Temporal Trend of Serum Perfluorooctanoic Acid and Perfluorooctane Sulfonic Acid among U.S. Adults with or without Comorbidities in NHANES 1999–2018
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Data
2.2. Serum PFOA and PFOS Measurement
2.3. Sociodemographic Characteristics
2.4. Definition of Variables
2.5. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
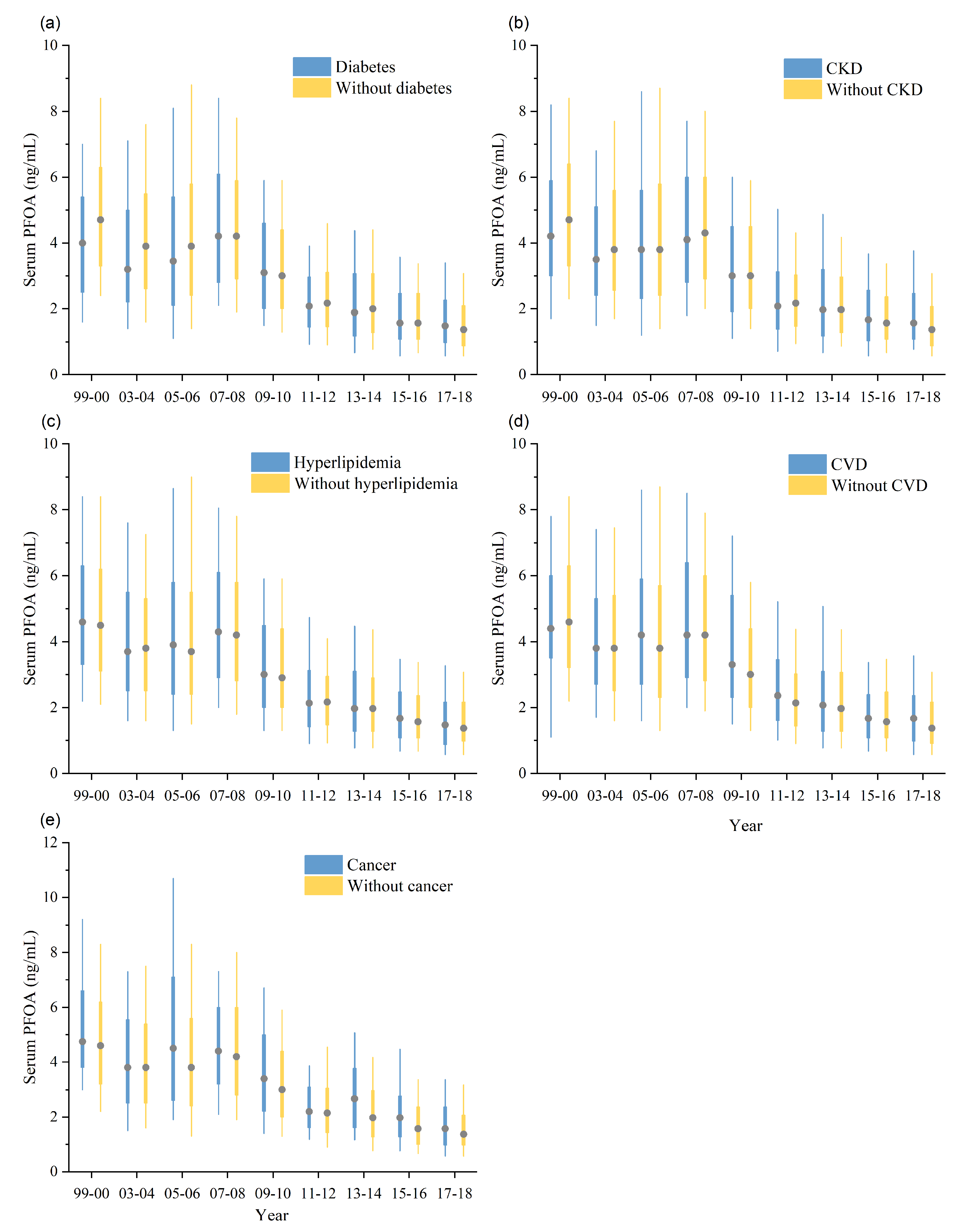
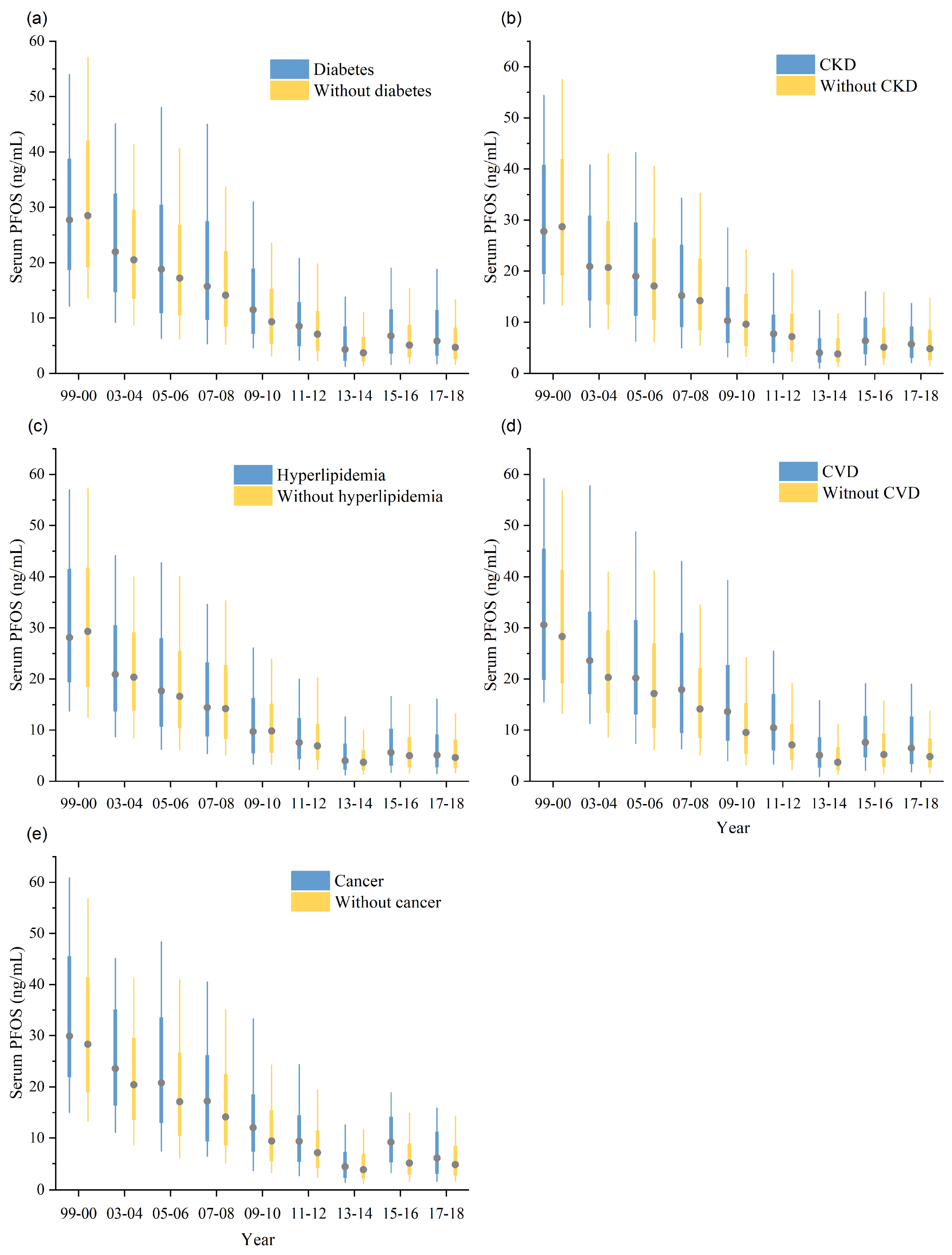
3.2. Subgroup Difference
3.3. Temporal Trend
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ATSDR. What Are PFAS? Available online: https://www.atsdr.cdc.gov/pfas/health-effects/overview.html (accessed on 6 April 2024).
- Lin, P.D.; Cardenas, A.; Hauser, R.; Gold, D.R.; Kleinman, K.P.; Hivert, M.F.; Calafat, A.M.; Webster, T.F.; Horton, E.S.; Oken, E. Temporal trends of concentrations of per- and polyfluoroalkyl substances among adults with overweight and obesity in the United States: Results from the Diabetes Prevention Program and NHANES. Environ. Int. 2021, 157, 106789. [Google Scholar] [CrossRef] [PubMed]
- Jane L Espartero, L.; Yamada, M.; Ford, J.; Owens, G.; Prow, T.; Juhasz, A. Health-related toxicity of emerging per- and polyfluoroalkyl substances: Comparison to legacy PFOS and PFOA. Environ. Res. 2022, 212, 113431. [Google Scholar] [CrossRef]
- Valvi, D.; Højlund, K.; Coull, B.A.; Nielsen, F.; Weihe, P.; Grandjean, P. Life-course Exposure to Perfluoroalkyl Substances in Relation to Markers of Glucose Homeostasis in Early Adulthood. J. Clin. Endocrinol. Metab. 2021, 106, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B.; Ducatman, A. Perfluoroalkyl acids serum concentrations and their relationship to biomarkers of renal failure: Serum and urine albumin, creatinine, and albumin creatinine ratios across the spectrum of glomerular function among US adults. Environ. Res. 2019, 174, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, A.; Gold, D.R.; Hauser, R.; Kleinman, K.P.; Hivert, M.F.; Calafat, A.M.; Ye, X.; Webster, T.F.; Horton, E.S.; Oken, E. Plasma Concentrations of Per- and Polyfluoroalkyl Substances at Baseline and Associations with Glycemic Indicators and Diabetes Incidence among High-Risk Adults in the Diabetes Prevention Program Trial. Environ. Health Perspect. 2017, 125, 107001. [Google Scholar] [CrossRef] [PubMed]
- Rosato, I.; Bonato, T.; Fletcher, T.; Batzella, E.; Canova, C. Estimation of per- and polyfluoroalkyl substances (PFAS) half-lives in human studies: A systematic review and meta-analysis. Environ. Res. 2024, 242, 117743. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Perfluoroalkyls. Available online: https://wwwn.cdc.gov/TSP/substances/ToxSubstance.aspx?toxid=237 (accessed on 6 April 2024).
- Xie, L.N.; Wang, X.C.; Su, L.Q.; Ji, S.S.; Dong, X.J.; Zhu, H.J.; Hou, S.S.; Wang, C.; Li, Z.H.; Dong, B.; et al. Serum concentrations of per-/polyfluoroalkyl substances and its association with renal function parameters among teenagers near a Chinese fluorochemical industrial plant: A cross-sectional study. Environ. Pollut. 2022, 302, 119020. [Google Scholar] [CrossRef]
- Steenland, K.; Winquist, A. PFAS and cancer, a scoping review of the epidemiologic evidence. Environ. Res. 2021, 194, 110690. [Google Scholar] [CrossRef] [PubMed]
- Ward-Caviness, C.K.; Moyer, J.; Weaver, A.; Devlin, R.; Diaz-Sanchez, D. Associations between PFAS occurrence and multimorbidity as observed in an electronic health record cohort. Environ. Epidemiol. 2022, 6, e217. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.W.; Hatch, E.E.; Webster, T.F. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ. Health Perspect. 2010, 118, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, L.; Zhou, Q.; Ding, J.; Yin, S.; Shang, X.; Tian, Y. Exposure to per- and polyfluoroalkyl substances as a risk factor for gestational diabetes mellitus through interference with glucose homeostasis. Sci. Total Environ. 2022, 838 Pt 4, 156561. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Wang, F.; Wang, R.; Zhang, S.; Zhang, Z.; Li, P.; Yao, J.; Bi, J.; He, J.; et al. Associations between serum PFOA and PFOS levels and incident chronic kidney disease risk in patients with type 2 diabetes. Ecotoxicol. Environ. Saf. 2022, 229, 113060. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. PFAS in the U.S. Population. Available online: https://www.atsdr.cdc.gov/pfas/health-effects/us-population.html (accessed on 6 April 2024).
- Sonnenberg, N.K.; Ojewole, A.E.; Ojewole, C.O.; Lucky, O.P.; Kusi, J. Trends in Serum Per- and Polyfluoroalkyl Substance (PFAS) Concentrations in Teenagers and Adults, 1999–2018 NHANES. Int. J. Environ. Res. Public Health 2023, 20, 6984. [Google Scholar] [CrossRef] [PubMed]
- van Gerwen, M.; Alpert, N.; Alsen, M.; Ziadkhanpour, K.; Taioli, E.; Genden, E. The Impact of Smoking on the Association between Perfluoroalkyl Acids (PFAS) and Thyroid Hormones: A National Health and Nutrition Examination Survey Analysis. Toxics 2020, 8, 116. [Google Scholar] [CrossRef]
- Melzer, D.; Rice, N.; Depledge, M.H.; Henley, W.E.; Galloway, T.S. Association between Serum Perfluorooctanoic Acid (PFOA) and Thyroid Disease in the US National Health and Nutrition Examination Survey. Environ. Health Perspect. 2010, 118, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Amer Diabet, A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.; Haines, S. Diabetes Mellitus. In Pharmacotherapy: A Pathophysiologic Approach, 11th ed.; DiPiro, J.T., Yee, G.C., Posey, L.M., Haines, S.T., Nolin, T.D., Ellingrod, V., Eds.; McGraw-Hill Education: New York, NY, USA, 2020. [Google Scholar]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Dharmarajan, S.H.; Bragg-Gresham, J.L.; Morgenstern, H.; Gillespie, B.W.; Li, Y.; Powe, N.R.; Tuot, D.S.; Banerjee, T.; Burrows, N.R.; Rolka, D.B.; et al. State-Level Awareness of Chronic Kidney Disease in the U.S. Am. J. Prev. Med. 2017, 53, 300–307. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Zhang, W.; Cheng, R.; Tan, A.; Shen, S.; Xiong, Y.; Zhao, L.; Lei, X. Association between blood lead levels and hyperlipidemiais: Results from the NHANES (1999–2018). Front. Public. Health 2022, 10, 981749. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, X.; Qin, Y.; Zhao, Y.; Zhang, G.; Liu, C. A cross-sectional study exploring the relationship between the dietary inflammatory index and hyperlipidemia based on the National Health and Nutrition Examination Survey (2005–2018). Lipids Health Dis. 2023, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Gori, M.; Gupta, D.K.; Claggett, B.; Selvin, E.; Folsom, A.R.; Matsushita, K.; Bello, N.A.; Cheng, S.; Shah, A.; Skali, H.; et al. Natriuretic Peptide and High-Sensitivity Troponin for Cardiovascular Risk Prediction in Diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes Care 2016, 39, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarski, K.R.; Sozio, S.M.; Chen, J.; Sang, Y.; Shafi, T. Resistant hypertension and cardiovascular disease mortality in the US: Results from the National Health and Nutrition Examination Survey (NHANES). BMC Nephrol. 2019, 20, 138. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Barrett, M.J.; Kim, H.-J.; Feuer, E.J. Estimating joinpoints in continuous time scale for multiple change-point models. Comput. Stat. Data Anal. 2007, 51, 2420–2427. [Google Scholar] [CrossRef]
- Wang, S.; Chen, J.; Li, Y.; Zhang, B.; Li, X.; Han, Y.; Zhang, J. Trends in sexually transmitted and blood-borne infections in China from 2005 to 2021: A joinpoint regression model. BMC Infect. Dis. 2023, 23, 741. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Fay, M.P.; Yu, B.; Barrett, M.J.; Feuer, E.J. Comparability of Segmented Line Regression Models. Biometrics 2004, 60, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Son, M.H.; Choi, S.D.; Lee, D.H.; Chang, Y.S. Influence of exposure to perfluoroalkyl substances (PFASs) on the Korean general population: 10-year trend and health effects. Environ. Int. 2018, 113, 149–161. [Google Scholar] [CrossRef]
- Gockener, B.; Weber, T.; Rudel, H.; Bucking, M.; Kolossa-Gehring, M. Human biomonitoring of per- and polyfluoroalkyl substances in German blood plasma samples from 1982 to 2019. Environ. Int. 2020, 145, 106123. [Google Scholar] [CrossRef] [PubMed]
- Toms, L.M.L.; Thompson, J.; Rotander, A.; Hobson, P.; Calafat, A.M.; Kato, K.; Ye, X.; Broomhall, S.; Harden, F.; Mueller, J.F. Decline in perfluorooctane sulfonate and perfluorooctanoate serum concentrations in an Australian population from 2002 to 2011. Environ. Int. 2014, 71, 74–80. [Google Scholar] [CrossRef]
- Bartolome, M.; Gallego-Pico, A.; Cutanda, F.; Huetos, O.; Esteban, M.; Perez-Gomez, B.; Castano, A.; Bioambient, E. Perfluorinated alkyl substances in Spanish adults: Geographical distribution and determinants of exposure. Sci. Total Environ. 2017, 603, 352–360. [Google Scholar] [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Jain, R.B. Time trends over 2003-2014 in the concentrations of selected perfluoroalkyl substances among US adults aged >/=20years: Interpretational issues. Sci. Total Environ. 2018, 645, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, H.; Sadia, M.; Krauss, T.; Baabish, A.; Yeung, L.W.Y. Perfluoroalkane acids in human milk under the global monitoring plan of the Stockholm Convention on Persistent Organic Pollutants (2008–2019). Front. Environ. Sci. Eng. 2022, 16, 132. [Google Scholar] [CrossRef]
- Li, Y.; Andersson, A.; Xu, Y.Y.; Pineda, D.; Nilsson, C.A.; Lindh, C.H.; Jakobsson, K.; Fletcher, T. Determinants of serum half-lives for linear and branched perfluoroalkyl substances after long-term high exposure-A study in Ronneby, Sweden. Environ. Int. 2022, 163, 107198. [Google Scholar] [CrossRef] [PubMed]
- von Ehrenstein, O.S.; Fenton, S.E.; Kato, K.; Kuklenyik, Z.; Calafat, A.M.; Hines, E.P. Polyfluoroalkyl chemicals in the serum and milk of breastfeeding women. Reprod. Toxicol. 2009, 27, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Harlow, S.D.; Batterman, S.; Mukherjee, B.; Park, S.K. Longitudinal trends in perfluoroalkyl and polyfluoroalkyl substances among multiethnic midlife women from 1999 to 2011: The Study of Women’s Health Across the Nation. Environ. Int. 2020, 135, 105381. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.W.; Hoffman, K.; Thayer, K.A.; Daniels, J.L. Polyfluoroalkyl Chemicals and Menopause among Women 20–65 Years of Age (NHANES). Environ. Health Perspect. 2014, 122, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Yu, N.Y.; Du, L.T.; Shi, W.; Yu, H.X.; Song, M.Y.; Wei, S. Transplacental Transfer of Per- and Polyfluoroalkyl Substances Identified in Paired Maternal and Cord Sera Using Suspect and Nontarget Screening. Environ. Sci. Technol. 2020, 54, 3407–3416. [Google Scholar] [CrossRef]
- Barton, K.E.; Starling, A.P.; Higgins, C.P.; McDonough, C.A.; Calafat, A.M.; Adgate, J.L. Sociodemographic and behavioral determinants of serum concentrations of per- and polyfluoroalkyl substances in a community highly exposed to aqueous film-forming foam contaminants in drinking water. Int. J. Hyg. Environ. Health 2020, 223, 256–266. [Google Scholar] [CrossRef]
- Cho, C.R.; Lam, N.H.; Cho, B.M.; Kannan, K.; Cho, H.S. Concentration and correlations of perfluoroalkyl substances in whole blood among subjects from three different geographical areas in Korea. Sci. Total Environ. 2015, 512–513, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Batzella, E.; Rosato, I.; Pitter, G.; Da Re, F.; Russo, F.; Canova, C.; Fletcher, T. Determinants of PFOA Serum Half-Life after End of Exposure: A Longitudinal Study on Highly Exposed Subjects in the Veneto Region. Environ. Health Perspect. 2024, 132, 027002. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Eliot, M.N.; Papandonatos, G.D.; Kelsey, K.T.; Fore, R.; Langevin, S.; Buckley, J.; Chen, A.; Lanphear, B.P.; Cecil, K.M.; et al. Gestational Perfluoroalkyl Substance Exposure and DNA Methylation at Birth and 12 Years of Age: A Longitudinal Epigenome-Wide Association Study. Environ. Health Perspect. 2022, 130, 37005. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jurkovic-Mlakar, S.; Li, Y.; Wahlberg, K.; Scott, K.; Pineda, D.; Lindh, C.H.; Jakobsson, K.; Engström, K. Association between serum concentrations of perfluoroalkyl substances (PFAS) and expression of serum microRNAs in a cohort highly exposed to PFAS from drinking water. Environ. Int. 2020, 136, 105446. [Google Scholar] [CrossRef]
- Stanifer, J.W.; Stapleton, H.M.; Souma, T.; Wittmer, A.; Zhao, X.; Boulware, L.E. Perfluorinated Chemicals as Emerging Environmental Threats to Kidney Health: A Scoping Review. Clin. J. Am. Soc. Nephrol. 2018, 13, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | 1999–2000 | 2003–2004 | 2005–2006 | 2007–2008 | 2009–2010 | 2011–2012 | 2013–2014 | 2015-2016 | 2017–2018 | Overall |
|---|---|---|---|---|---|---|---|---|---|---|
| 948 (%) | 1451 (%) | 1479 (%) | 1738 (%) | 1868 (%) | 1558 (%) | 1603 (%) | 1634 (%) | 1608 (%) | 13,887 (%) | |
| Sex | ||||||||||
| Male | 440 (46.4) | 707 (48.7) | 720 (48.7) | 858 (49.4) | 875 (46.8) | 789 (50.6) | 748 (46.7) | 767 (46.9) | 784 (48.8) | 6688 (48.2) |
| Female | 508 (53.6) | 744 (51.3) | 759 (51.3) | 880 (50.6) | 993 (53.2) | 769 (49.4) | 855 (53.3) | 867 (53.1) | 824 (51.2) | 7199 (51.8) |
| Race | ||||||||||
| Hispanic | 371 (39.1) | 330 (22.7) | 331 (22.4) | 500 (28.8) | 542 (29) | 310 (19.9) | 364 (22.7) | 496 (30.4) | 376 (23.4) | 3620 (26.1) |
| White | 398 (42.0) | 777 (53.5) | 774 (52.3) | 820 (47.2) | 912 (48.8) | 584 (37.5) | 701 (43.7) | 520 (31.8) | 573 (35.6) | 6059 (43.6) |
| Black | 153 (16.1) | 297 (20.5) | 324 (21.9) | 330 (19.0) | 321 (17.2) | 382 (24.5) | 310 (19.3) | 370 (22.6) | 367 (22.8) | 2854 (20.6) |
| All other races | 26 (2.7) | 47 (3.2) | 50 (3.4) | 88 (5.1) | 93 (5) | 282 (18.1) | 228 (14.2) | 248 (15.2) | 292 (18.2) | 1354 (9.8) |
| Age | ||||||||||
| 20–39 | 339 (35.8) | 488 (33.6) | 542 (36.6) | 558 (32.1) | 598 (32.0) | 572 (36.7) | 516 (32.2) | 548 (33.5) | 496 (30.8) | 4657 (33.5) |
| 40–59 | 270 (28.5) | 387 (26.7) | 467 (31.6) | 567 (32.6) | 644 (34.5) | 501 (32.2) | 532 (33.2) | 553 (33.8) | 510 (31.7) | 4431 (31.9) |
| ≥60 | 339 (35.8) | 576 (39.7) | 470 (31.8) | 613 (35.3) | 626 (33.5) | 485 (31.1) | 555 (34.6) | 533 (32.6) | 602 (37.4) | 4799 (34.6) |
| Smoking status | ||||||||||
| Never | 498 (52.5) | 741 (51.1) | 774 (52.3) | 907 (52.2) | 1024 (54.8) | 902 (57.9) | 914 (57.0) | 945 (57.8) | 939 (58.4) | 3435 (24.7) |
| Former | 258 (27.2) | 414 (28.5) | 393 (26.6) | 432 (24.9) | 457 (24.5) | 362 (23.2) | 370 (23.1) | 367 (22.5) | 382 (23.8) | 7644 (55.0) |
| Current | 192 (20.3) | 296 (20.4) | 312 (21.1) | 399 (23.0) | 387 (20.7) | 294 (18.9) | 319 (19.9) | 322 (19.7) | 287 (17.8) | 2808 (20.2) |
| DM | ||||||||||
| No | 825 (87.0) | 1237 (85.3) | 1267 (85.7) | 1413 (81.3) | 1560 (83.5) | 1263 (81.1) | 1285 (80.2) | 1308 (80.0) | 1274 (79.2) | 11,432 (82.3) |
| Yes | 123 (13.0) | 214 (14.7) | 212 (14.3) | 325 (18.7) | 308 (16.5) | 295 (18.9) | 318 (19.8) | 326 (20.0) | 334 (20.8) | 2455 (17.7) |
| CKD | ||||||||||
| No | 734 (77.4) | 1052 (72.5) | 1115 (75.4) | 1291 (74.3) | 1447 (77.5) | 1217 (78.1) | 1174 (73.2) | 1238 (75.8) | 1380 (85.8) | 10,648 (76.7) |
| Yes | 214 (22.6) | 399 (27.5) | 364 (24.6) | 447 (25.7) | 421 (22.5) | 341 (21.9) | 429 (26.8) | 396 (24.2) | 228 (14.2) | 3239 (23.3) |
| Hyperlipidemia | ||||||||||
| No | 187 (19.7) | 390 (26.9) | 429 (29.0) | 688 (39.6) | 763 (40.8) | 666 (42.7) | 658 (41.0) | 743 (45.5) | 719 (44.7) | 5243 (37.8) |
| Yes | 761 (80.3) | 1061 (73.1) | 1050 (71.0) | 1050 (60.4) | 1105 (59.2) | 892 (57.3) | 945 (59.0) | 891 (54.5) | 889 (55.3) | 8644 (62.2) |
| Any CVD | ||||||||||
| No | 881 (92.9) | 1280 (88.2) | 1338 (90.5) | 1575 (90.6) | 1721 (92.1) | 1448 (92.9) | 1457 (90.9) | 1488 (91.1) | 1454 (90.4) | 12,642 (91.0) |
| Yes | 67 (7.1) | 171 (11.8) | 141 (9.5) | 163 (9.4) | 147 (7.9) | 110 (7.1) | 146 (9.1) | 146 (8.9) | 154 (9.6) | 1245 (9.0) |
| Cancer | ||||||||||
| No | 882 (93.0) | 1307 (90.1) | 1344 (90.9) | 1573 (90.5) | 1683 (90.1) | 1432 (91.9) | 1445 (90.1) | 1481 (90.6) | 1453 (90.4) | 12,600 (90.7) |
| Yes | 66 (7.0) | 144 (9.9) | 135 (9.1) | 165 (9.5) | 185 (9.9) | 126 (8.1) | 158 (9.9) | 153 (9.4) | 155 (9.6) | 1287 (9.3) |
| Group | Sample Size | PFOA (ng/mL) | PFOS (ng/mL) | ||
| Median | p-Value | Median | p-Value | ||
| Overall | 13,887 | 2.7 | - | 9.8 | - |
| Sex | 0.000 | 0.000 | |||
| Male | 6688 | 3.1 | 12.3 | ||
| Female | 7199 | 2.3 | 7.8 | ||
| Smoking status | 0.000 | 0.000 | |||
| Never | 7644 | 2.5 | 9.1 | ||
| Former | 3435 | 2.9 | 11.9 | ||
| Now | 2808 | 2.8 | 9.3 | ||
| DM | 0.000 | 0.010 | |||
| No | 11,432 | 2.7 | 9.6 | ||
| Yes | 2455 | 2.5 | 10.4 | ||
| CKD | 0.031 | 0.000 | |||
| No | 10,648 | 2.6 | 9.5 | ||
| Yes | 3239 | 2.8 | 10.8 | ||
| Hyperlipidemia | 0.000 | 0.000 | |||
| No | 5243 | 2.4 | 8.3 | ||
| Yes | 8644 | 2.8 | 11.0 | ||
| Any CVD | 0.002 | 0.000 | |||
| No | 12,642 | 2.7 | 9.5 | ||
| Yes | 1245 | 3.0 | 13.2 | ||
| Cancer | 0.000 | 0.000 | |||
| No | 12,600 | 2.6 | 9.6 | ||
| Yes | 1287 | 2.9 | 12.0 | ||
| Group | PFOA | PFOS | ||||||
|---|---|---|---|---|---|---|---|---|
| AAPC | 95%CI | Test Statistic (t) | p-Value | AAPC | 95%CI | Test Statistic (t) | p-Value | |
| Overall | −6.7 * | (−9.0, −4.3) | −6.6 | 0.000 | −8.8 * | (−10.8, −6.7) | −7.9 | 0.000 |
| DM | ||||||||
| No | −6.4 * | (−8.7, −4.2) | −6.6 | 0.000 | −8.7 * | (−12.4, −4.9) | −4.4 | 0.000 |
| Yes | −7.3 * | (−10.4, −4.0) | −5.1 | 0.001 | −11.1 * | (−13.5, −8.5) | −9.9 | 0.000 |
| CKD | ||||||||
| No | −6.7 * | (−9.0, −4.4) | −6.6 | 0.000 | −10.6 * | (−13.0, −8.2) | −10.0 | 0.000 |
| Yes | −6.9 * | (−10.4, −3.1) | −4.3 | 0.004 | −9.6 * | (−11.2, −7.9) | −10.9 | 0.000 |
| Hyperlipidemia | ||||||||
| No | −5.0 * | (−9.4, −0.4) | −2.1 | 0.034 | −9.0 * | (−10.4, −7.5) | −11.9 | 0.000 |
| Yes | −6.3 * | (−10.3, −2.0) | −2.9 | 0.004 | −8.3 * | (−12.0, −4.4) | −4.1 | 0.000 |
| Any CVD | ||||||||
| No | −6.3 * | (−8.4, −4.2) | −5.6 | 0.000 | −8.8 * | (−12.4, −5.0) | −4.4 | 0.000 |
| Yes | −7.3 * | (−11.3, −3.1) | −3.4 | 0.001 | −9.2 * | (−11.1, −7.3) | −9.2 | 0.000 |
| Cancer | ||||||||
| No | −6.3 * | (−8.2, −4.3) | −6.1 | 0.000 | −8.7 * | (−11.1, −6.4) | −7.0 | 0.000 |
| Yes | −7.4 * | (−10.4, −4.3) | −4.6 | 0.000 | −9.8 * | (−18.2, −0.6) | −2.1 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, J.; Ouyang, C.; Zhou, S.; Wang, X.; Liu, H.; Zhang, J.; Wang, X.; Shi, X.; Yang, A.; Hu, X. Temporal Trend of Serum Perfluorooctanoic Acid and Perfluorooctane Sulfonic Acid among U.S. Adults with or without Comorbidities in NHANES 1999–2018. Toxics 2024, 12, 314. https://doi.org/10.3390/toxics12050314
Pan J, Ouyang C, Zhou S, Wang X, Liu H, Zhang J, Wang X, Shi X, Yang A, Hu X. Temporal Trend of Serum Perfluorooctanoic Acid and Perfluorooctane Sulfonic Acid among U.S. Adults with or without Comorbidities in NHANES 1999–2018. Toxics. 2024; 12(5):314. https://doi.org/10.3390/toxics12050314
Chicago/Turabian StylePan, Jinhua, Changping Ouyang, Shengze Zhou, Xuemei Wang, Heming Liu, Jia Zhang, Xiao Wang, Xiaoru Shi, Aimin Yang, and Xiaobin Hu. 2024. "Temporal Trend of Serum Perfluorooctanoic Acid and Perfluorooctane Sulfonic Acid among U.S. Adults with or without Comorbidities in NHANES 1999–2018" Toxics 12, no. 5: 314. https://doi.org/10.3390/toxics12050314
APA StylePan, J., Ouyang, C., Zhou, S., Wang, X., Liu, H., Zhang, J., Wang, X., Shi, X., Yang, A., & Hu, X. (2024). Temporal Trend of Serum Perfluorooctanoic Acid and Perfluorooctane Sulfonic Acid among U.S. Adults with or without Comorbidities in NHANES 1999–2018. Toxics, 12(5), 314. https://doi.org/10.3390/toxics12050314







