Cadmium-Induced Physiological Responses, Biosorption and Bioaccumulation in Scenedesmus obliquus
Abstract
1. Introduction
2. Materials and Methods
2.1. The Algal Cultivation
2.2. Cd Toxicity Test
2.3. Algal Growth
2.4. Photosynthesis and Respiration
2.5. Chlorophyll Fluorescence
2.6. QA− Reoxidation Kinetics
2.7. Total Protein Quantification
2.8. Reactive Oxygen Species Measurement
2.9. Determination of Antioxidant Enzyme Activities
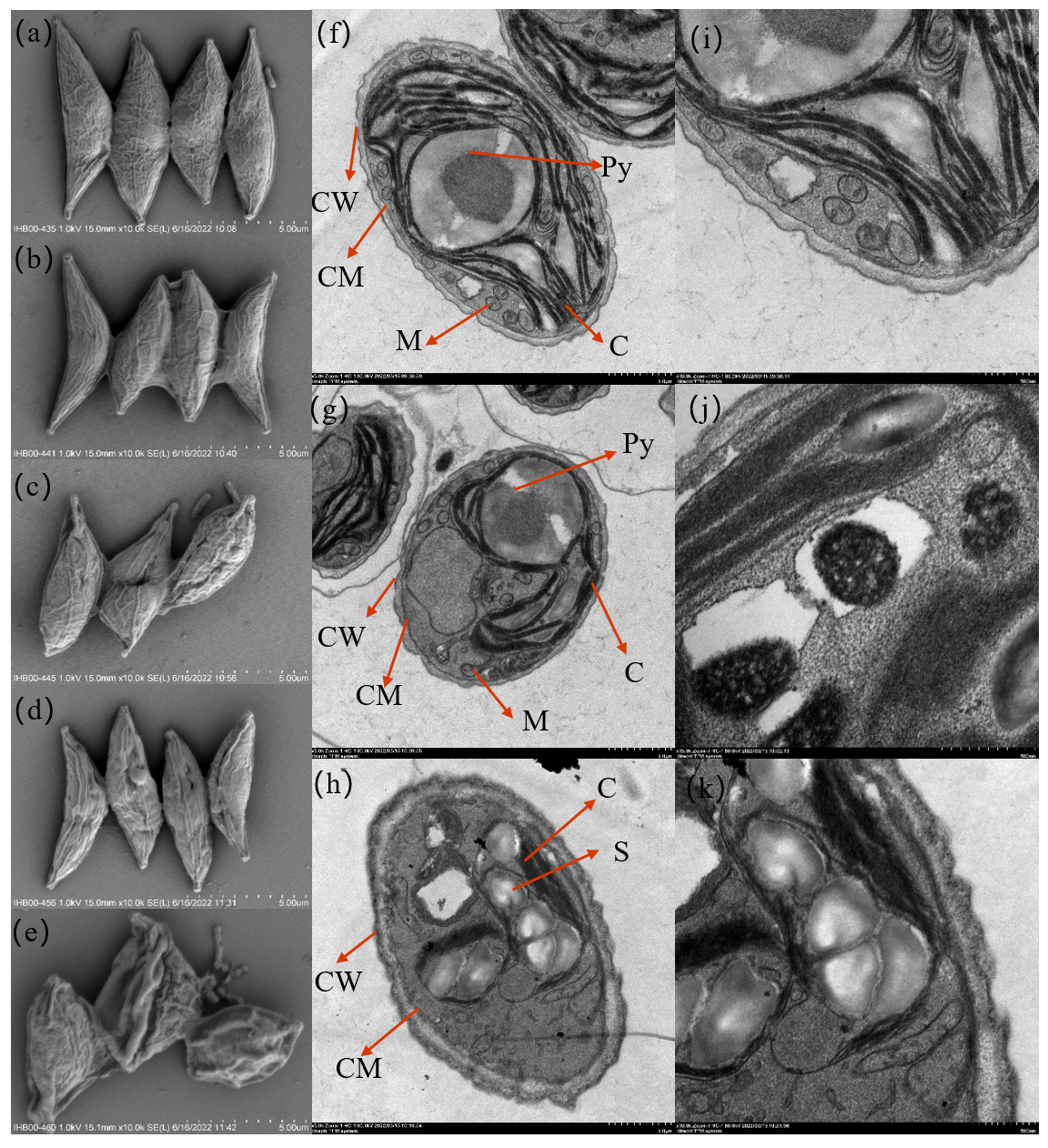
2.10. Cellular Morphology
2.11. Fourier Transform Infrared Spectroscopy
2.12. Extraction and Compositional Analysis of EPS
2.13. Cadmium Distribution
2.14. Statistical Analysis
3. Results
3.1. Cellular Morphology
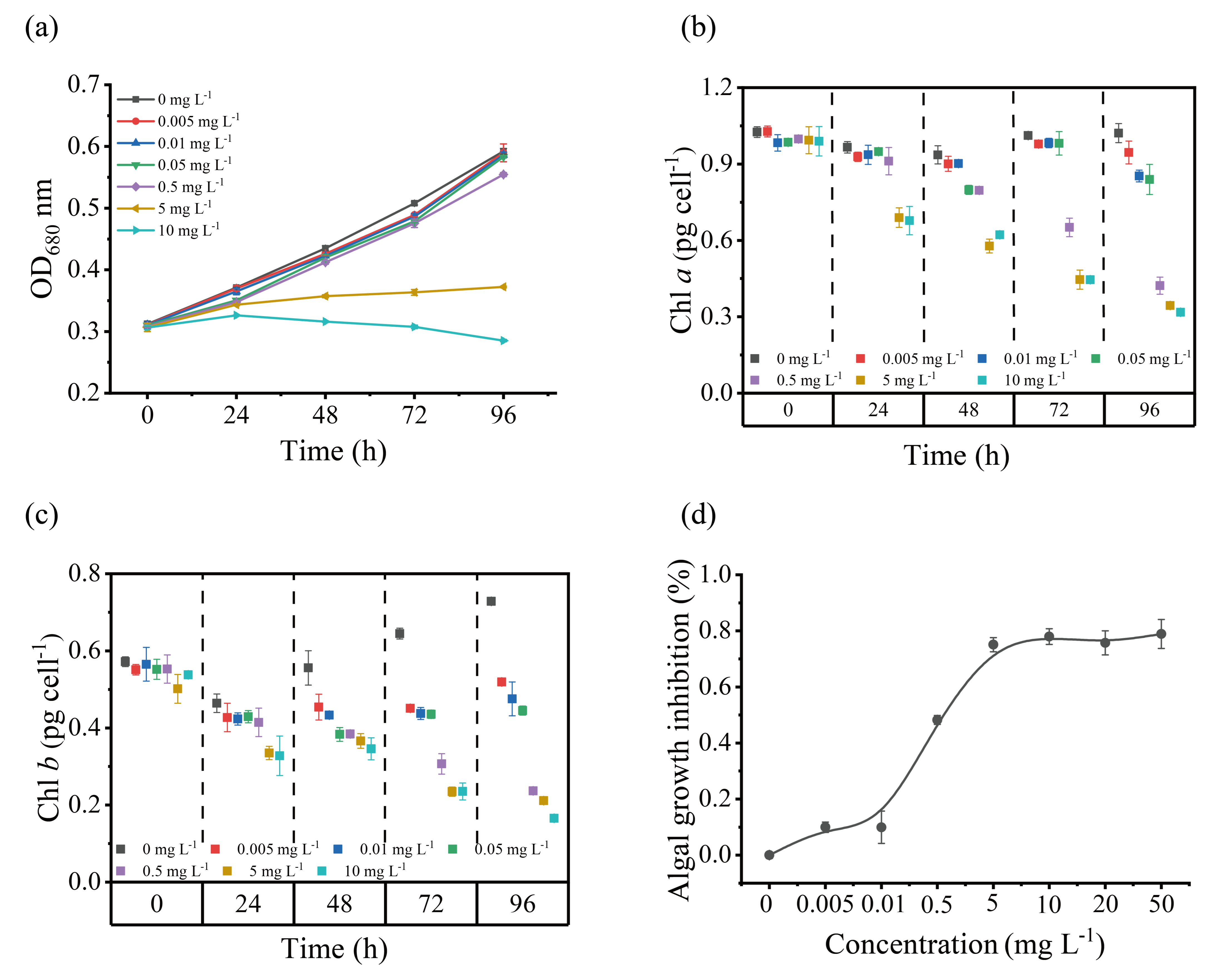
3.2. Growth and Tolerance
3.3. Photosynthesis and Respiration
3.4. Antioxidant Enzymes
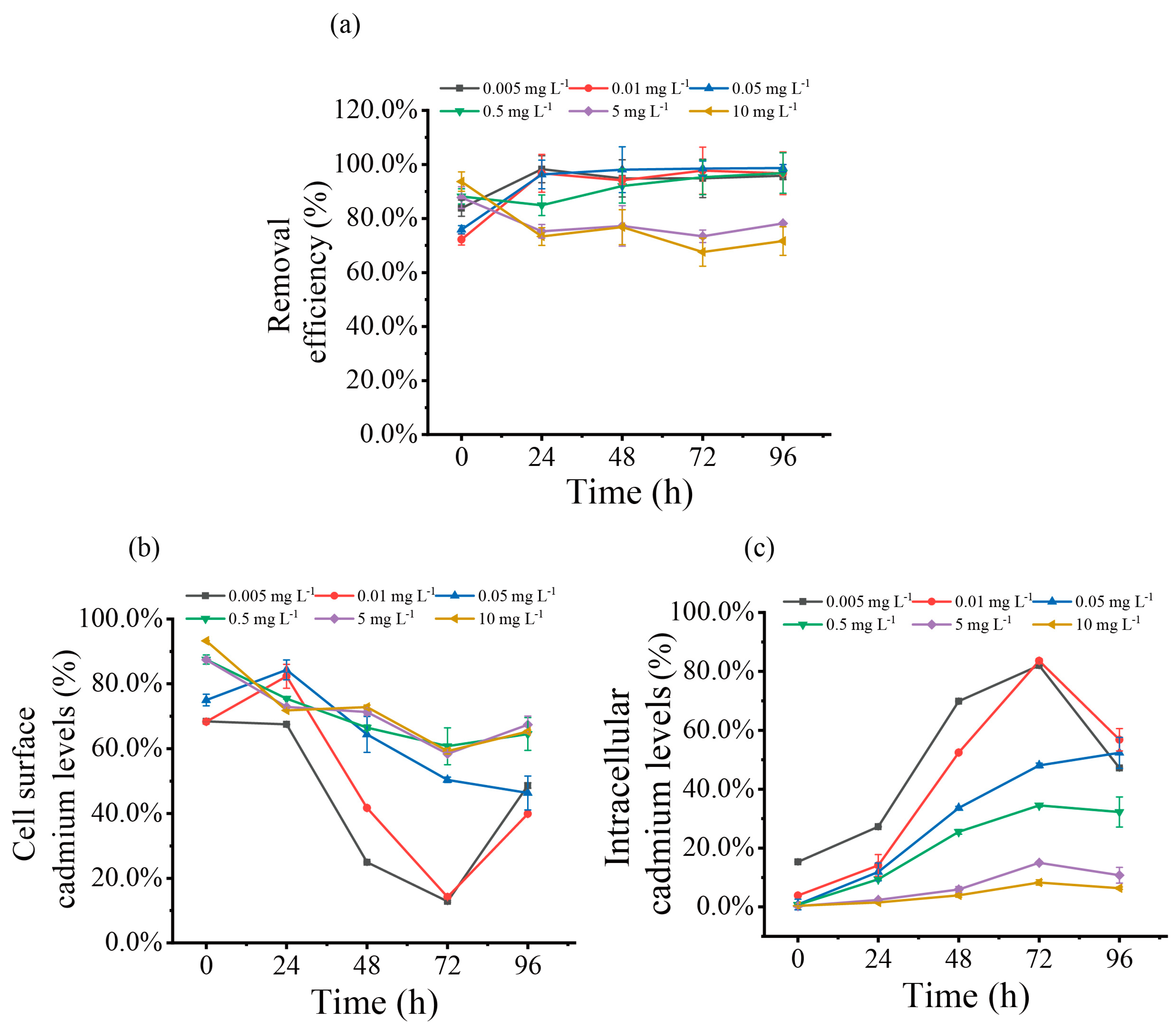
3.5. Biosorption and Accumulation
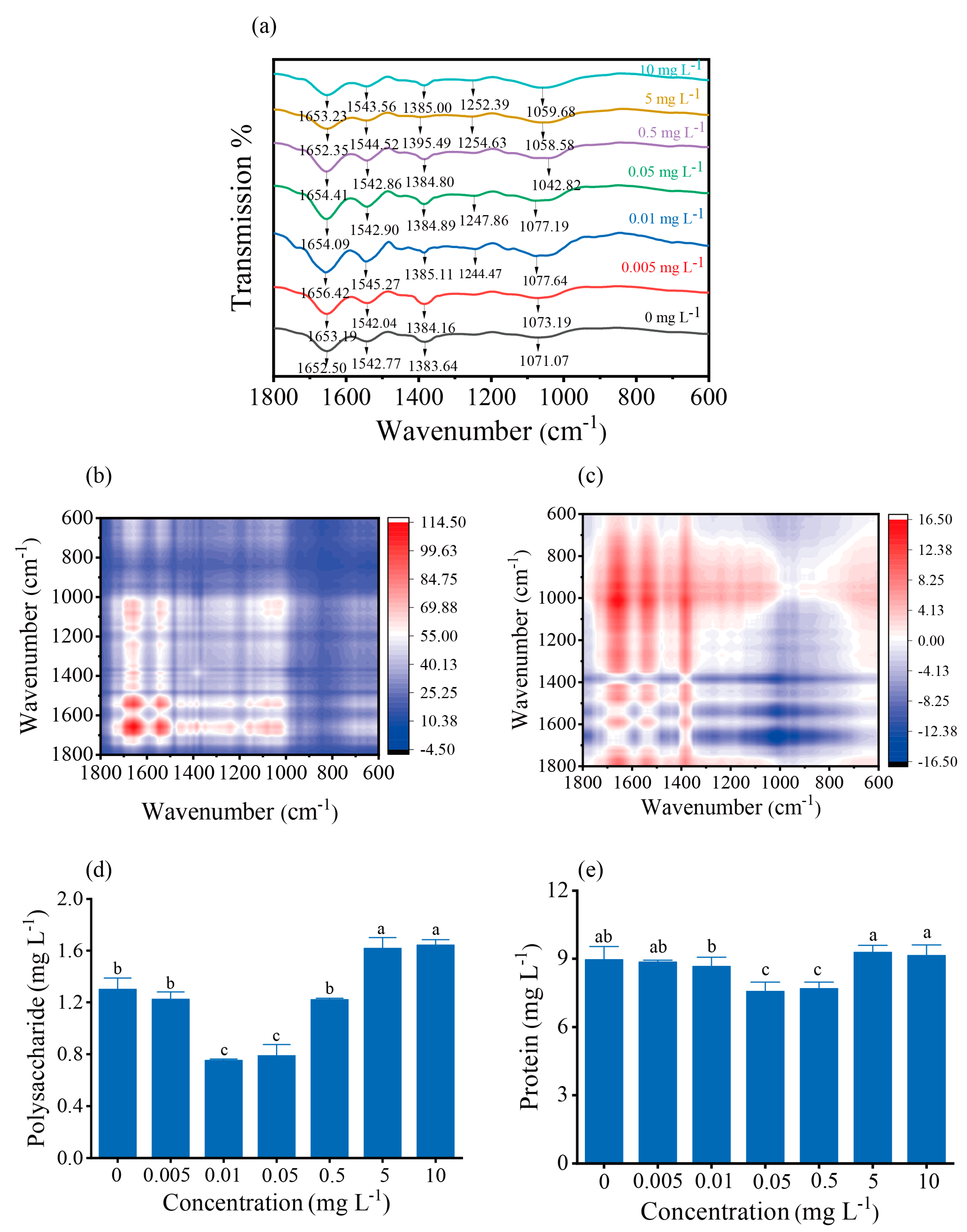
3.6. Composition of Functional Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Chen, D.; Xue, R.; Long, J.; Lin, X.; Lin, Y.; Jia, L.; Zeng, R.; Song, Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 2019, 367, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ai, H.; Cao, L.; Sui, R.; Ye, H.; Du, D.; Sun, J.; Yao, J.; Chen, K.; Chen, L. Transcriptome analysis providing novel insights for Cd-resistant tall fescue responses to Cd stress. Ecotoxicol. Environ. Saf. 2018, 160, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Wang, J.; Cui, L.; Zeng, W.; Qiu, G.; Hu, Q.; Peng, A.; Zhang, D.; Shen, L. Longitudinal physiological and transcriptomic analyses reveal the short term and long term response of Synechocystis sp. PCC6803 to cadmium stress. Chemosphere 2022, 303, 134727. [Google Scholar] [CrossRef]
- Shen, L.; Chen, R.; Wang, J.; Fan, L.; Cui, L.; Zhang, Y.; Cheng, J.; Wu, X.; Li, J.; Zeng, W. Biosorption behavior and mechanism of cadmium from aqueous solutions by Synechocystis sp. PCC6803. RSC Adv. 2021, 11, 18637–18650. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhang, M.; Bao, J.; Liu, J. Physiological, metabolomic, and transcriptomic analyses reveal the dynamic redox homeostasis upon extended exposure of Dunaliella salina GY-H13 cells to Cd. Ecotoxicol. Environ. Saf. 2021, 223, 112593. [Google Scholar] [CrossRef] [PubMed]
- Volgusheva, A.; Todorenko, D.; Baizhumanov, A.; Chivkunova, O.; Solovchenko, A.; Antal, T. Cadmium- and chromium-induced damage and acclimation mechanisms in Scenedesmus quadricauda and Chlorella sorokiniana. J. Appl. Phycol. 2022, 34, 1435–1446. [Google Scholar] [CrossRef]
- Kováčik, J.; Babula, P.; Peterková, V.; Hedbavny, J. Long-term impact of cadmium shows little damage in Scenedesmus acutiformis cultures. Algal Res. 2017, 25, 184–190. [Google Scholar] [CrossRef]
- Zhu, Q.; Guo, S.; Wen, F.; Zhang, X.; Wang, C.; Si, L.; Zheng, J.; Liu, J. Transcriptional and physiological responses of Dunaliella salina to cadmium reveals time-dependent turnover of ribosome, photosystem, and ROS-scavenging pathways. Aquat. Toxicol. 2019, 207, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, J.; Xue, M.; Wang, S.; Li, Q.; Qin, K.; Jiang, J.; Ding, J.; Zhao, Q. Adsorption behaviors of Cu2+, Zn2+ and Cd2+ onto proteins, humic acid, and polysaccharides extracted from sludge EPS: Sorption properties and mechanisms. Bioresour. Technol. 2019, 291, 121868. [Google Scholar] [CrossRef]
- Li, M.; Ma, C.; Yin, X.; Zhang, L.; Tian, X.; Chen, Q.; Wang, L. Investigating trivalent chromium biosorption-driven extracellular polymeric substances changes of Synechocystis sp. PCC 7806 by parallel factor analysis (PARAFAC) analysis. Bioresour. Technol. Rep. 2019, 7, 100249. [Google Scholar] [CrossRef]
- Lavoie, M.; Le Faucheur, S.; Fortin, C.; Campbell, P.G.C. Cadmium detoxification strategies in two phytoplankton species: Metal binding by newly synthesized thiolated peptides and metal sequestration in granules. Aquat. Toxicol. 2009, 92, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; Culaba, A.B.; Chen, W.; Chang, J. Microalgal biosorption of heavy metals: A comprehensive bibliometric review. J. Hazard. Mater. 2021, 402, 123431. [Google Scholar] [CrossRef] [PubMed]
- Mangal, V.; Nguyen, T.Q.; Fiering, Q.; Guéguen, C. An untargeted metabolomic approach for the putative characterization of metabolites from Scenedesmus obliquus in response to cadmium stress. Environ. Pollut. 2020, 266, 115123. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Xu, P.; Zhu, Y.; Mi, W.; Bi, Y. Molecular complexation properties of Cd2+ by algal organic matter from Scenedesmus obliquus. Ecotoxicol. Environ. Saf. 2023, 263, 115378. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, K.; Zhang, L.; Wang, Q.; Guo, R.; Yan, Z.; Chen, J. A novel cadmium-containing wastewater treatment method: Bio-immobilization by microalgae cell and their mechanism. J. Hazard. Mater. 2019, 374, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Chen, C.Y.; Guo, W.Q.; Chang, H.W.; Chen, W.M.; Lee, D.J.; Huang, C.C.; Ren, N.Q.; Chang, J.S. Fixed-bed biosorption of cadmium using immobilized Scenedesmus obliquus CNW-N cells on loofa (Luffa cylindrica) sponge. Bioresour. Technol. 2014, 160, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Arora, N.; Pruthi, V.; Poluri, K.M. Elucidating the bioremediation mechanism of Scenedesmus sp. IITRIND2 under cadmium stress. Chemosphere 2021, 283. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Wu, G.; Cheng, J.; Wei, J.; Huang, J.; Sun, Y.; Zhang, L.; Huang, Y.; Yang, Z. Growth and photosynthetic responses of Ochromonas gloeopara to cadmium stress and its capacity to remove cadmium. Environ. Pollut. 2021, 273, 116496. [Google Scholar] [CrossRef]
- Fan, P.; Xu, P.; Zhu, Y.; Tu, X.; Song, G.; Zuo, Y.; Bi, Y. Addition of humic acid accelerates the growth of Euglena pisciformis AEW501 and the accumulation of lipids. J. Appl. Phycol. 2022, 34, 51–63. [Google Scholar] [CrossRef]
- Li, C.; Zheng, C.; Fu, H.; Zhai, S.; Hu, F.; Naveed, S.; Zhang, C.; Ge, Y. Contrasting detoxification mechanisms of Chlamydomonas reinhardtii under Cd and Pb stress. Chemosphere 2021, 274, 129771. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A.; Hartel, H.; Hubschmann, T.; Hoffmann, P.; Shestakov, S.V.; Borner, T. Inactivation of a Synechocystis sp. strain PCC-6803 gene with homology to conserved chloroplast open reading frame-184 increases the photosystem-II-to-photosystem-I ratio. Plant Cell 1995, 7, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, F.; Zhu, X.; Zhu, J.Y.; Rong, J.F.; Zhan, J.; Chen, H.; He, C.L.; Wang, Q. The acceptor side of Photosystem II is the initial target of nitrite stress in Synechocystis sp. strain PCC 6803. Appl. Environ. Microb. 2017, 83. [Google Scholar] [CrossRef] [PubMed]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Beauchemin, R.; Gauthier, A.; Harnois, J.; Boisvert, S.; Govindachary, S.; Carpentier, R. Spermine and spermidine inhibition of photosystem II: Disassembly of the oxygen evolving complex and consequent perturbation in electron donation from TyrZ to P680+ and the quinone acceptors QA− to QB. Biochim. Biophys. Acta (BBA)-Bioenerg. 2007, 1767, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, R.; Yu, D.; Chang, P.; Guo, S.; Yang, X.; Liu, X.; Xu, C.; Hu, Y. The calcium signaling module CaM–IQM destabilizes IAA–ARF interaction to regulate callus and lateral root formation. Proc. Natl. Acad. Sci. USA 2022, 119, e2092298177. [Google Scholar] [CrossRef]
- Rogatsky, E. Pandora box of BCA assay. Investigation of the accuracy and linearity of the microplate bicinchoninic protein assay: Analytical challenges and method modifications to minimize systematic errors. Anal. Biochem. 2021, 631, 114321. [Google Scholar] [CrossRef]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. Vitr. 2013, 27, 954–963. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, N.; Lin, D.; Qu, R.; Zhou, Q.; Ge, F. The complexation with proteins in extracellular polymeric substances alleviates the toxicity of Cd (II) to Chlorella vulgaris. Environ. Pollut. 2020, 263, 114102. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for setermination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.; Chen, J.; Peng, D.; He, F. Composition and functional group characterization of extracellular polymeric substances (EPS) in activated sludge: The impacts of polymerization degree of proteinaceous substrates. Water Res. 2018, 129, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef] [PubMed]
- Terry, P.A.; Stone, W. Biosorption of cadmium and copper contaminated water by Scenedesmus abundans. Chemosphere 2002, 47, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Seiyaboh, E.; Izah, S.; Oweibi, S. Assessment of water quality from Sagbama Creek, Niger Delta, Nigeria. Biotechnol. Res. 2017, 3, 20–24. [Google Scholar]
- Malea, P.; Kevrekidis, T.; Chatzipanagiotou, K.; Mogias, A. Cadmium uptake kinetics in parts of the seagrass Cymodocea nodosa at high exposure concentrations. J. Biol. Res.-Thessal. 2018, 25, 5. [Google Scholar] [CrossRef] [PubMed]
- Plöhn, M.; Escudero-Oñate, C.; Funk, C. Biosorption of Cd(II) by Nordic microalgae: Tolerance, kinetics and equilibrium studies. Algal Res. 2021, 59, 102471. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.; Malcata, F.X. Cadmium removal by two strains of Desmodesmus pleiomorphus cells. Water Air Soil Pollut. 2010, 208, 17–27. [Google Scholar] [CrossRef]
- Magdaleno, A.; Vélez, C.G.; Wenzel, M.T.; Tell, G. Effects of cadmium, copper and zinc on growth of four isolated algae from a highly polluted Argentina River. Bull. Environ. Contam. Toxicol. 2014, 92, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Perales-Vela, H.V.; Peña-Castro, J.M.; Cañizares-Villanueva, R.O. Heavy metal detoxification in eukaryotic microalgae. Chemosphere 2006, 64, 1–10. [Google Scholar] [CrossRef]
- Bauenova, M.O.; Sadvakasova, A.K.; Mustapayeva, Z.O.; Kokociński, M.; Zayadan, B.K.; Wojciechowicz, M.K.; Balouch, H.; Akmukhanova, N.R.; Alwasel, S.; Allakhverdiev, S.I. Potential of microalgae Parachlorella kessleri Bh-2 as bioremediation agent of heavy metals cadmium and chromium. Algal Res. 2021, 59, 102463. [Google Scholar] [CrossRef]
- León-Vaz, A.; León, R.; Giráldez, I.; Vega, J.M.; Vigara, J. Impact of heavy metals in the microalga Chlorella sorokiniana and assessment of its potential use in cadmium bioremediation. Aquat. Toxicol. 2021, 239, 105941. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wufuer, R.; Duo, J.; Li, W.; Pan, X. Cadmium caused different toxicity to Photosystem I and Photosystem II of freshwater unicellular algae Chlorella pyrenoidosa (Chlorophyta). Toxics 2022, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Küpper, H.; Šetlík, I.; Spiller, M.; Küpper, F.C.; Prášil, O. Heavy metal—Induced inhibition of photosynthesis: Targets of in vivo heavy metal chlorophyll formation1. J. Phycol. 2002, 38, 429–441. [Google Scholar] [CrossRef]
- Küpper, H.; Küpper, F.C.; Spiller, M. [Heavy metal]-chlorophylls formed in vivo during heavy metal stress and degradation products formed during digestion, extraction and storage of plant material. In Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications; Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 67–77. [Google Scholar]
- Grajek, H.; Rydzyński, D.; Piotrowicz-Cieślak, A.; Herman, A.; Maciejczyk, M.; Wieczorek, Z. Cadmium ion-chlorophyll interaction—Examination of spectral properties and structure of the cadmium-chlorophyll complex and their relevance to photosynthesis inhibition. Chemosphere 2020, 261, 127434. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, M.M.S.; Said, A.A. Tolerance of Pseudochlorella pringsheimii to Cd and Pb stress: Role of antioxidants and biochemical contents in metal detoxification. Ecotoxicol. Environ. Saf. 2018, 164, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Holmgren, A.; Larsson, N.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D.; et al. Unraveling the Biological Roles of Reactive Oxygen Species. Cell Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Gao, Y.; Chang, M.; Ma, H.; Han, K.; Tao, X.; Li, Y. Colony formation by the green alga Chlorella vulgaris in response to the competitor Ceratophyllum demersum. Hydrobiologia 2018, 805, 177–187. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Ge, J.; Wang, W.; Chen, Y.; Montagnes, D. Aggregate formation and polysaccharide content of Chlorella pyrenoidosa Chick (Chlorophyta) in response to simulated nutrient stress. Bioresour. Technol. 2010, 101, 8336–8341. [Google Scholar] [CrossRef]
- Duque, D.; Montoya, C.; Botero, L.R. Cadmium (Cd) tolerance evaluation of three strains of microalgae of the genus Ankistrodesmus, Chlorella and Scenedesmus. Rev. Fac. Ing. Univ. Antioq. 2019, 88–95. [Google Scholar] [CrossRef]
- Naveed, S.; Yu, Q.; Zhang, C.; Ge, Y. Extracellular polymeric substances alter cell surface properties, toxicity, and accumulation of arsenic in Synechocystis PCC6803. Environ. Pollut. 2020, 261, 114233. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekharaiah, P.S.; Sanyal, D.; Dasgupta, S.; Banik, A. Cadmium biosorption and biomass production by two freshwater microalgae Scenedesmus acutus and Chlorella pyrenoidosa: An integrated approach. Chemosphere 2021, 269, 128755. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.G.; Lalonde, S.V.; Konhauser, K.O.; Foght, J.M. Role of extracellular polymeric substances in the surface chemical reactivity of Hymenobacter aerophilus, a psychrotolerant bacterium. Appl. Environ. Microb. 2010, 76, 102–109. [Google Scholar] [CrossRef]
- Teng, Z.; Shao, W.; Zhang, K.; Huo, Y.; Zhu, J.; Li, M. Pb biosorption by Leclercia adecarboxylata: Protective and immobilized mechanisms of extracellular polymeric substances. Chem. Eng. J. 2019, 375, 122113. [Google Scholar] [CrossRef]
- Gao, X.; Deng, R.; Lin, D. Insights into the regulation mechanisms of algal extracellular polymeric substances secretion upon the exposures to anatase and rutile TiO2 nanoparticles. Environ. Pollut. 2020, 263, 114608. [Google Scholar] [CrossRef]
- Koukal, B.; Rossé, P.; Reinhardt, A.; Ferrari, B.; Wilkinson, K.J.; Loizeau, J.; Dominik, J. Effect of Pseudokirchneriella subcapitata (Chlorophyceae) exudates on metal toxicity and colloid aggregation. Water Res. 2007, 41, 63–70. [Google Scholar] [CrossRef]


| Formulae and Terms | Illustrations |
|---|---|
| Fo | Minimal record fluorescence intensity |
| Fm | Maximal recorded fluorescence intensity |
| Approximated initial slope of the fluorescence transient | |
| Normalized total complementary area above the O-J-I-P transie (reflecting single-turnover QA reduction events) | |
| N | The number of times QA was restored from the time illumination began until Fm arrived. |
| Maximum quantum yield for primary photochemistry (at t = 0) | |
| The probability that a trapped exciton moves an electron into the electron transport chain beyond QA− (at t = 0) | |
| Quantum yield for electron transport (at t = 0) | |
| Quantum yield for dissipated energy (at t = 0) | |
| Absorption flux per RC | |
| Dissipated energy flux per RC (at t = 0) | |
| Trapped energy flux per RC (at t = 0) | |
| Electron transport flux per RC (at t = 0) | |
| Absorption flux per CS (at t = 0) | |
| Trapped energy flux per CS (at t = 0) | |
| Electron transport flux per CS (at t = 0) | |
| Dissipated energy flux per CS (at t = 0) | |
| Density of RCs (QA− reducing PSII reaction centers) | |
| Performance index on absorption basis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, P.; Tu, X.; An, Z.; Mi, W.; Wan, D.; Bi, Y.; Song, G. Cadmium-Induced Physiological Responses, Biosorption and Bioaccumulation in Scenedesmus obliquus. Toxics 2024, 12, 262. https://doi.org/10.3390/toxics12040262
Xu P, Tu X, An Z, Mi W, Wan D, Bi Y, Song G. Cadmium-Induced Physiological Responses, Biosorption and Bioaccumulation in Scenedesmus obliquus. Toxics. 2024; 12(4):262. https://doi.org/10.3390/toxics12040262
Chicago/Turabian StyleXu, Pingping, Xiaojie Tu, Zhengda An, Wujuan Mi, Dong Wan, Yonghong Bi, and Gaofei Song. 2024. "Cadmium-Induced Physiological Responses, Biosorption and Bioaccumulation in Scenedesmus obliquus" Toxics 12, no. 4: 262. https://doi.org/10.3390/toxics12040262
APA StyleXu, P., Tu, X., An, Z., Mi, W., Wan, D., Bi, Y., & Song, G. (2024). Cadmium-Induced Physiological Responses, Biosorption and Bioaccumulation in Scenedesmus obliquus. Toxics, 12(4), 262. https://doi.org/10.3390/toxics12040262








