7.2 × 10−3 | 2.4 × 10−3 | 1.1 × 10−3 | 9.9 × 10−3 |
| Cu | 1.3 × 10−3 | 10.6 × 10−3 | 10.6 × 10−3 | 8.7 × 10−3 | 1.4 × 10−2 | 1.1 × 10−2 | 7.1 × 10−3 | 1.1 × 10−3 | 1.9 × 10−3 | 0.10 |
| HI | ||||||||||
| G1 | G2 | G3 | G4 | G5 | G6 | G7 | G8 | G9 | G10 | |
| 0.154 | 0.149 | 0.139 | 0.145 | 0.187 | 0.330 | 0.510 | 0.299 | 0.146 | 0.239 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dippong, T.; Resz, M.-A. Chemical Assessment of Drinking Water Quality and Associated Human Health Risk of Heavy Metals in Gutai Mountains, Romania. Toxics 2024, 12, 168. https://doi.org/10.3390/toxics12030168
Dippong T, Resz M-A. Chemical Assessment of Drinking Water Quality and Associated Human Health Risk of Heavy Metals in Gutai Mountains, Romania. Toxics. 2024; 12(3):168. https://doi.org/10.3390/toxics12030168
Chicago/Turabian StyleDippong, Thomas, and Maria-Alexandra Resz. 2024. "Chemical Assessment of Drinking Water Quality and Associated Human Health Risk of Heavy Metals in Gutai Mountains, Romania" Toxics 12, no. 3: 168. https://doi.org/10.3390/toxics12030168
APA StyleDippong, T., & Resz, M.-A. (2024). Chemical Assessment of Drinking Water Quality and Associated Human Health Risk of Heavy Metals in Gutai Mountains, Romania. Toxics, 12(3), 168. https://doi.org/10.3390/toxics12030168







