Mechanistic Evidence for Hg Removal from Wastewater by Biologically Produced Sulfur
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of BPS
2.2. Hg Removal Efficiency
2.3. Hg Adsorption Isotherm
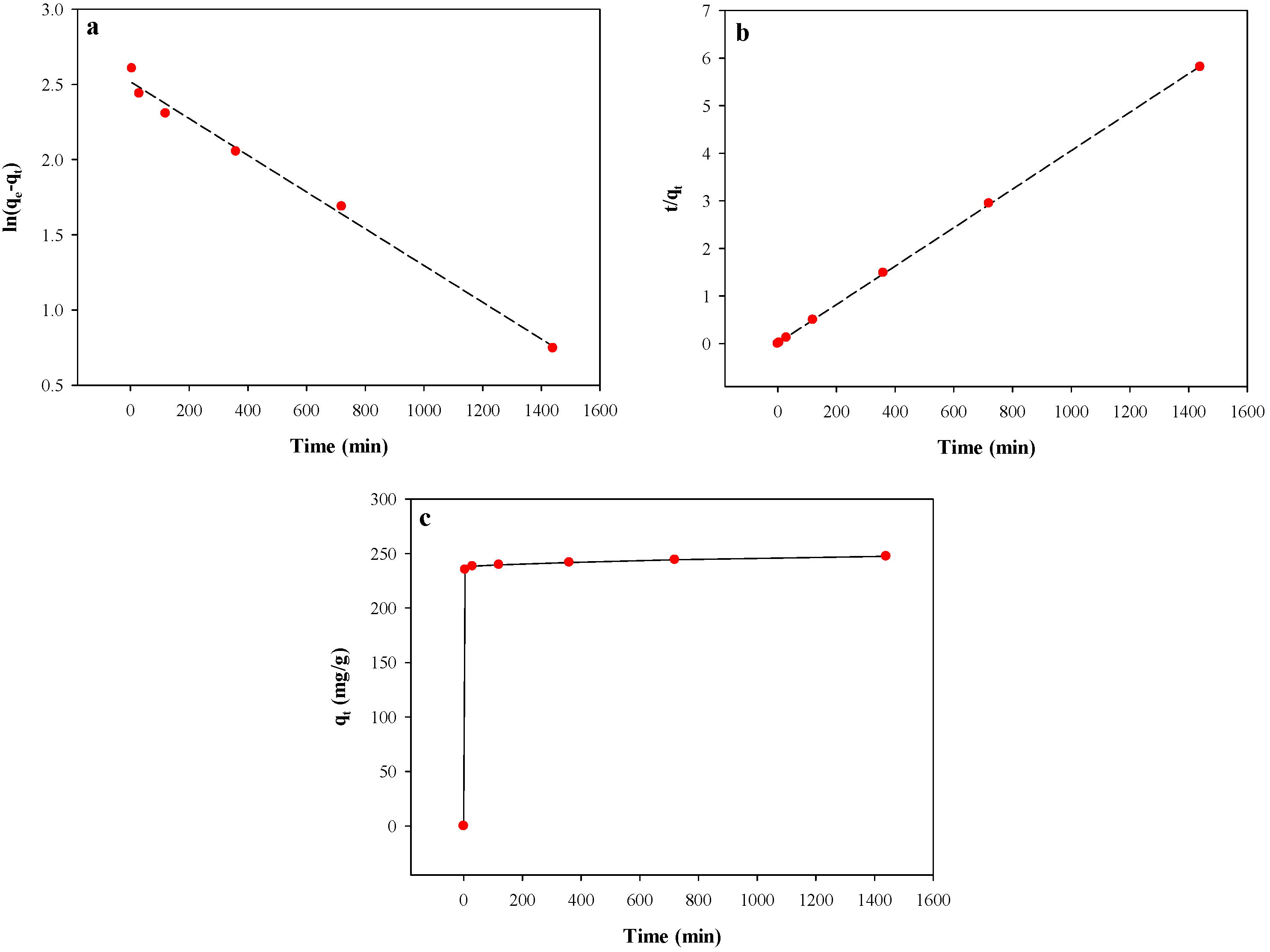
2.4. Hg Adsorption Kinetics
2.5. Pseudo-Thermodynamic Parameters of Hg Adsorption
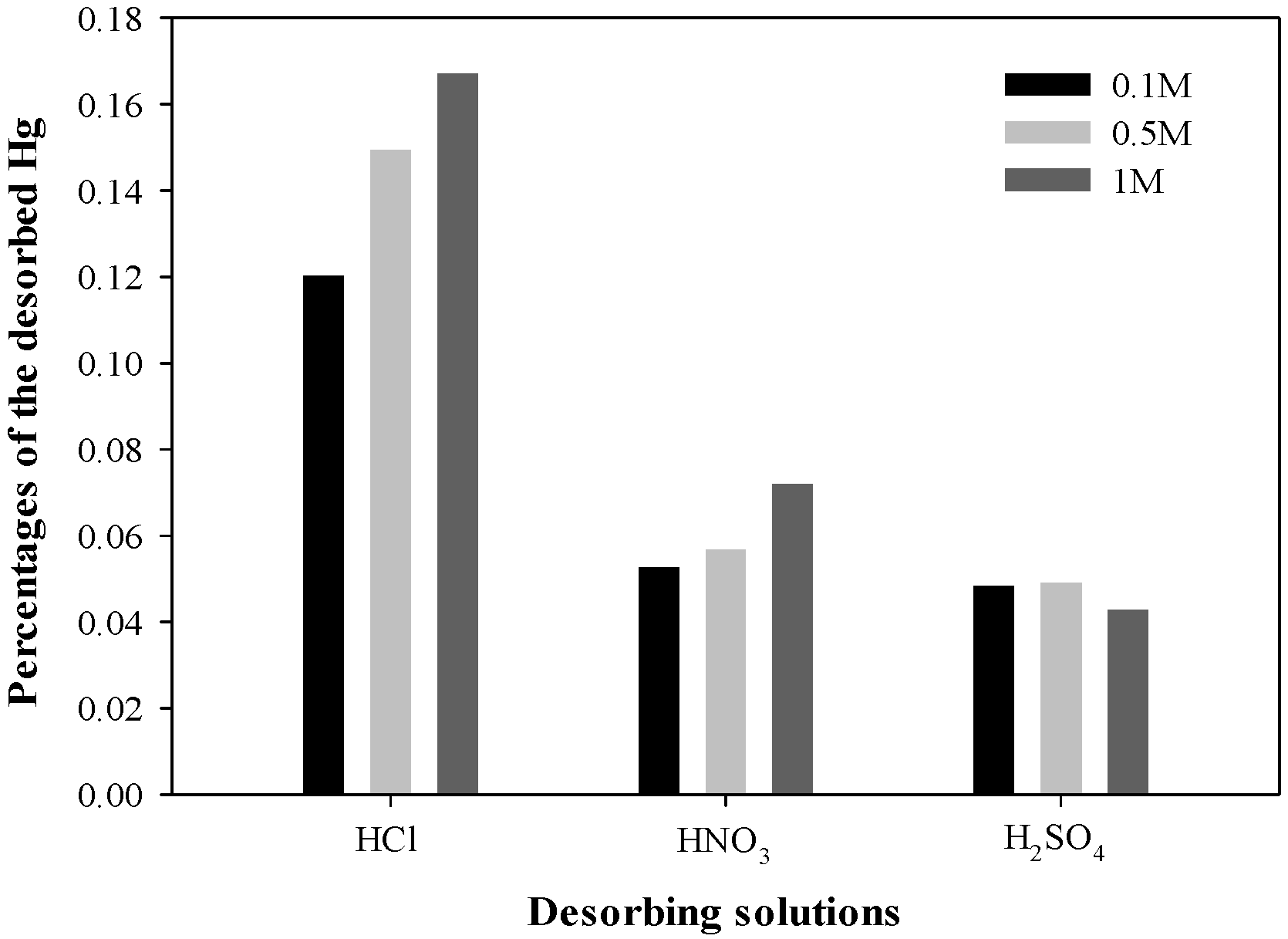
2.6. Hg Desorption
2.7. Hg Removal from Waste Water
3. Results and Discussion
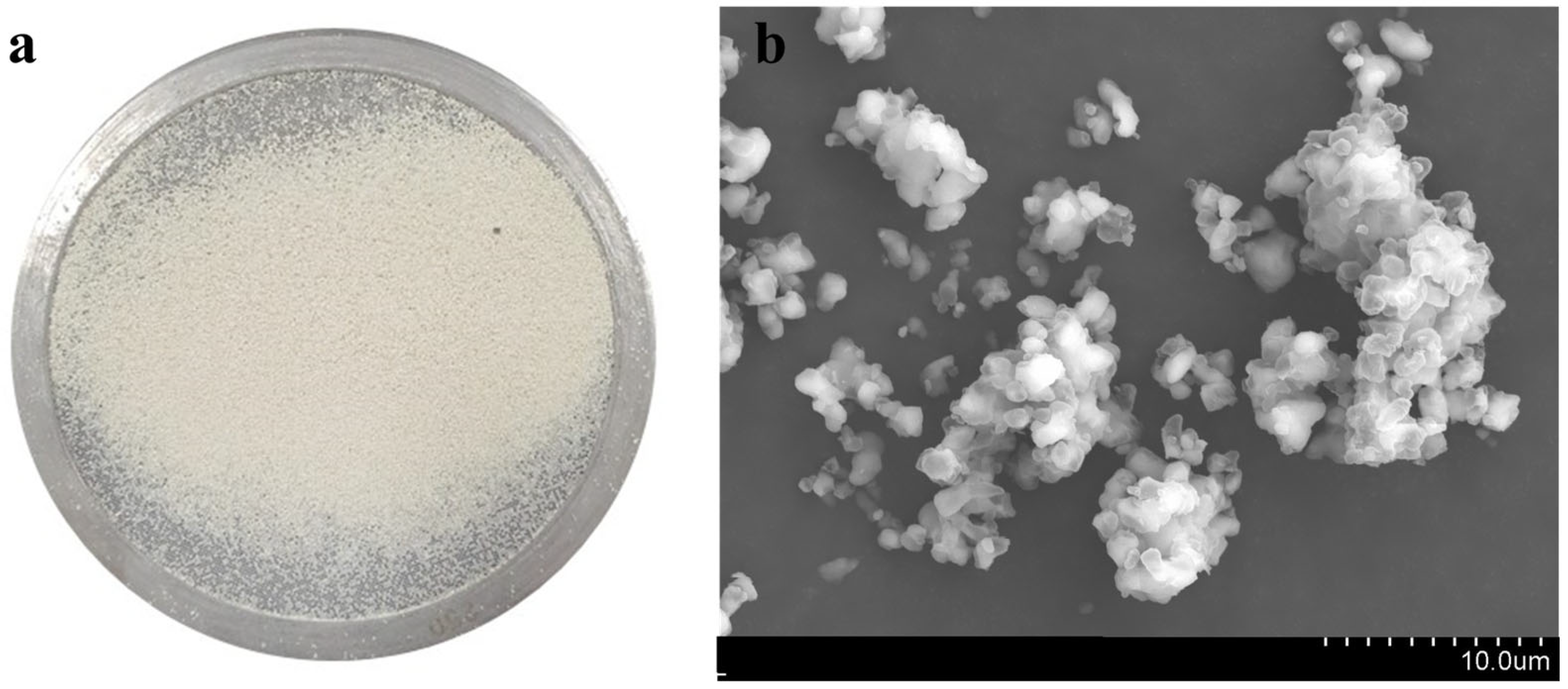
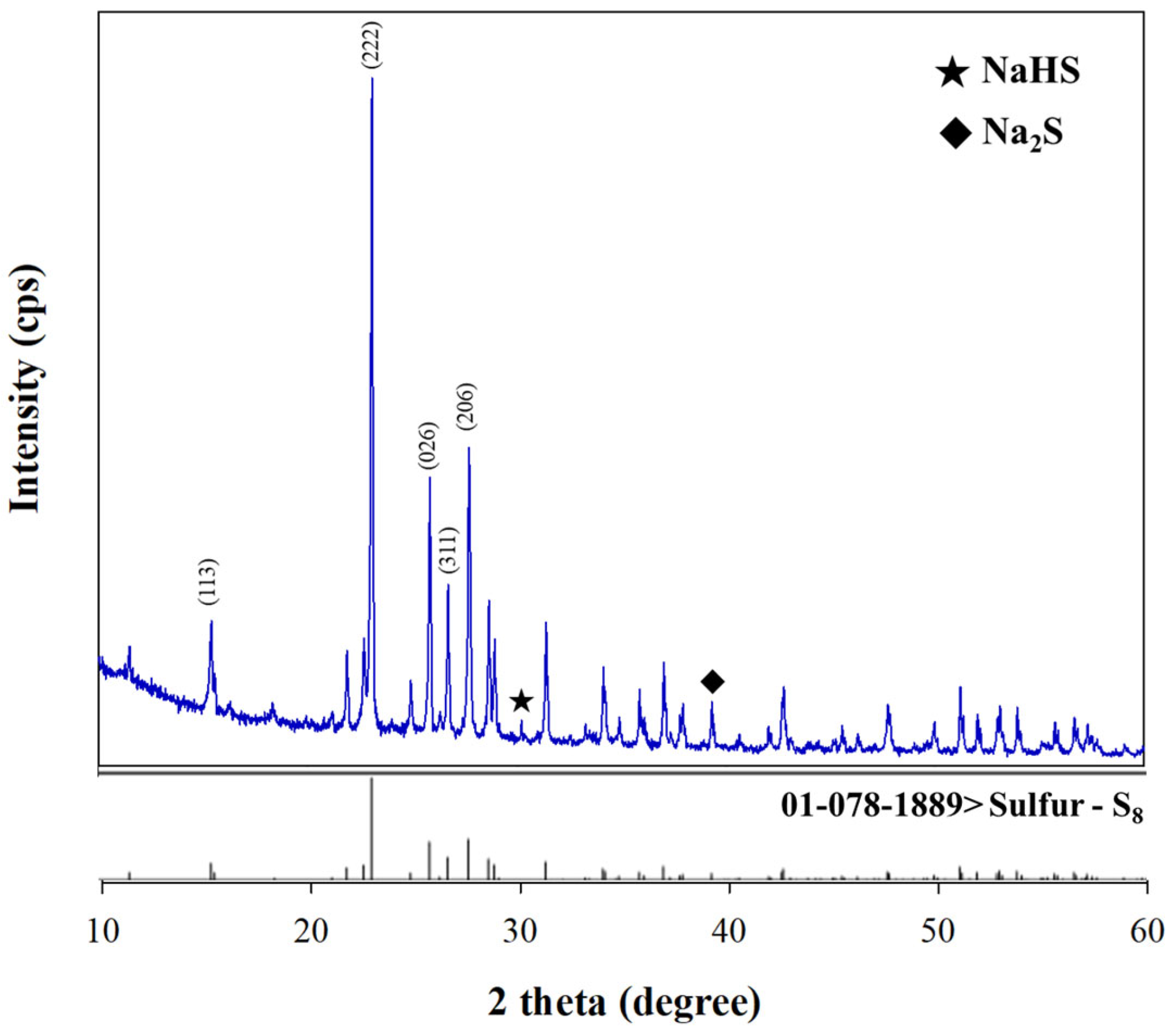
3.1. Biologically Produced S Characteristics
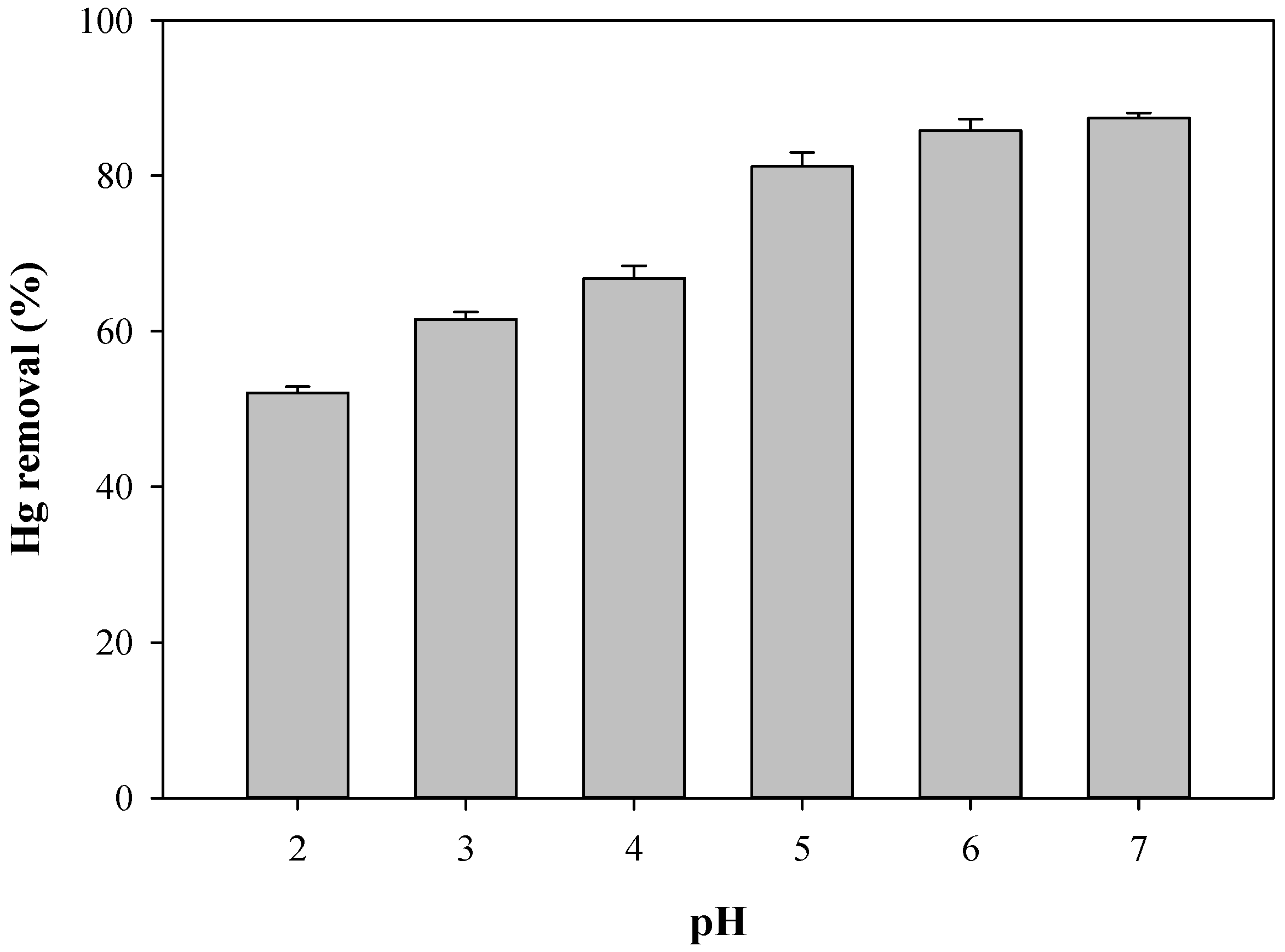
3.2. Effect of pH and Adsorbent Dose on Hg Removal
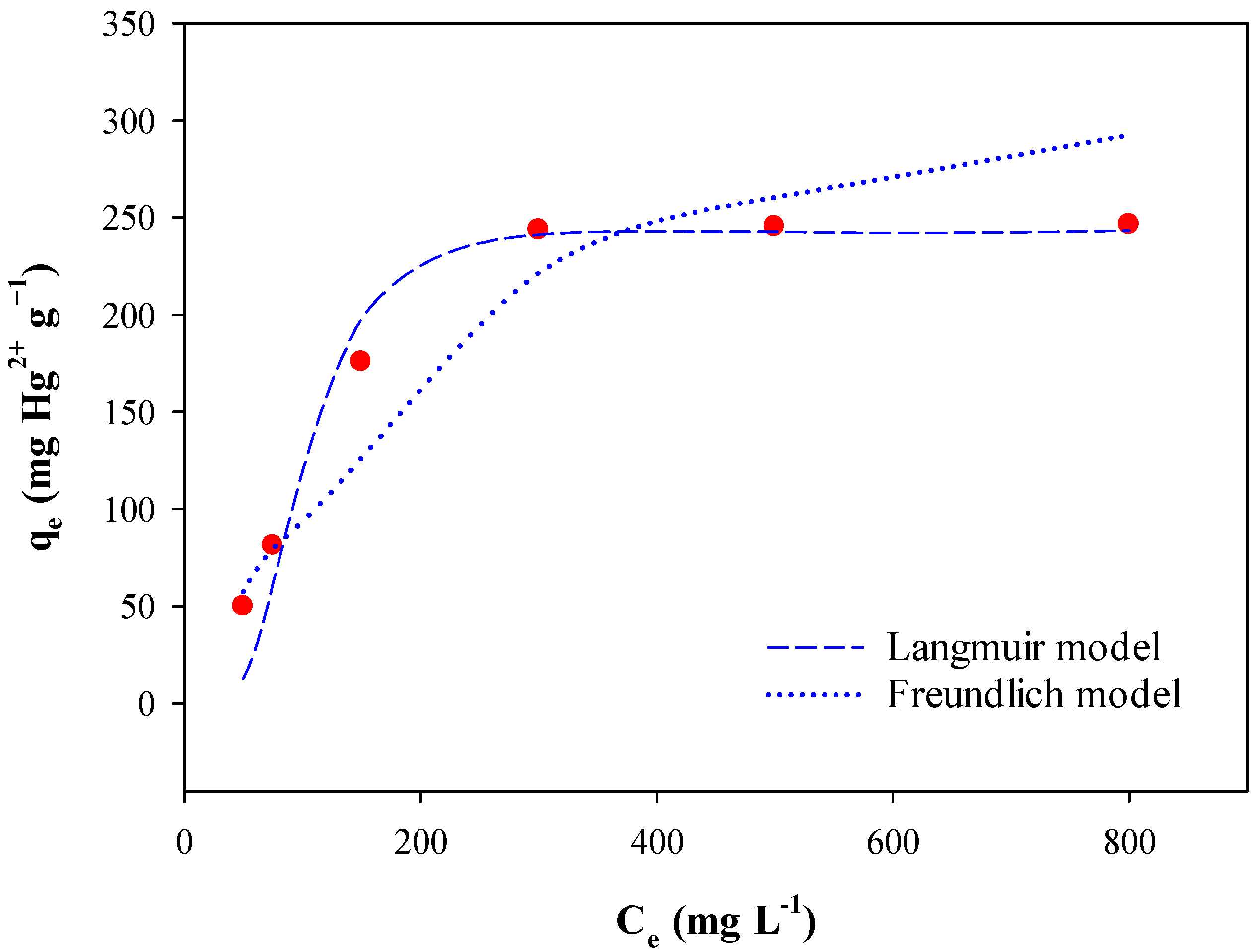
3.3. Adsorption Isotherms
3.4. Adsorption Kinetics
3.5. Adsorption Thermodynamics
3.6. Desorption of Hg from HgS Complex
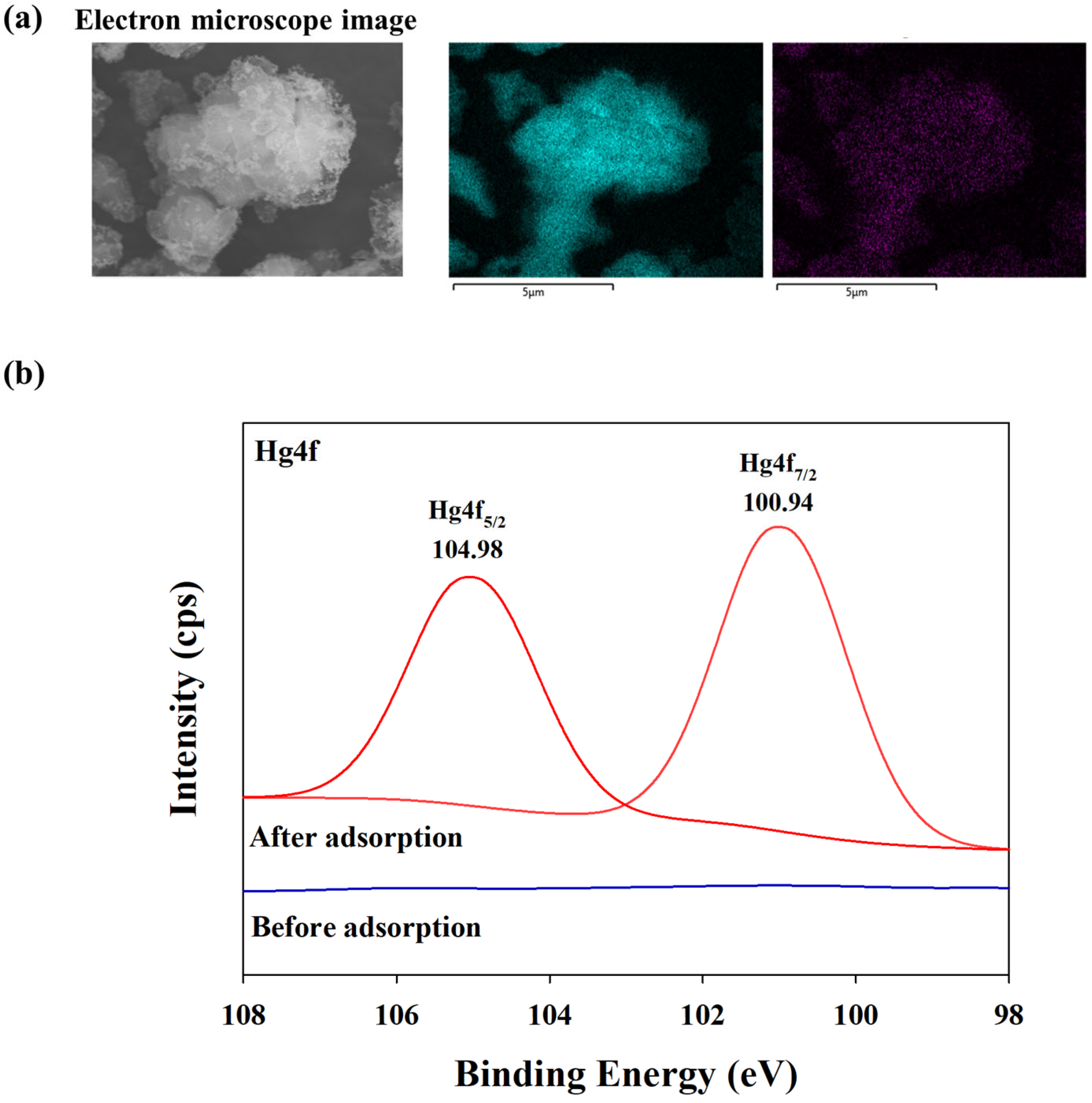
3.7. BPS Surface Morphology after Hg Adsorption
3.8. Application of BPS for Wastewater Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Mercury and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/mercury-and-health (accessed on 5 January 2024).
- Counter, S.A.; Buchanan, L.H. Mercury exposure in children: A review. Toxicol. Appl. Pharmacol. 2004, 198, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.; James, K.A.F.; Levy, L.S. Is low-level environmental mercury exposure of concern to human health? Sci. Total Environ. 2009, 408, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Paduraru, E.; Iacob, D.; Rarinca, V.; Rusu, A.; Jijie, R.; Ilie, O.D.; Ciobica, A.; Nicoara, M.; Doroftei, B. Comprehensive review regarding mercury poisoning and its complex involvement in Alzheimer’s disease. Int. J. Mol. Sci. 2022, 23, 1992. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Gill, S.S.; Gupta, G.D.; Verma, S.K. Water toxicants: A comprehension on their health concerns, detection, and remediation. Environ. Sci. Pollut. Res. 2022, 29, 53934–53953. [Google Scholar] [CrossRef] [PubMed]
- Naija, A.; Yalcin, H.C. Evaluation of cadmium and mercury on cardiovascular and neurological systems: Effects on humans and fish. Toxicol. Rep. 2023, 10, 498–508. [Google Scholar] [CrossRef]
- Albatrni, H.; Qiblawey, H.; El-Naas, M.H. Comparative study between adsorption and membrane technologies for the removal of mercury. Separ. Purif. Technol. 2021, 257, 117833. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, A.; Arya, R.K. Removal of mercury (II) from aqueous solution: A review of recent work. Separ. Sci. Technol. 2015, 50, 1310–1320. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, W.; Chang, M.; Wen, S.; Liu, D.; Han, G. Advances in depressants for flotation separation of Cu-Fe sulfide minerals at low alkalinity: A critical review. Int. J. Miner. Metall. Mater. 2024, 31, 1–17. [Google Scholar] [CrossRef]
- Feng, Q.; Zhang, G.; Zhang, Q.; Zhao, W. Synergistic activation of sulfidized hemimorphite with copper-lead species for improving surface hydrophobicity and floatability. Sep. Purif. Technol. 2024, 332, 125854. [Google Scholar] [CrossRef]
- Yu, J.G.; Yue, B.Y.; Wu, X.W.; Liu, Q.; Jiao, F.P.; Jiang, X.Y.; Chen, X.Q. Removal of mercury by adsorption: A review. Environ. Sci. Pollut. Res. 2016, 23, 5056–5076. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, Y.; Xu, X.; Qu, J.; Qu, B. Adsorption of Hg(II) in an aqueous solution by activated carbon prepared from rice husk using KOH activation. ACS Omega 2020, 5, 29231–29242. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Giri, S.; Muliwa, A.M.; Maity, A. High-performance Hg(II) removal using thiol-functionalized polypyrrole (PPy/MAA) composite and effective catalytic activity of Hg(II)-adsorbed waste material. ACS Sustain. Chem. Eng. 2017, 5, 7524–7536. [Google Scholar] [CrossRef]
- Velempini, T.; Pillay, K. Sulphur functionalized materials for Hg(II) adsorption: A review. J. Environ. Chem. Eng. 2019, 7, 103350. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, Z.; Zhang, T.; Zhang, P. Sulfide modifies physicochemical properties and mercury adsorption of microplastics. Sci. Total Environ. 2022, 848, 157802. [Google Scholar] [CrossRef]
- Huang, L.; Shen, R.; Liu, R.; Shuai, Q. Thiol-functionalized magnetic covalent organic frameworks by a cutting strategy for efficient removal of Hg2+ from water. J. Hazard. Mater. 2020, 392, 122320. [Google Scholar] [CrossRef]
- Xu, D.; Wu, W.D.; Zi, H.J.; Yang, R.X.; Deng, W.Q. Sulfur rich microporous polymer enables rapid and efficient removal of mercury(II) from water. Chemosphere 2018, 196, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Attari, M.; Bukhari, S.S.; Kazemian, H.; Rohani, S. A low-cost adsorbent from coal fly ash for mercury removal from industrial wastewater. J. Environ. Chem. Eng. 2017, 5, 391–399. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, Y.; Hussain, A. A review on coal fly ash-based adsorbents for mercury and arsenic removal. J. Clean. Prod. 2020, 267, 122143. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Cao, Y.; Ok, Y.S.; Tack, F.M.G.; Rinklebe, J.; O’Connor, D. Remediation of mercury contaminated soil, water, and air: A review of emerging materials and innovative technologies. Environ. Int. 2020, 134, 105281. [Google Scholar] [CrossRef]
- Hu, L.; Du, Y.; Long, Y. Relationship between H2S emissions and the migration of sulfur-containing compounds in landfill sites. Ecol. Eng. 2017, 106, 17–23. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeong, S.S.; Lee, J.G.; Yoon, J.H.; Lee, S.P.; Kim, K.R.; Kim, S.C.; Kirkham, M.B.; Yang, J.E. Biologically produced sulfur as a novel adsorbent to remove Cd2+ from aqueous solutions. J. Hazard. Mater. 2021, 419, 126470. [Google Scholar] [CrossRef]
- Southern Research Institute. Environmental Technology Verification Report—NATCO Group, Inc.—Paques THIOPAQ Gas Purification Technology; EPA/600/R-04/165; U.S. Environmental Protection Agency: Washington, DC, USA, 2004.
- Heo, J.; Lee, B.; Kim, S.; Kim, J.N.; Lim, H. Techno-economic analysis of a biological desulfurization process for a landfill gas in Korea. Sep. Sci. Technol. 2018, 53, 2769–2781. [Google Scholar] [CrossRef]
- Shen, Y.; Li, H.; Zhu, W.; Ho, S.H.; Yuan, W.; Chen, J.; Xie, Y. Microalgal-biochar immobilized complex: A novel efficient biosorbent for cadmium removal from aqueous solution. Bioresour. Technol. 2017, 244, 1031–1038. [Google Scholar] [CrossRef]
- Chiron, N.; Guilet, R.; Deydier, E. Adsorption of Cu(II) and Pb(II) onto a grafted silica: Isotherms and kinetic models. Water Res. 2003, 37, 3079–3086. [Google Scholar] [CrossRef]
- Georgieva, V.G.; Gonsalvesh, L.; Tavlieva, M.P. Thermodynamics and kinetics of the removal of nickel (II) ions from aqueous solutions by biochar adsorbent made from agro-waste walnut shells. J. Mol. Liq. 2020, 312, 112788. [Google Scholar] [CrossRef]
- Venkiteshwaran, K.; Wells, E.; Mayer, B.K. Kinetics, affinity, thermodynamics, and selectivity of phosphate removal using immobilized phosphate-binding proteins. Environ. Sci. Technol. 2020, 54, 10885–10894. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environment. Water Environment Conservation Act. Available online: https://www.law.go.kr/LSW//lsInfoP.do?lsiSeq=231465&urlMode=engLsInfoR&viewCls=engLsInfoR#0000 (accessed on 10 January 2024).
- Janssen, A.J.H.; Lettinga, G.; de Keizer, A. Removal of hydrogen sulphide from wastewater and waste gases by biological conversion to elemental Sulphur Colloidal and interfacial aspects of biologically produced sulphur particles. Colloids Surf. A Physicochem. Eng. Asp. 1999, 151, 389–397. [Google Scholar] [CrossRef]
- Kleinjan, W.E.; de Keizer, A.; Janssen, A.J.H. Kinetics of the reaction between dissolved sodium sulfide and biologically produced sulfur. Ind. Eng. Chem. Res. 2005, 44, 309–317. [Google Scholar] [CrossRef]
- Kuklińska, K.; Wolska, L.; Namieśnik, J.; Cieszynska, M. Analytical and bio-analytical problems associated with the toxicity of elemental sulfur in the environment. Trends Anal. Chem. 2013, 48, 14–21. [Google Scholar] [CrossRef]
- Aranzabe, E.; Villasante, P.M.; March, R.; Arriortua, M.I.; Larrañaga, A.; Aranzabe, A. More than color: Pigments with thermal storage capacity; processing and degradation behavior. Adv. Mater. Phys. Chem. 2015, 5, 171–184. [Google Scholar] [CrossRef]
- Li, X.; Morrish, R.M.; Yang, Y.; Wolden, C.A.; Yang, Y. Thermodynamically favorable conversion of hydrogen sulfide into valuable products through reaction with sodium naphthalenide. ChemPlusChem 2015, 80, 1508–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Gutha, Y.; Xu, J. Adsorption of Pb(II) ions from aqueous environment using eco-friendly chitosan schiff’s base@Fe3O4 (CSB@Fe3O4) as an adsorbent; kinetics, isotherm and thermodynamic studies. Int. J. Biol. Macromol. 2017, 105, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Zhou, A.; Zhong, L.; Zhang, Z.; Liu, Y. Selective and effective adsorption of Hg(II) from aqueous solution over wide pH range by thiol functionalized magnetic carbon nanotubes. Chemosphere 2019, 226, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Xu, J.; Lu, R.; Wo, J.; Xu, X. Enhanced Hg2+ removal and Hg0 re-emission control from wet fuel gas desulfurization liquors with additives. Fuel 2010, 89, 3613–3617. [Google Scholar] [CrossRef]
- Qu, Z.; Fang, L.; Chen, D.; Xu, H.; Yan, N. Effective and regenerable Ag/graphene adsorbent for Hg(II) removal from aqueous solution. Fuel 2017, 203, 128–134. [Google Scholar] [CrossRef]
- Caicedo Saceldo, O.D.; Varga, D.P.; Giraldo, L.; Moreno-Piraján, J.C. Study of mercury [Hg(II)] adsorption from aqueous solution on functionalized activated carbon. ACS Omega 2021, 6, 11849–11856. [Google Scholar] [CrossRef] [PubMed]
- Yardim, M.F.; Budinova, T.; Ekinci, E.; Petrove, N.; Razvigorova, M.; Minkova, V. Removal of mercury (II) from aqueous solution by activated carbon obtained from furfural. Chemosphere 2003, 52, 835–841. [Google Scholar] [CrossRef]
- Aguado, J.; Arsuaga, J.M.; Arencibia, A. Adsorption of aqueous mercury(II) on propylthiol-functionalized mesoporous silica obtained by cocondensation. Ind. Eng. Chem. Res. 2005, 44, 3665–3671. [Google Scholar] [CrossRef]
- Zabihi, M.; Asl, A.H.; Ahmadpour, A. Studies on adsorption of mercury from aqueous solution on activated carbons prepared from walnut shell. J. Hazard. Mater. 2010, 174, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Bai, R.; Liu, C. Enhanced and selective adsorption of mercury ions on chitosan beads grafted with polyacrylamide via surface-initiated atom transfer radical polymerization. Langmuir 2005, 21, 11780–11787. [Google Scholar] [CrossRef] [PubMed]
- Şahan, T.; Erol, F.; Yilmaz, Ş. Mercury(II) adsorption by a novel adsorbent mercapto-modified bentonite using ICP-OES and use of response surface methodology for optimization. Microchem. J. 2018, 138, 360–368. [Google Scholar] [CrossRef]
- Shahzad, A.; Jang, J.; Lim, S.R.; Lee, D.A. Unique selectivity and rapid uptake of molybdenum-disulfide-functionalized MXene nanocomposite for mercury adsorption. Environ. Res. 2020, 182, 109005. [Google Scholar] [CrossRef] [PubMed]
- Namasivayam, C.; Kadirvelu, K. Uptake of mercury (II) from wastewater by activated carbon from an unwated agricultural solid by-product: Coirpith. Carbon 1999, 37, 79–84. [Google Scholar] [CrossRef]
- Rao, M.M.; Reddy, D.H.K.K.; Venkateswarlu, P.; Seshaiah, K. Removal of mercury from aqueous solutions using activated carbon prepared from agricultural by-product/waste. J. Environ. Manag. 2009, 90, 634–643. [Google Scholar] [CrossRef]
- Wadi, V.S.; Mittal, H.; Fosso-Kankeu, E.; Jena, K.K.; Alhassan, S.M. Mercury removal by porous sulfur copolymers: Adsorption isotherm and kinetics studies. Colloids Surf. A 2020, 606, 125333. [Google Scholar] [CrossRef]
- Johari, K.; Saman, N.; Song, S.T.; Mat, H.; Stuckey, D.C. Utilization of coconut milk processing waste as a low-cost mercury sorbent. Ind. Eng. Chem. Res. 2013, 52, 15648–15657. [Google Scholar] [CrossRef]
- Inbaraj, B.S.; Sulochana, N. Mercury adsorption on a carbon sorbent derived from fruit shell of Terminalia catappa. J. Hazard. Mater. 2006, 133, 283–290. [Google Scholar] [CrossRef]
- Ho, Y.S.; Wang, C.C. Sorption equilibrium of mercury onto ground-up tree fern. J. Hazard. Mater. 2008, 156, 398–404. [Google Scholar] [CrossRef]
- Alvarez, N.M.M.; Pastrana, J.M.; Lagos, Y.; Lozada, J.J. Evaluation of mercury (Hg2+) adsorption capacity using exhausted coffee waste. Sustain. Chem. Pharm. 2018, 10, 60–70. [Google Scholar] [CrossRef]
- Gharabaghi, M.; Irannajad, M.; Azadmehr, A.R. Selective sulphide precipitation of heavy metals from acidic polymetallic aqueous solution by thioacetamide. Ind. Eng. Chem. Res. 2012, 51, 954–963. [Google Scholar] [CrossRef]
- Molavi, H.; Hakimian, A.; Shojaei, A.; Raeiszadeh, M. Selective dye adsorption by highly water stable metal-organic framework: Long term stability analysis in aqueous media. Appl. Surf. Sci. 2018, 445, 424–436. [Google Scholar] [CrossRef]
- Li, H.; Jin, R.; Hu, H.; Kalkhajeh, Y.K.; Zhao, Y.; Gao, Y.; Zhang, B. Adsorption of As(III), Pb(II), and Zn(II) from wastewater by sodium alginate modified materials. J. Anal. Methods Chem. 2021, 2021, 7527848. [Google Scholar] [CrossRef]
- Asiabi, H.; Yamini, Y.; Shamsayei, M.; Molaei, K.; Shamsipur, M. Functionalized layered double hydroxide with nitrogen and sulfur co-decorated carbondots for highly selective and efficient removal of soft Hg2+ and Ag+ ions. J. Hazard. Mater. 2018, 357, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Chen, T.; Liu, H.; Li, P.; Peng, S.; Yang, Y. Green preparation of nanoporous pyrrhotite by thermal treatment of pyrite as an effective Hg(II) adsorbent: Performance and mechanism. Minerals 2019, 9, 74. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Mei, J.; Hu, Q.; Yang, S. Outstanding performance of magnetically separable sulfureted MoO3/Fe−Ti spinel for gaseous Hg0 recovery from smelting flue gas: Mechanism and adsorption kinetics. Environ. Sci. Technol. 2020, 54, 7659–7668. [Google Scholar] [CrossRef]
- Song, S.; Li, Y.; Liu, Q.S.; Wang, H.; Li, P.; Shi, J.; Hu, L.; Zhang, H.; Liu, Y.; Li, K.; et al. Interaction of mercury ion (Hg2+) with blood and cytotoxicity attenuation by serum albumin binding. J. Hazard. Mater. 2021, 412, 125158. [Google Scholar] [CrossRef]
- Singh, N.; Patil, K.R.; Khanna, P.K. Nano-sized HgSe powder: Single-step preparation and characterization. Mater. Sci. Eng. B 2007, 142, 31–36. [Google Scholar] [CrossRef]
- Duan, L.; Hu, X.; Sun, D.; Liu, Y.; Guo, Q.; Zhang, T.; Zhang, B. Rapid removal of low concentrations of mercury from wastewater using coal gasification slag. Korean J. Chem. Eng. 2020, 37, 1166–1173. [Google Scholar] [CrossRef]
- Fu, W.; Wang, X.; Huang, Z. Remarkable reusability of magnetic Fe3O4-encapsulated C3N3S3 polymer/reduced graphene oxide composite: A highly effective adsorbent for Pb and Hg ions. Sci. Total Environ. 2019, 659, 895–904. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Li, C.; Meng, H.; Lu, Y.; Li, Y. A novel Sulfur-functionalized alkynyl carbon material for highly efficient removal of Hg(II) from water. Sep. Purif. Technol. 2022, 290, 120891. [Google Scholar] [CrossRef]
- Košak, A.; Lobnik, A.; Bauman, M. Adsorption of mercury (II), lead (II), cadmium (II) and zinc (II) from aqueous solutions using mercapto-modified silica particles. Int. J. Appl. Ceram. Technol. 2015, 12, 461–472. [Google Scholar] [CrossRef]

| pH | Adsorbent Dose (g L−1) | Contact Time (min) | Initial Hg2+ Concentration (mg L−1) | Temperature (K) | |
|---|---|---|---|---|---|
| Effect of pH | 2, 3, 4, 5, 6 and 7 | 1 | 1440 | 300 | 298 |
| Effect of time | 5 | 1 | 5, 30, 120, 360, 720, 1440 | 300 | 298 |
| Effect of Hg2+ concentration | 5 | 1 | 1440 | 50, 150, 300, 500, 800 | 288, 298, 308 |
| As | Cd | Cr | Cu | Pb | Zn | Hg | |
|---|---|---|---|---|---|---|---|
| Wastewater (mg L−1) | 4.2 | 118 | 0.61 | 49 | 1.45 | 22,727 | 0.13 |
| Allowable limit † (mg L−1) | 0.25 | 0.1 | 2 | 3 | 0.5 | 5 | 0.005 |
| S | O | Na | C | K | Si | P | |
|---|---|---|---|---|---|---|---|
| BPS (% by mass) | 76.1 | 15.9 | 5.55 | 2.41 | 0.02 | 0.01 | 0.01 |
| Langmuir Model † | Freundlich Model | |||||
|---|---|---|---|---|---|---|
| Qmax (mg g−1) | b (L mg−1) | r2 | Kf (mg g−1) | n | r2 | |
| BPS | 243.9 | 0.56 | 0.99 ** | 87.3 | 5.52 | 0.92 ** |
| Adsorbents | Temperature (°C) | Dose (g/L) | Concentration (mg/L) | pH | Qmax (mg/g) | r2 | References |
|---|---|---|---|---|---|---|---|
| Activated carbon (from mango seed) activated with CaCl2 or H2SO4 | Room | 3.33 | 10–150 | 5.0 | 74.45 79.11 | 0.905 0.903 | [40] |
| Activated carbon (from mango seed) activated with CaCl2 or H2SO4 and functionalized with Na2S | Room | 3.33 | 10–150 | 5.0 | 92.16 124.13 | 0.925 0.910 | [40] |
| Activated carbon (from furfural) activated with steam | Room | 0.2 | 10–40 | 5.5 | 174 | - | [41] |
| Mesoporous silica functionalized with propylthiol | 20 °C | 0.57 | 30–600 | - | 110.32–577.70 | - | [42] |
| Activated carbon (from walnut shell) activated with ZnCl2 | 29 °C | 1 | 9.7–107 | 5.0 | 100.9 151.5 | 0.998 0.999 | [43] |
| Chitosan beads grafted with polyacrylamide | Room | 0.25 | 10–200 | 4.0 | 322.6 | 0.997 | [44] |
| Bentonite modified with mercapto | 37.28 °C | 1.9 | 5–40 | 6.17 | 32.89 | 0.99 | [45] |
| Ti3C2Tx MXene functionalized with thioacetamnide and sodium molybdate | - | 1 | 50–2000 | 6.5 | 1446.26 | 0.984 | [46] |
| Activated carbon (from coir pith) | Room | 0.2 | 10–40 | 5.0 | 154 | - | [47] |
| Activated carbon (from Ceiba pentandra hulls) | 30 °C | - | 10–140 | 6.0 | 25.88 | 0.8167 | [48] |
| Activated carbon (from Phaseolus aureus hulls) | 30 °C | - | 10–140 | 7.0 | 23.66 | 0.9016 | [48] |
| Activated carbon (from Cicer arietinum waste) | 30 °C | - | 10–140 | 7.0 | 22.88 | 0.9273 | [48] |
| Zeolitized coal fly ash | Room | 10–100 | 10 | 2.5 | 0.44 | 0.96 | [19] |
| Porous sulfur copolymer | 25 °C | 0.1 | 2–10 | - | 0.37 | 0.999 | [49] |
| Desiccated coconut waste | 30 °C | 1 | 25–500 | 7.4 | 500 | 0.970 | [50] |
| Activated carbon (from fruit shell of Terminalia catappa L.) activated with H2SO4 | 32 °C | 0.05–5.0 | 30 | 5.0 | 94.43 | 0.9956 | [51] |
| Tree fern | 10–25 °C | 5 | 55–145 | - | 20.2–26.5 | - | [52] |
| Exhausted coffee waste | 33 °C | 4 | 50–110 | 7.0 | 31.75 | 0.99 | [53] |
| Kinetic Models | Parameters † | Values |
|---|---|---|
| Pseudo-first-order | qe (mg g−1) | 12.41 |
| K1 (min−1) | 0.0012 | |
| r2 | 0.99 ** | |
| RMSE | 394.73 | |
| Pseudo-second-order | qe (mg g−1) | 250.00 |
| K2 (g mg−1min−1) | 0.0013 | |
| r2 | 0.99 ** | |
| RMSE | 31.54 | |
| Double-exponential | qe (mg g−1) | 248.89 |
| D1 (g L−1) | 237.17 | |
| KD1 (min−1) | 4.9438 | |
| D2 (g L−1) | 11.72 | |
| KD2 (min−1) | 0.0015 | |
| r2 | 0.99 ** | |
| RMSE | 0.63 |
| Temperature (K) | (kJ mol−1) | (kJ mol−1) | (J mol−1 K−1) | |
|---|---|---|---|---|
| BPS | 288 | −12.6 | 61.2 | 256.7 |
| 298 | −15.7 | |||
| 308 | −17.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.-S.; Park, B.-J.; Yoon, J.-H.; Kirkham, M.B.; Yang, J.-E.; Kim, H.-S. Mechanistic Evidence for Hg Removal from Wastewater by Biologically Produced Sulfur. Toxics 2024, 12, 278. https://doi.org/10.3390/toxics12040278
Jeong S-S, Park B-J, Yoon J-H, Kirkham MB, Yang J-E, Kim H-S. Mechanistic Evidence for Hg Removal from Wastewater by Biologically Produced Sulfur. Toxics. 2024; 12(4):278. https://doi.org/10.3390/toxics12040278
Chicago/Turabian StyleJeong, Seok-Soon, Byung-Jun Park, Jung-Hwan Yoon, Mary Beth Kirkham, Jae-E. Yang, and Hyuck-Soo Kim. 2024. "Mechanistic Evidence for Hg Removal from Wastewater by Biologically Produced Sulfur" Toxics 12, no. 4: 278. https://doi.org/10.3390/toxics12040278





