Chlorogenic Acid Intravesical Therapy Changes Acute Voiding Behavior of Systemic Lipopolysaccharide Inflammation-Induced Cystitis Bladder in Mice
Abstract
:1. Introduction
2. Results
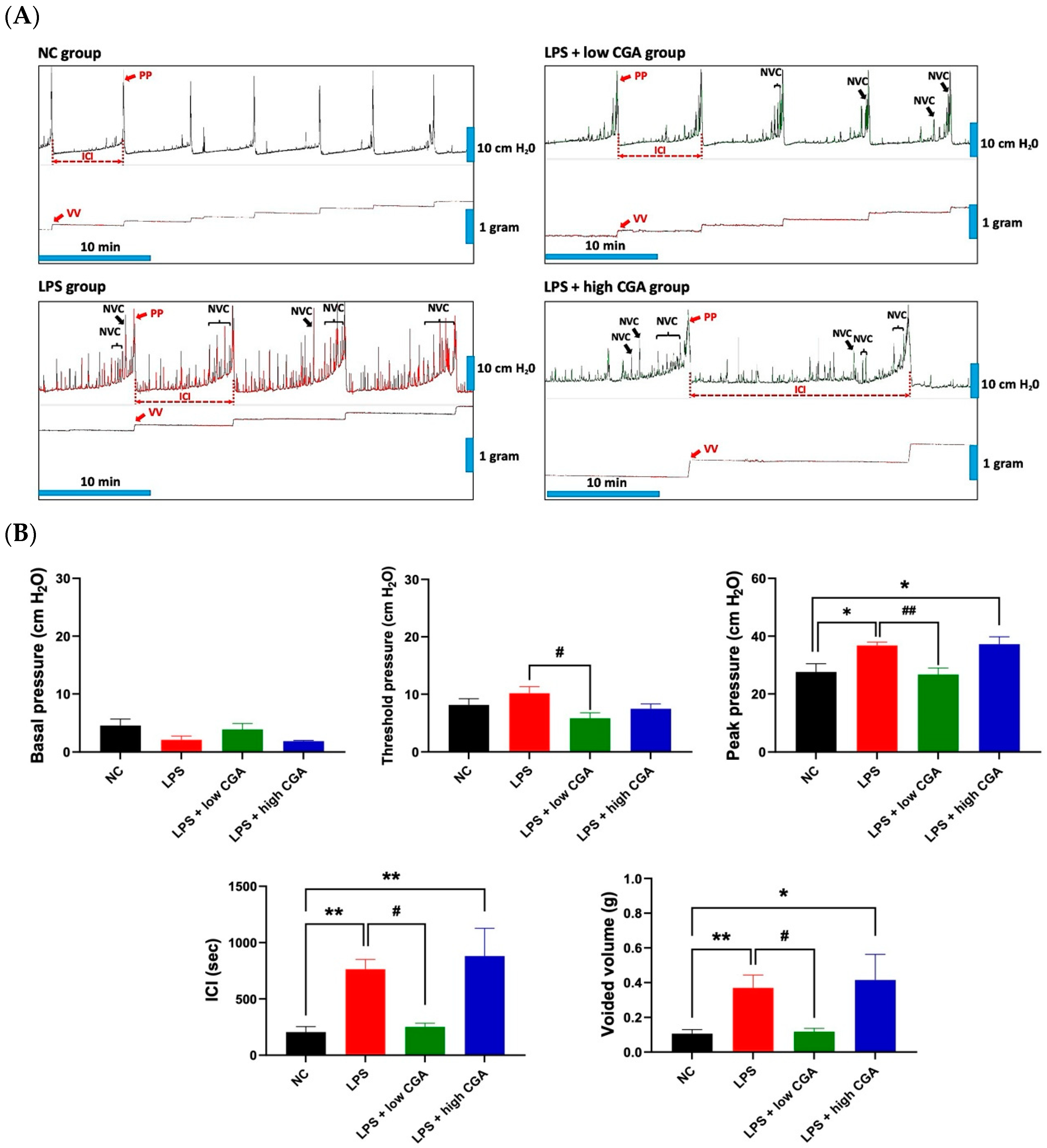
2.1. Low CGA Administration Changes the Voiding Function in IP Injection LPS-Induced IC Mice
2.2. Low CGA Administration Attenuates Bladder Interstitial Edema and Inflammation in LPS-Induced IC
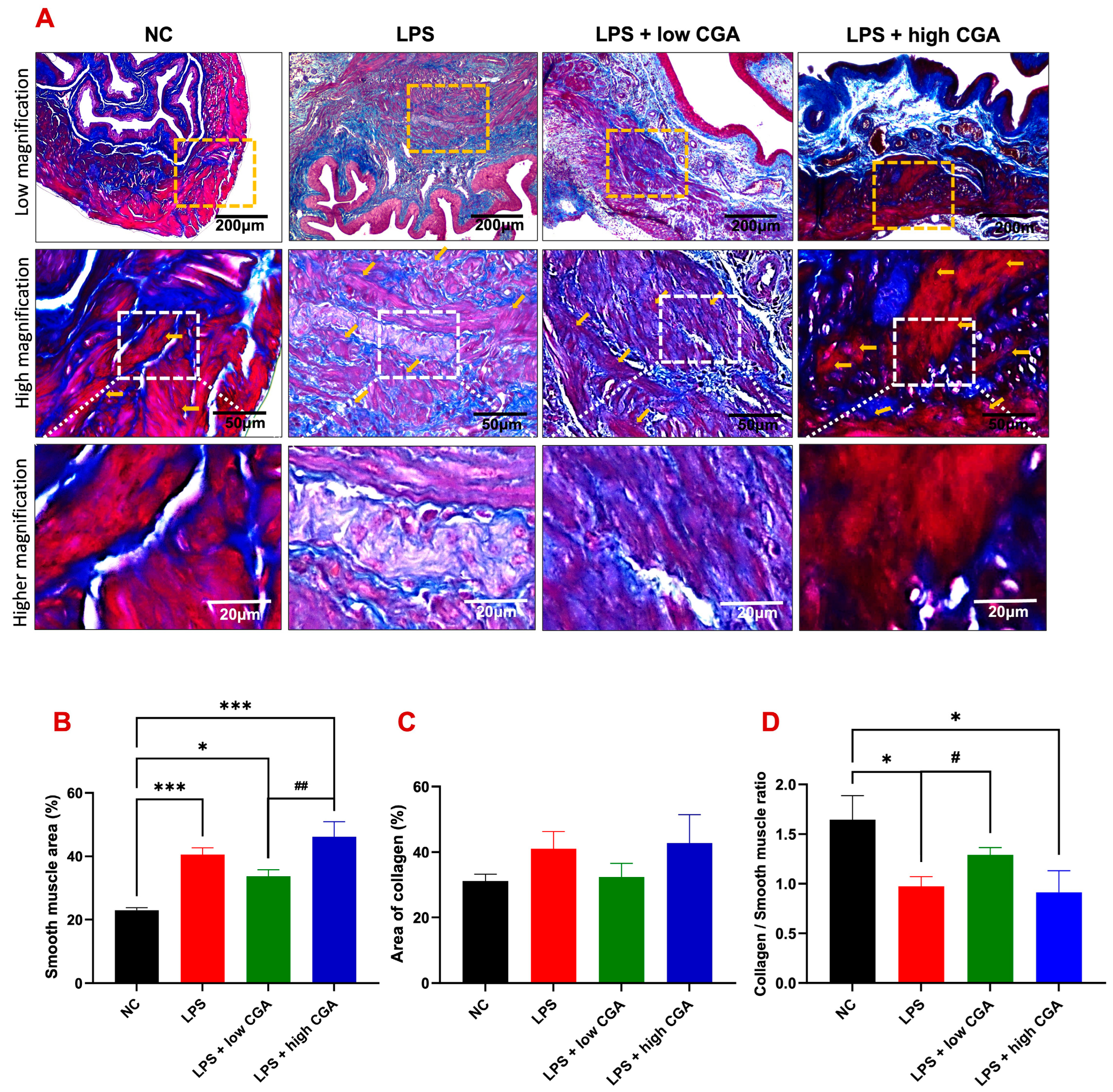
2.3. Low CGA Slightly Attenuates Bladder Wall Remodeling after LPS-Induced Inflammatory Bladder
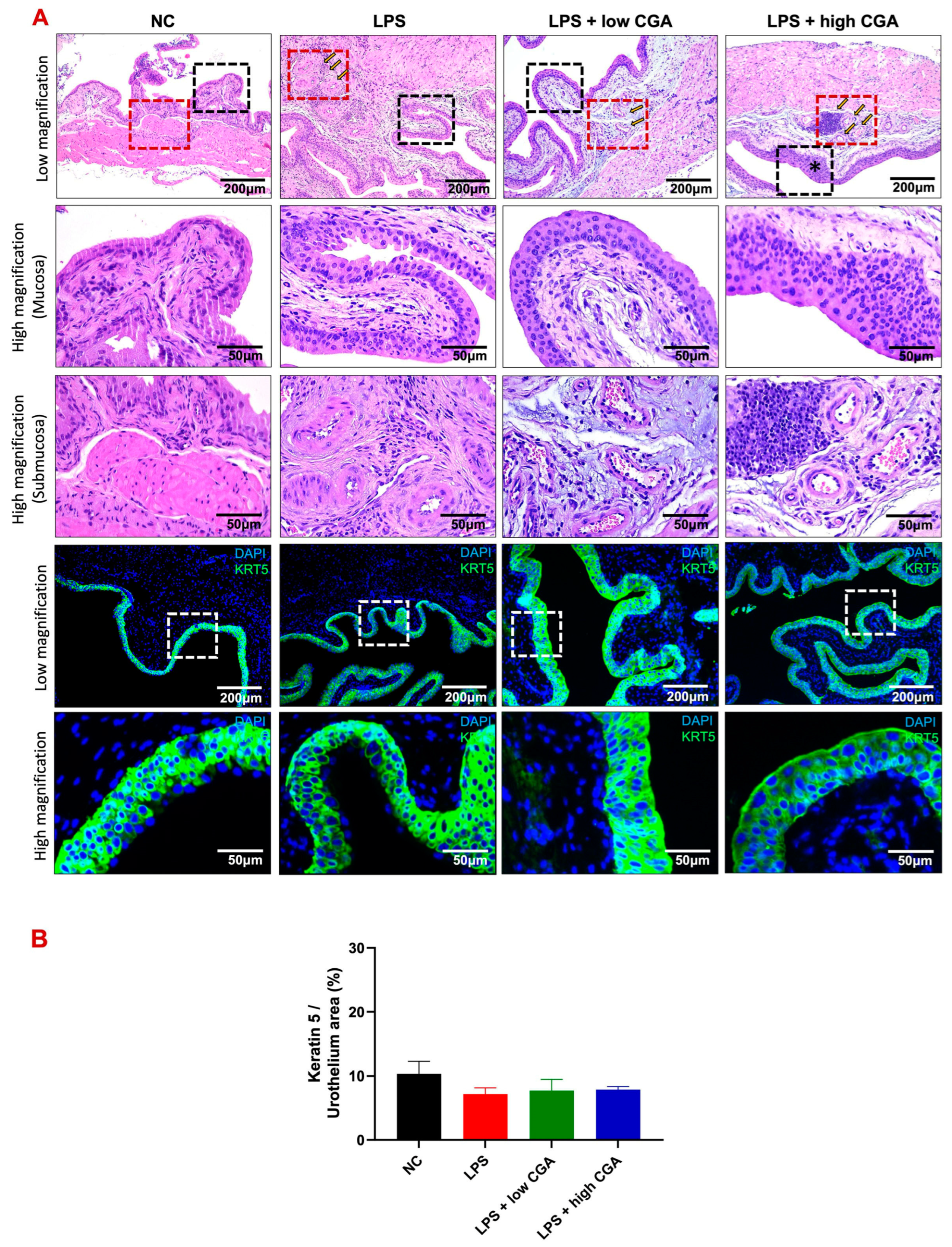
2.4. High CGA Administration Causes the Loss of the Superficial Umbrella Cells of the Bladder
3. Discussions
4. Materials and Methods
4.1. Experimental Setup
4.2. Animal Selection and Housing
4.3. Induction of Interstitial Cystitis through E. coli Lipopolysaccharide
4.4. Direct Instillation of CGA into the Bladder
4.5. Surgical Procedure for Cystometric Measurements and Bladder Function Evaluation
4.6. Preparation of Bladder Tissue Homogenates and Western Blotting
4.7. Histology Evaluation, Hematoxylin, Eosin (H&E), and Masson’s Trichrome Staining
4.8. Immunofluorescence Staining
4.9. Quantitative Assessment
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pires, M.V.; de Lima, C.J.; Carvalho, H.C.; Moreira, L.H.; Fernandes, A.B. Effectiveness of Intravesical Ozone in Interstitial Cystitis by the O’Leary–Sant Symptom Index. Int. Urogynecol. J. 2023, 34, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Youn, S. Intravesical Bladder Treatment and Deep Learning Applications to Improve Irritative Voiding Symptoms Caused by Interstitial Cystitis: A Literature Review. Int. Neurourol. J. 2023, 27, S13–S20. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Liao, C.H.; Chen, K.C.; Wang, K.L.; Tseng, X.W.; Tsai, W.K.; Chiang, H.S.; Wu, Y.N. B6 Mouse Strain: The Best Fit for LPS-Induced Interstitial Cystitis Model. Int. J. Mol. Sci. 2021, 22, 12053. [Google Scholar] [CrossRef] [PubMed]
- Garzon, S.; Laganà, A.S.; Casarin, J.; Raffaelli, R.; Cromi, A.; Sturla, D.; Franchi, M.; Ghezzi, F. An Update on Treatment Options for Interstitial Cystitis. Prz Menopauzalny 2020, 19, 35–43. [Google Scholar] [CrossRef]
- Giusto, L.L.; Zahner, P.M.; Shoskes, D.A. An Evaluation of the Pharmacotherapy for Interstitial Cystitis. Exp. Opin. Pharmacother. 2018, 19, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide Modification in Gram-Negative Bacteria During Chronic Infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.M.; Shin, J.H.; Yu, H.Y.; Ju, H.; Kim, S.; Lim, J.; Heo, J.; Lee, S.; Shin, D.M.; Choo, M.S. N-acetylcysteine Prevents Bladder Tissue Fibrosis in a Lipopolysaccharide-Induced Cystitis Rat Model. Sci. Rep. 2019, 9, 8134. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.C.; Pham, H.; Ito, T.; Parsons, C.L. Bladder Injury Model Induced in Rats by Exposure to Protamine Sulfate Followed by Bacterial Endotoxin. J. Urol. 1996, 155, 1133–1138. [Google Scholar] [CrossRef]
- Bjorling, D.E.; Wang, Z.Y.; Bushman, W. Models of inflammation of the lower urinary tract. Neurourol. Urodyn. 2011, 30, 673–682. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Frezza, C.; Conti, F.; Bini, L.M.; Giuliani, C.; Bramucci, M.; Quassinti, L.; Damiano, S.; Lupidi, G.; et al. Essential Oil Composition, Polar Compounds, Glandular Trichomes and Biological Activity of Hyssopus officinalis subsp. aristatus (Godr.) Nyman from Central Italy. Ind. Crops Prod. 2015, 77, 353–363. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bhandarkar, N.S.; Brown, L.; Panchal, S.K. Chlorogenic acid attenuates high-carbohydrate, high-fat diet–induced cardiovascular, liver, and metabolic changes in rats. Nutr. Res. 2019, 62, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Hida, M.; Matsumoto, T.; Ikeuchi-Takahashi, Y.; Onishi, H.; Kobayashi, T. Effect of Short-term Polyphenol Treatment on Endothelial Dysfunction and Thromboxane A2 Levels in Streptozotocin-induced Diabetic Mice. Biol. Pharm. Bull. 2014, 37, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic Acid Prevents Diabetic Nephropathy by Inhibiting Oxidative Stress and Inflammation through Modulation of the Nrf2/HO-1 and NF-ĸB Pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, T.; Takeda, H.; Nishihira, J. Protective Effect of Chlorogenic Acid on the Inflammatory Damage of Pancreas and Lung in Mice with L-arginine-induced Pancreatitis. Life Sci. 2017, 190, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, C.; Luo, X.; Yang, Y.; Li, J.; Song, B.; Zhao, J.; Li, L. Chlorogenic Acid Attenuates Cyclophosphamide-induced Rat Interstitial Cystitis. Life Sci. 2020, 254, 117590. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.T.; Krlin, R.M.; Winters, J.C. Urodynamics: Examining the Current Role of UDS Testing. What is the Role of Urodynamic Testing in Light of Recent AUA Urodynamics and Overactive Bladder Guidelines and the VALUE Study? Curr. Urol. Rep. 2013, 14, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, N.; Kamijo, T.C.; Wada, N.; Chiba, H.; Shinohara, N.; Miyazato, M. Effects of Low-intensity Extracorporeal Shock Wave Therapy on Lipopolysaccharide Cystitis in a Rat Model of Interstitial Cystitis/Bladder Pain Syndrome. Int. Urol. Nephrol. 2023, 56, 77–86. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical Against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Liu, N.; Shimizu, S.; Shimizu, T.; Nakamura, K.; Yamamoto, M.; Higashi, Y.; Saito, M. Protective Effects of the Selective Alpha1A-adrenoceptor Antagonist Silodosin Against Cyclophosphamide-induced Cystitis in Rats. J. Pharmacol. Sci. 2016, 132, 71–77. [Google Scholar] [CrossRef]
- Kullmann, F.A.; McDonnell, B.M.; Wolf-Johnston, A.S.; Lynn, A.M.; Getchell, S.E.; Ruiz, W.G.; Zabbarova, I.V.; Ikeda, Y.; Kanai, A.J.; Roppolo, J.R.; et al. Inflammation and Tissue Remodeling in the Bladder and Urethra in Feline Interstitial Cystitis. Front. Syst. Neurosci. 2018, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Biermann, M.H.; Brauner, J.M.; Liu, Y.; Zhao, Y.; Herrmann, M. New Insights Into Neutrophil Extracellular Traps: Mechanisms of Formation and Role in Inflammation. Front. Immunol. 2016, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- Du, W.Y.; Chang, C.; Zhang, Y.; Liu, Y.Y.; Sun, K.; Wang, C.S.; Wang, M.X.; Liu, Y.; Wang, F.; Fan, J.Y.; et al. High-dose Chlorogenic Acid Induces Inflammation Reactions and Oxidative Stress Injury in Rats Without Implication of Mast Cell Degranulation. J. Ethanopharmacol. 2013, 147, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Felts, P.A.; Woolston, A.M.; Fernando, H.B.; Asquith, S.; Gregson, N.A.; Mizzi, O.J.; Smith, K.J. Inflammation and Primary Demyelination Induced by the Intraspinal Injection of Lipopolysaccharide. Brain 2005, 128, 1649–1666. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Rosenberg, D.; Hardy, C.; Crocker, S.; Smith, P. Effects of CNS Demyelination and Myelin Recovery on Urinary Physiology. Innov. Aging 2020, 4, 119–120. [Google Scholar] [CrossRef]
- Vaidyanathan, S.; McDicken, I.W.; Ikin, A.J.; Mansour, P.; Soni, B.M.; Singh, G.; Sett, P. A Study of Cytokeratin 20 Immunostaining in the Urothelium of Neuropathic Bladder of Patients with Spinal Cord Injury. BMC Urol. 2002, 2, 7. [Google Scholar] [CrossRef]
- Bruch, P.G.; Plage, H.; Hofbauer, S.; Kornienko, K.; Weinberger, S.; Roßner, F.; Schallenberg, S.; Kluth, M.; Lennartz, M.; Blessin, N.C.; et al. Cytokeratin 20 Expression is Linked to Stage Progression and Poor Prognosis in Advanced (pT4) Urothelial Carcinoma of the Bladder. Exp. Mol. Pathol. 2023, 131, 104860. [Google Scholar] [CrossRef] [PubMed]
- Hauser, P.J.; VanGordon, S.B.; Seavey, J.; Sofinowski, T.M.; Ramadan, M.; Abdullah, S.; Buffington, C.T.; Hurst, R.E. Abnormalities in Expression of Structural, Barrier and Differentiation Related Proteins, and Chondroitin Sulfate in Feline and Human Interstitial Cystitis. J. Urol. 2015, 194, 571–577. [Google Scholar] [CrossRef]
- Shih, H.J.; Chang, C.Y.; Lai, C.H.; Huang, C.J. Therapeutic Effect of Modulating the NLRP3-regulated Transforming Growth Factor-β Signaling Pathway on Interstitial Cystitis/Bladder Pain Syndrome. Biomed. Pharmacother. 2021, 138, 111522. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Lin, Y.H.; Wu, Y.N.; Chen, Y.L.; Lin, Y.C.; Cheng, C.Y.; Chiang, H.S. Loss of SLC9A3 decreases CFTR protein and causes obstructed azoospermia in mice. PLoS Genet. 2017, 13, e1006715. [Google Scholar] [CrossRef]
- Juszczak, K.; Drewa, T. Pharmacotherapy in detrusor underactivity: A new challenge for urologists and pharmacologists (from lab to clinic). Pharmacol. Rep. 2016, 68, 703–706. [Google Scholar] [CrossRef]
- Merrill, L.; Girard, B.; Arms, L.; Guertin, P.; Vizzard, M.A. Neuropeptide/Receptor expression and plasticity in micturition pathways. Curr. Pharmaceuti. Des. 2013, 19, 4411–4422. [Google Scholar] [CrossRef]
- Merrill, L.; Vizzard, M.A. Intravesical TRPV4 blockade reduces repeated variate stress-induced bladder dysfunction by increasing bladder capacity and decreasing voiding frequency in male rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2014, 307, R471–R480. [Google Scholar] [CrossRef]
- Yeh, C.H.; Chen, B.H.; Tseng, X.W.; Liao, C.H.; Tsai, W.K.; Chiang, H.S.; Wu, Y.N. Intravesical Instillation of Norketamine, a Ketamine Metabolite, and Induced Bladder Functional Changes in Rats. Toxics 2021, 9, 154. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-H.; Praveen Rajneesh, C.; Liao, C.-H.; You, W.-C.; Chen, K.-C.; Wu, Y.-N.; Chiang, H.-S. Chlorogenic Acid Intravesical Therapy Changes Acute Voiding Behavior of Systemic Lipopolysaccharide Inflammation-Induced Cystitis Bladder in Mice. Toxics 2024, 12, 239. https://doi.org/10.3390/toxics12040239
Yeh C-H, Praveen Rajneesh C, Liao C-H, You W-C, Chen K-C, Wu Y-N, Chiang H-S. Chlorogenic Acid Intravesical Therapy Changes Acute Voiding Behavior of Systemic Lipopolysaccharide Inflammation-Induced Cystitis Bladder in Mice. Toxics. 2024; 12(4):239. https://doi.org/10.3390/toxics12040239
Chicago/Turabian StyleYeh, Chung-Hsin, Chellappan Praveen Rajneesh, Chun-Hou Liao, Wen-Chen You, Kuo-Chiang Chen, Yi-No Wu, and Han-Sun Chiang. 2024. "Chlorogenic Acid Intravesical Therapy Changes Acute Voiding Behavior of Systemic Lipopolysaccharide Inflammation-Induced Cystitis Bladder in Mice" Toxics 12, no. 4: 239. https://doi.org/10.3390/toxics12040239





