Amended Vegetation Filters as Nature-Based Solutions for the Treatment of Pharmaceuticals: Infiltration Experiments Coupled to Reactive Transport Modelling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Column Experiments
2.2. Analytical Method
2.3. Numerical Modelling
2.3.1. Governing Water Flow and Tracer Transport Formulations
2.3.2. Reactive Solute Transport Formulations
2.3.3. Model Set-Up
2.3.4. Model Calibration and Adjustments
2.4. Sensitivity Analysis
3. Results
3.1. Water Flow, Woodchip Hydraulic Parameters and Conservative Transport
3.2. Reactive Transport through the Soil Layer
3.3. Effects of Using Woodchips as Soil Amendments in Vegetation Filters on Flow and Contaminant Attenuation
3.4. Sensitivity Analysis
3.5. Qualitative Description of the Attenuation of Other Target Pharmaceuticals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD. Health at a Glance 2021: OECD Indicators; Health at a Glance; OECD Publishing: Paris, France, 2021; ISBN 9789264961012. [Google Scholar]
- Boxall, A.B.A.; Wilkinson, J.L.; Bouzas-Monroy, A. Medicating Nature: Are Human-Use Pharmaceuticals Poisoning the Environment? One Earth 2022, 5, 1080–1084. [Google Scholar] [CrossRef]
- Aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the Environment-Global Occurrences and Perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Barra Caracciolo, A.; Topp, E.; Grenni, P. Pharmaceuticals in the Environment: Biodegradation and Effects on Natural Microbial Communities. A Review. J. Pharm. Biomed. Anal. 2015, 106, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Mejías, C.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of Pharmaceuticals and Their Metabolites in Sewage Sludge and Soil: A Review on Their Distribution and Environmental Risk Assessment. Trends Environ. Anal. Chem. 2021, 30, e00125. [Google Scholar] [CrossRef]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, Sources and Fate of Pharmaceuticals and Personal Care Products in the Groundwater: A Review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef]
- Biel-Maeso, M.; Corada-Fernández, C.; Lara-Martín, P.A. Monitoring the Occurrence of Pharmaceuticals in Soils Irrigated with Reclaimed Wastewater. Environ. Pollut. 2018, 235, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Meffe, R.; de Santiago-Martín, A.; Teijón, G.; Martínez-Hernández, V.; López-Heras, I.; Nozal, L.; de Bustamante, I. Pharmaceutical and Transformation Products during Unplanned Water Reuse: Insights into Natural Attenuation, Plant Uptake and Human Health Impact under Field Conditions. Environ. Int. 2021, 157, 106835. [Google Scholar] [CrossRef]
- de Santiago-Martín, A.; Meffe, R.; Teijón, G.; Martínez-Hernández, V.; López-Heras, I.; Alonso, C.A.; Romasanta, M.A.; de Bustamante, I. Pharmaceuticals and Trace Metals in the Surface Water Used for Crop Irrigation: Risk to Health or Natural Attenuation? Sci. Total Environ. 2020, 705, 135825. [Google Scholar] [CrossRef]
- Sleight, H.; Boxall, A.B.A.; Toet, S. Uptake of Pharmaceuticals by Crops: A Systematic Review and Meta-analysis. Environ. Toxicol. Chem. 2023, 42, 2091–2104. [Google Scholar] [CrossRef]
- Carvalho, P.N.; Basto, M.C.P.; Almeida, C.M.R.; Brix, H. A Review of Plant–Pharmaceutical Interactions: From Uptake and Effects in Crop Plants to Phytoremediation in Constructed Wetlands. Environ. Sci. Pollut. Res. 2014, 21, 11729–11763. [Google Scholar] [CrossRef]
- Ebele, A.J.; Oluseyi, T.; Drage, D.S.; Harrad, S.; Abou-Elwafa Abdallah, M. Occurrence, Seasonal Variation and Human Exposure to Pharmaceuticals and Personal Care Products in Surface Water, Groundwater and Drinking Water in Lagos State, Nigeria. Emerg. Contam. 2020, 6, 124–132. [Google Scholar] [CrossRef]
- Bexfield, L.M.; Toccalino, P.L.; Belitz, K.; Foreman, W.T.; Furlong, E.T. Hormones and Pharmaceuticals in Groundwater Used As a Source of Drinking Water Across the United States. Environ. Sci. Technol. 2019, 53, 2950–2960. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.B.; Lange, A.; Nicol, E.; Bickley, L.K.; De-Bastos, E.S.R.; Jobling, S.; Tyler, C.R. Effects of Exposure to WwTW Effluents over Two Generations on Sexual Development and Breeding in Roach Rutilus Rutilus. Environ. Sci. Technol. 2015, 49, 12994–13002. [Google Scholar] [CrossRef]
- Menon, N.G.; Mohapatra, S.; Padhye, L.P.; Tatiparti, S.S.V.; Mukherji, S. Review on Occurrence and Toxicity of Pharmaceutical Contamination in Southeast Asia. In Emerging Issues in the Water Environment during Anthropocene. Springer Transactions in Civil and Environmental Engineering; Springer: Singapore, 2020; pp. 63–91. [Google Scholar]
- Niemuth, N.J.; Klaper, R.D. Emerging Wastewater Contaminant Metformin Causes Intersex and Reduced Fecundity in Fish. Chemosphere 2015, 135, 38–45. [Google Scholar] [CrossRef]
- Mezzelani, M.; Gorbi, S.; Fattorini, D.; D’Errico, G.; Consolandi, G.; Milan, M.; Bargelloni, L.; Regoli, F. Long-Term Exposure of Mytilus Galloprovincialis to Diclofenac, Ibuprofen and Ketoprofen: Insights into Bioavailability, Biomarkers and Transcriptomic Changes. Chemosphere 2018, 198, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Santos, L. Antibiotics in the Aquatic Environments: A Review of the European Scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and Antibiotic Resistant Genes (ARGs) in Groundwater: A Global Review on Dissemination, Sources, Interactions, Environmental and Human Health Risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef]
- Keerthanan, S.; Jayasinghe, C.; Biswas, J.K.; Vithanage, M. Pharmaceutical and Personal Care Products (PPCPs) in the Environment: Plant Uptake, Translocation, Bioaccumulation, and Human Health Risks. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1221–1258. [Google Scholar] [CrossRef]
- Ziylan-Yavas, A.; Santos, D.; Flores, E.M.M.; Ince, N.H. Pharmaceuticals and Personal Care Products (PPCPs): Environmental and Public Health Risks. Environ. Prog. Sustain. Energy 2022, 41, e13821. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Proto, A.; Bianco, P.M.; Montano, L.; Motta, O. Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure. Water 2021, 13, 1347. [Google Scholar] [CrossRef]
- de Aquino, S.F.; Brandt, E.M.F.; Bottrel, S.E.C.; Gomes, F.B.R.; Silva, S.d.Q. Occurrence of Pharmaceuticals and Endocrine Disrupting Compounds in Brazilian Water and the Risks They May Represent to Human Health. Int. J. Environ. Res. Public Health 2021, 18, 11765. [Google Scholar] [CrossRef] [PubMed]
- Parra-Saldivar, R.; Castillo-Zacarías, C.; Bilal, M.; Iqbal, H.M.N.; Barceló, D. Sources of Pharmaceuticals in Water. In Interaction and Fate of Pharmaceuticals in Soil-Crop Systems: The Impact of Reclaimed Wastewater; Springer: Berlin/Heidelberg, Germany, 2021; pp. 33–47. [Google Scholar]
- Pereira, A.; Silva, L.; Laranjeiro, C.; Lino, C.; Pena, A. Selected Pharmaceuticals in Different Aquatic Compartments: Part I—Source, Fate and Occurrence. Molecules 2020, 25, 1026. [Google Scholar] [CrossRef]
- Council of the European Union. Proposal for a Directive of the European Parliament and of the Council Concerning Urban Wastewater Treatment (Recast). 2022/0345(COD); Council of the European Union: Brussels, Belgium, 2024.
- Bagheri, A.; Mahvi, A.H.; Nabizadeh, R.; Dehghani, M.H.; Mahmoudi, B.; Akbari- Adergani, B.; Yaghmaeian, K. Rapid Destruction of the Non-Steroidal Anti-Inflammatory Drug Diclofenac Using Advanced Nano-Fenton Process in Aqueous Solution. Acta Medica Mediterr. 2017, 33, 879–883. [Google Scholar]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment Technologies for Emerging Contaminants in Water: A Review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Bojanowska-czajka, A.; Trojanowicz, M. Comparison of Different Advanced Degradation Processes for the Removal of the Pharmaceutical Compounds Diclofenac and Carbamazepine from Liquid Solutions. Environ. Sci. Pollut. Res. 2018, 25, 27704–27723. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.N.W.; Mohamed, M.A.; Jaafar, J.; Ismail, A.F.; Harun, Z. Hybrid Membrane Filtration-Advanced Oxidation Processes for Removal of Pharmaceutical Residue. J. Colloid. Interface Sci. 2018, 532, 236–260. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, V.; Leal, M.; Meffe, R.; de Miguel, A.; Alonso-Alonso, C.; de Bustamante, I.; Lillo, J.; Martín, I.; Salas, J.J. Removal of Emerging Organic Contaminants in a Poplar Vegetation Filter. J. Hazard. Mater. 2018, 342, 482–491. [Google Scholar] [CrossRef]
- Pradana, R.; Hernández-Martín, J.A.; Martínez-Hernández, V.; Meffe, R.; de Santiago-Martín, A.; Pérez Barbón, A.; de Bustamante, I. Attenuation Mechanisms and Key Parameters to Enhance Treatment Performance in Vegetation Filters: A Review. J. Environ. Manag. 2021, 300, 113752. [Google Scholar] [CrossRef]
- de Miguel, A.; Meffe, R.; Leal, M.; González-Naranjo, V.; Martínez-Hernández, V.; Lillo, J.; Martín, I.; Salas, J.J.; de Bustamante, I. Treating Municipal Wastewater through a Vegetation Filter with a Short-Rotation Poplar Species. Ecol. Eng. 2014, 73, 560–568. [Google Scholar] [CrossRef]
- Martínez-Hernández, V.; Meffe, R.; Kohfahl, C.; de Bustamante, I. Investigating Natural Attenuation of Pharmaceuticals through Unsaturated Column Tests. Chemosphere 2017, 177, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Meffe, R.; de Miguel, A.; Martínez-Hernández, V.; Lillo, J.; de Bustamante, I. Soil Amendment Using Poplar Woodchips to Enhance the Treatment of Wastewater-Originated Nutrients. J. Environ. Manag. 2016, 180, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, V.; Meffe, R.; Hernández-Martín, J.; Alonso González, A.; de Santiago-Martín, A.; de Bustamante, I. Sustainable Soil Amendments to Improve Nature-Based Solutions for Wastewater Treatment and Resource Recovery. J. Environ. Manag. 2020, 261, 110255. [Google Scholar] [CrossRef] [PubMed]
- Robertson, W.D.; Ptacek, C.J.; Brown, S.J. Rates of Nitrate and Perchlorate Removal in a 5-Year-Old Wood Particle Reactor Treating Agricultural Drainage. Groundw. Monit. Remediat. 2009, 29, 87–94. [Google Scholar] [CrossRef]
- Schipper, L.A.; Robertson, W.D.; Gold, A.J.; Jaynes, D.B.; Cameron, S.C. Denitrifying Bioreactors-An Approach for Reducing Nitrate Loads to Receiving Waters. Ecol. Eng. 2010, 36, 1532–1543. [Google Scholar] [CrossRef]
- Qu, J.; Xue, J.; Sun, M.; Li, K.; Wang, J.; Zhang, G.; Wang, L.; Jiang, Z.; Zhang, Y. Superefficient Non-Radical Degradation of Benzo[a]Pyrene in Soil by Fe-Biochar Composites Activating Persulfate. Chem. Eng. J. 2024, 481, 148585. [Google Scholar] [CrossRef]
- García-Santiago, X.; Garrido, J.M.; Lema, J.M.; Franco-Uría, A. Fate of Pharmaceuticals in Soil after Application of STPs Products: Influence of Physicochemical Properties and Modelling Approach. Chemosphere 2017, 182, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Kiecak, A.; Breuer, F.; Stumpp, C. Column Experiments on Sorption Coefficients and Biodegradation Rates of Selected Pharmaceuticals in Three Aquifer Sediments. Water 2020, 12, 14. [Google Scholar] [CrossRef]
- Lyu, S.; Chen, W.; Qian, J.; Wen, X.; Xu, J. Prioritizing Environmental Risks of Pharmaceuticals and Personal Care Products in Reclaimed Water on Urban Green Space in Beijing. Sci. Total Environ. 2019, 697, 133850. [Google Scholar] [CrossRef]
- Rauch-Williams, T.; Hoppe-Jones, C.; Drewes, J.E. The Role of Organic Matter in the Removal of Emerging Trace Organic Chemicals during Managed Aquifer Recharge. Water Res. 2010, 44, 449–460. [Google Scholar] [CrossRef]
- Park, J.Y.; Huwe, B. Effect of PH and Soil Structure on Transport of Sulfonamide Antibiotics in Agricultural Soils. Environ. Pollut. 2016, 213, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Archundia, D.; Duwig, C.; Spadini, L.; Morel, M.C.; Prado, B.; Perez, M.P.; Orsag, V.; Martins, J.M.F. Assessment of the Sulfamethoxazole Mobility in Natural Soils and of the Risk of Contamination of Water Resources at the Catchment Scale. Environ. Int. 2019, 130, 104905. [Google Scholar] [CrossRef] [PubMed]
- Guillemoto, Q.; Picot-Colbeaux, G.; Valdes, D.; Devau, N.; Mathurin, F.A.; Pettenati, M.; Kloppmann, W.; Mouchel, J.M. Transfer of Trace Organic Compounds in an Operational Soil-Aquifer Treatment System Assessed through an Intrinsic Tracer Test and Transport Modelling. Sci. Total Environ. 2022, 836, 155643. [Google Scholar] [CrossRef] [PubMed]
- Valhondo, C.; Duporté, G.; Cabaret, G.; Rosain, D.; Gomez, E.; Luquot, L. Assessing the Feasibility of Sustainable Materials to Boost the Sorption of Pharmaceutical Active Compounds When Included in Reactive Barriers in Soil Aquifer Treatment for Water Reuse. Water 2023, 15, 1393. [Google Scholar] [CrossRef]
- Huidobro-López, B.; León, C.; López-Heras, I.; Martínez-Hernández, V.; Nozal, L.; Crego, A.L.; de Bustamante, I. Untargeted Metabolomic Analysis to Explore the Impact of Soil Amendments in a Non-Conventional Wastewater Treatment. Sci. Total Environ. 2023, 870, 161890. [Google Scholar] [CrossRef] [PubMed]
- Vithanage, M.; Rajapaksha, A.U.; Tang, X.; Thiele-Bruhn, S.; Kim, K.H.; Lee, S.E.; Ok, Y.S. Sorption and Transport of Sulfamethazine in Agricultural Soils Amended with Invasive-Plant-Derived Biochar. J. Environ. Manag. 2014, 141, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Keerthanan, S.; Gunawardane, C.; Somasundaram, T.; Jayampathi, T.; Jayasinghe, C.; Vithanage, M. Immobilization and Retention of Caffeine in Soil Amended with Ulva Reticulata Biochar. J. Environ. Manag. 2021, 281, 111852. [Google Scholar] [CrossRef] [PubMed]
- Pan, M. Biochar Adsorption of Antibiotics and Its Implications to Remediation of Contaminated Soil. Water Air Soil. Pollut. 2020, 231, 221. [Google Scholar] [CrossRef]
- Šimůnek, J.; Šejna, A.M.; Saito, H.; Sakai, M.; Van Genuchten, M.T. The HYDRUS-1D Software Package for Simulating the Movement of Water, Heat, and Multiple Solutes in Variably Saturated Media, Version 4.17. In HYDRUS Software Series 3; Department of Environmental Sciences, University of California Riverside: Riverside, CA, USA, 2013; p. 343. Available online: https://www.pc-progress.com/Downloads/Pgm_hydrus1D/HYDRUS1D-4.17.pdf (accessed on 25 April 2024).
- Rassam, D.; Simunek, J.; Mallants, D.; Van Genuchten, M.T. The HYDRUS-1D Software Package for Simulating the One-Dimensional Movement of Water, Heat, and Multiple Solutes in Variably-Saturated Media: Tutorial; CSIRO Land and Water: Australia, 2018; ISBN 8096780808. Available online: https://www.pc-progress.com/Downloads/Public_Lib_H1D/HYDRUS-1D_Tutorial_V1.00_2018.pdf (accessed on 25 April 2024).
- Šimůnek, J.; Van Genuchten, M.T. Modeling Nonequilibrium Flow and Transport Processes Using HYDRUS. Vadose Zone J. 2008, 7, 782–797. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Postma, D. (Eds.) Geochemistry, Groundwater and Pollution; CRC Press: Rotterdam, The Netherlands, 2005; ISBN 9780429152320. [Google Scholar]
- Millington, R.J.; Quirk, J.P. Permeability of Porous Solids. Trans. Faraday Soc. 1961, 57, 1200. [Google Scholar] [CrossRef]
- Johnson, R.L.; Palmer, C.D.; Fish, W. Subsurface Chemical Processes. In Transport and Fate of Contaminants in the Subsurface. Report EPA/625/4-89/019; EPA: Washington, DC, USA, 1989; pp. 41–56. [Google Scholar]
- Mualem, Y. A New Model for Predicting the Hydraulic Conductivity of Unsaturated Porous Media. Water Resour. Res. 1976, 12, 513–522. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A Closed-Form Equation for Predicting the Hydraulic Conductivity of Unsaturated Soils. Soil. Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Schaap, M.G.; Leij, F.J.; Van Genuchten, M.T. Rosetta: A Computer Program for Estimating Soil Hydraulic Parameters with Hierarchical Pedotransfer Functions. J. Hydrol. 2001, 251, 163–176. [Google Scholar] [CrossRef]
- Meffe, R.; Kohfahl, C.; Hamann, E.; Greskowiak, J.; Massmann, G.; Dünnbier, U.; Pekdeger, A. Fate of Para-Toluenesulfonamide (p-TSA) in Groundwater under Anoxic Conditions: Modelling Results from a Field Site in Berlin (Germany). Environ. Sci. Pollut. Res. 2014, 21, 568–583. [Google Scholar] [CrossRef] [PubMed]
- Subroy, V.; Giménez, D.; Qin, M.; Krogmann, U.; Strom, P.F.; Miskewitz, R.J. Hydraulic Properties of Coarsely and Finely Ground Woodchips. J. Hydrol. 2014, 517, 201–212. [Google Scholar] [CrossRef]
- Fetter, C.W. Applied Hydrogeology, 4th ed.; Merrill Publishing Company: Florham Park, NJ, USA, 2001. [Google Scholar]
- Van Driel, P.W.; Robertson, W.D.; Merkley, L.C. Denitrification of Agricultural Drainage Using Wood-Based Reactors. Trans. ASABE 2006, 49, 565–573. [Google Scholar] [CrossRef]
- Vanderborght, J.; Vereecken, H. Review of Dispersivities for Transport Modeling in Soils. Vadose Zone J. 2007, 6, 29–52. [Google Scholar] [CrossRef]
- Freeze, R.A.; Cherry, J.A. Groundwater; Prentice-Hall: Englewoods Cliffs, NJ, USA, 1979. [Google Scholar]
- Lynn, T.J.; Ergas, S.J.; Nachabe, M.H. Effect of Hydrodynamic Dispersion in Denitrifying Wood-Chip Stormwater Biofilters. J. Sustain. Water Built Environ. 2016, 2, 04016004. [Google Scholar] [CrossRef]
- Jaynes, D.B.; Moorman, T.B.; Parkin, T.B.; Kaspar, T.C. Simulating Woodchip Bioreactor Performance Using a Dual-Porosity Model. J. Environ. Qual. 2016, 45, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Nordström, A.; Herbert, R.B. Denitrification in a Low-Temperature Bioreactor System at Two Different Hydraulic Residence Times: Laboratory Column Studies. Environ. Technol. 2017, 38, 1362–1375. [Google Scholar] [CrossRef]
- Halaburka, B.J.; Lefevre, G.H.; Luthy, R.G. Evaluation of Mechanistic Models for Nitrate Removal in Woodchip Bioreactors. Environ. Sci. Technol. 2017, 51, 5156–5164. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.A.; Cooke, R.A.; Eheart, J.W.; Cho, J. Estimation of Flow and Transport Parameters for Woodchip-Based Bioreactors: II. Field-Scale Bioreactor. Biosyst. Eng. 2010, 105, 95–102. [Google Scholar] [CrossRef]
- Chun, J.A.; Cooke, R.A.; Eheart, J.W.; Kang, M.S. Estimation of Flow and Transport Parameters for Woodchip-Based Bioreactors: I. Laboratory-Scale Bioreactor. Biosyst. Eng. 2009, 104, 384–395. [Google Scholar] [CrossRef]
- Amato, M.T.; Giménez, D.; Kannepalli, S.; Strom, P.F.; Krogmann, U.; Miskewitz, R.J. Forecasting Leachate Generation from Pilot Woodchip Stockpiles Using a Three-Dimensional Transient Flow Model. J. Environ. Manag. 2020, 262, 110379. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, D.E.; Simunek, J. Soil Physics with HYDRUS; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781315275666. [Google Scholar]
- Flury, M.; Gimmi, T.F. 6.2 Solute Diffusion. In Methods of Soil Analysis: Part 4 Physical Methods; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; pp. 1323–1351. [Google Scholar]
- Li, Y.-H.; Gregory, S. Diffusion of Ions in Sea Water and in Deep-Sea Sediments. Geochim. Cosmochim. Acta 1974, 38, 703–714. [Google Scholar] [CrossRef]
- Xu, J.; Chen, W.; Wu, L.; Chang, A.C. Adsorption and Degradation of Ketoprofen in Soils. J. Environ. Qual. 2009, 38, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Breuer, F. Investigation of Sorption and Degradation Parameters of Selected Emerging Organic Contaminants under Different Hydrological Conditions. Master’s Thesis, University of Freiburg, Freiburg, Germany, 2016. [Google Scholar]
- Styszko, K. Sorption of Emerging Organic Micropollutants onto Fine Sediments in a Water Supply Dam Reservoir, Poland. J. Soils Sediments 2016, 16, 677–686. [Google Scholar] [CrossRef]
- Ilhan, Z.E.; Ong, S.K.; Moorman, T.B. Herbicide and Antibiotic Removal by Woodchip Denitrification Filters: Sorption Processes. Water Air Soil. Pollut. 2012, 223, 2651–2662. [Google Scholar] [CrossRef]
- Tseng, Y.J.; Lai, W.W.P.; Tung, H.H.; Lin, A.Y.C. Pharmaceutical and Anticorrosive Substance Removal by Woodchip Column Reactor: Removal Process and Effects of Operational Parameters. Environ. Sci. Process. Impacts 2020, 22, 187–196. [Google Scholar] [CrossRef]
- Marín-Benito, J.M.; Herrero-Hernández, E.; Rodríguez-Cruz, M.S.; Arienzo, M.; Sánchez-Martín, M.J. Study of Processes Influencing Bioavailability of Pesticides in Wood-Soil Systems: Effect of Different Factors. Ecotoxicol. Environ. Saf. 2017, 139, 454–462. [Google Scholar] [CrossRef]
- Mackay, A.A.; Gschwend, P.M. Sorption of Monoaromatic Hydrocarbons to Wood. Environ. Sci. Technol. 2000, 34, 839–845. [Google Scholar] [CrossRef]
- Rodríguez-Cruz, M.S.; Valderrábano, M.; del Hoyo, C.; Sánchez-Martín, M.J. Physicochemical Study of the Sorption of Pesticides by Wood Components. J. Environ. Qual. 2009, 38, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Price, G.W.; Jamieson, R.; Burton, D.; Khosravi, K. Sorption and Desorption of Selected Non-Steroidal Anti-Inflammatory Drugs in an Agricultural Loam-Textured Soil. Chemosphere 2017, 174, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.B.; Weiss, S.; Reemtsma, T. Pathways and Metabolites of Microbial Degradation of Selected Acidic Pharmaceutical and Their Occurrence in Municipal Wastewater Treated by a Membrane Bioreactor. Water Res. 2005, 39, 2654–2664. [Google Scholar] [CrossRef] [PubMed]
- Domaradzka, D.; Guzik, U.; Wojcieszyńska, D. Biodegradation and Biotransformation of Polycyclic Non-Steroidal Anti-Inflammatory Drugs. Rev. Environ. Sci. Biotechnol. 2015, 14, 229–239. [Google Scholar] [CrossRef]
- Nazhakaiti, P.; Tsutsui, H.; Urase, T. Aerobic and Anaerobic Biological Degradation of Pharmaceutically Active Compounds in Rice Paddy Soils. Appl. Sci. 2019, 9, 2505. [Google Scholar] [CrossRef]
- Liang, C.; Lan, Z.; Zhang, X.; Liu, Y. Mechanism for the Primary Transformation of Acetaminophen in a Soil/Water System. Water Res. 2016, 98, 215–224. [Google Scholar] [CrossRef]
- Lin, A.Y.C.; Lin, C.A.; Tung, H.H.; Chary, N.S. Potential for Biodegradation and Sorption of Acetaminophen, Caffeine, Propranolol and Acebutolol in Lab-Scale Aqueous Environments. J. Hazard. Mater. 2010, 183, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, V.; Meffe, R.; Herrera López, S.; de Bustamante, I. The Role of Sorption and Biodegradation in the Removal of Acetaminophen, Carbamazepine, Caffeine, Naproxen and Sulfamethoxazole during Soil Contact: A Kinetics Study. Sci. Total Environ. 2016, 559, 232–241. [Google Scholar] [CrossRef]
- Phong Vo, H.N.; Le, G.K.; Hong Nguyen, T.M.; Bui, X.T.; Nguyen, K.H.; Rene, E.R.; Vo, T.D.H.; Thanh Cao, N.D.; Mohan, R. Acetaminophen Micropollutant: Historical and Current Occurrences, Toxicity, Removal Strategies and Transformation Pathways in Different Environments. Chemosphere 2019, 236, 124391. [Google Scholar] [CrossRef]
- Koroša, A.; Brenčič, M.; Mali, N. Sorption and Degradation of Pharmaceuticals in the Unsaturated Zone. In Proceedings of the EGU General Assembly 2020, Online, 4–8 May 2020. EGU2020-22431. [Google Scholar] [CrossRef]
- Maeng, S.K.; Sharma, S.K.; Abel, C.D.T.; Magic-Knezev, A.; Amy, G.L. Role of Biodegradation in the Removal of Pharmaceutically Active Compounds with Different Bulk Organic Matter Characteristics through Managed Aquifer Recharge: Batch and Column Studies. Water Res. 2011, 45, 4722–4736. [Google Scholar] [CrossRef] [PubMed]
- Fram, M.S.; Belitz, K. Occurrence and Concentrations of Pharmaceutical Compounds in Groundwater Used for Public Drinking-Water Supply in California. Sci. Total Environ. 2011, 409, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, E.; Woessner, W.W.; Benotti, M.J. Pharmaceuticals in On-Site Sewage Effluent and Ground Water, Western Montana. Groundwater 2007, 45, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Koroša, A.; Auersperger, P.; Mali, N. Determination of Micro-Organic Contaminants in Groundwater (Maribor, Slovenia). Sci. Total Environ. 2016, 571, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C. Occurrence, Sources, and Fate of Pharmaceuticals in Aquatic Environment and Soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Banzhaf, S.; Nödler, K.; Licha, T.; Krein, A.; Scheytt, T. Redox-Sensitivity and Mobility of Selected Pharmaceutical Compounds in a Low Flow Column Experiment. Sci. Total Environ. 2012, 438, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Scheytt, T.; Mersmann, P.; Lindstädt, R.; Heberer, T. Determination of Sorption Coefficients of Pharmaceutically Active Substances Carbamazepine, Diclofenac, and Ibuprofen, in Sandy Sediments. Chemosphere 2005, 60, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Arye, G.; Dror, I.; Berkowitz, B. Fate and Transport of Carbamazepine in Soil Aquifer Treatment (SAT) Infiltration Basin Soils. Chemosphere 2011, 82, 244–252. [Google Scholar] [CrossRef]
- Koba, O.; Golovko, O.; Kodešová, R.; Klement, A.; Grabic, R. Transformation of Atenolol, Metoprolol, and Carbamazepine in Soils: The Identification, Quantification, and Stability of the Transformation Products and Further Implications for the Environment. Environ. Pollut. 2016, 218, 574–585. [Google Scholar] [CrossRef]
- Li, J.; Dodgen, L.; Ye, Q.; Gan, J. Degradation Kinetics and Metabolites of Carbamazepine in Soil. Environ. Sci. Technol. 2013, 47, 3678–3684. [Google Scholar] [CrossRef]
- Kodešová, R.; Grabic, R.; Kočárek, M.; Klement, A.; Golovko, O.; Fér, M.; Nikodem, A.; Jakšík, O. Pharmaceuticals’ Sorptions Relative to Properties of Thirteen Different Soils. Sci. Total Environ. 2015, 511, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, V.; Meffe, R.; Herrera, S.; Arranz, E.; de Bustamante, I. Sorption/Desorption of Non-Hydrophobic and Ionisable Pharmaceutical and Personal Care Products from Reclaimed Water onto/from a Natural Sediment. Sci. Total Environ. 2014, 472, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Vulava, V.M.; Cory, W.C.; Murphey, V.L.; Ulmer, C.Z. Sorption, Photodegradation, and Chemical Transformation of Naproxen and Ibuprofen in Soils and Water. Sci. Total Environ. 2016, 565, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Price, G.W.; Jamieson, R.; Lake, C. Biodegradation Kinetics of Individual and Mixture Non-Steroidal Anti-Inflammatory Drugs in an Agricultural Soil Receiving Alkaline Treated Biosolids. Sci. Total Environ. 2021, 755, 142520. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Gan, J. Sorption and Degradation of Wastewater-Associated Non-Steroidal Anti-Inflammatory Drugs and Antibiotics in Soils. Chemosphere 2011, 83, 240–246. [Google Scholar] [CrossRef]
- Schmidt, C.K.; Lange, F.T.; Brauch, H.-J. Assessing the Impact of Different Redox Conditions and Residence Times on the Fate of Organic Micropollutants during Riverbank Filtration. In Proceedings of the 4th International Conference on Pharmaceuticals and Endocrine Disrupting Chemicals in Water, Minneapolis, MN, USA, 13 October 2004. [Google Scholar]
- Barbieri, M.; Carrera, J.; Ayora, C.; Sanchez-Vila, X.; Licha, T.; Nödler, K.; Osorio, V.; Pérez, S.; Köck-Schulmeyer, M.; López de Alda, M.; et al. Formation of Diclofenac and Sulfamethoxazole Reversible Transformation Products in Aquifer Material under Denitrifying Conditions: Batch Experiments. Sci. Total Environ. 2012, 426, 256–263. [Google Scholar] [CrossRef]
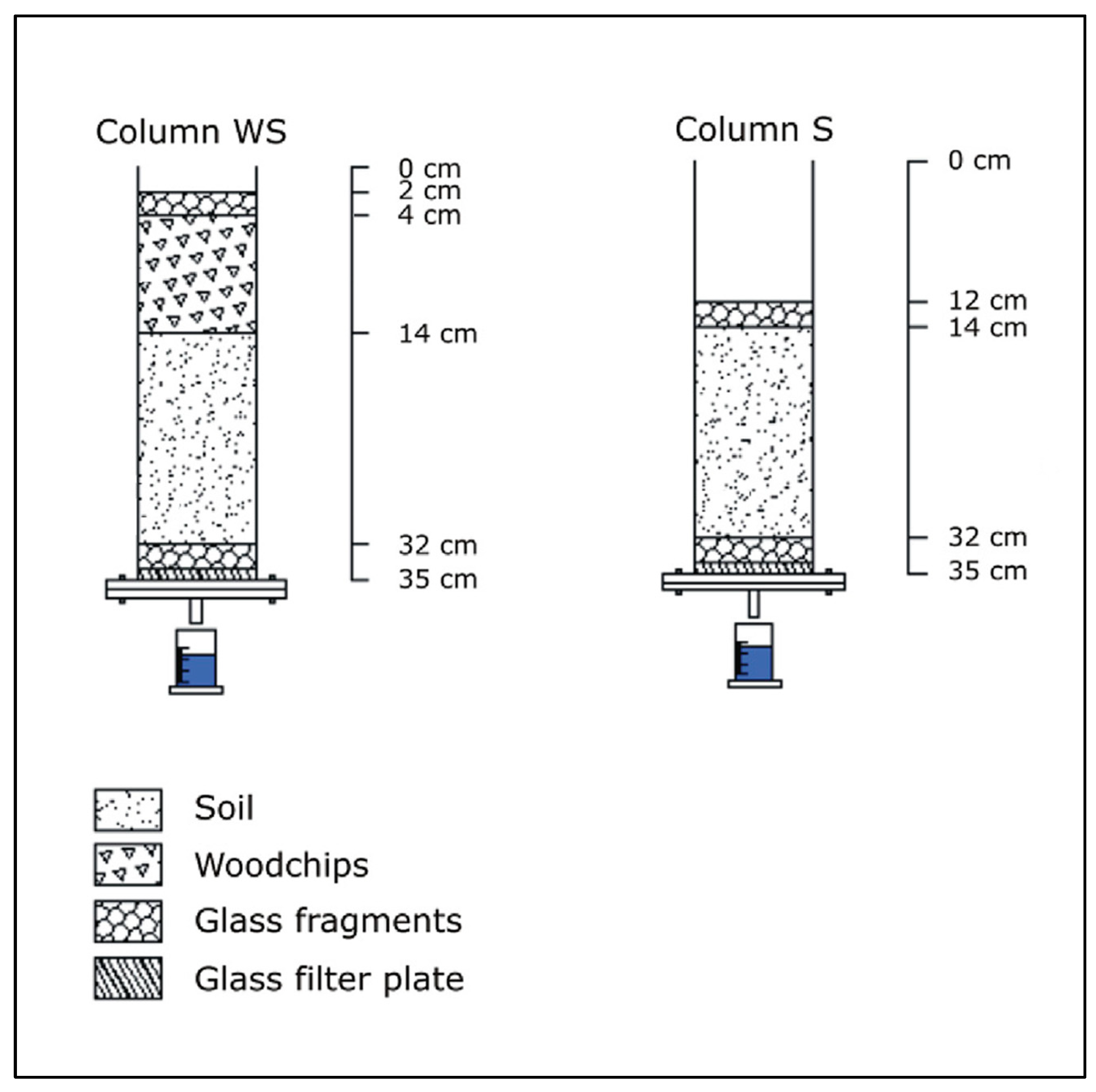

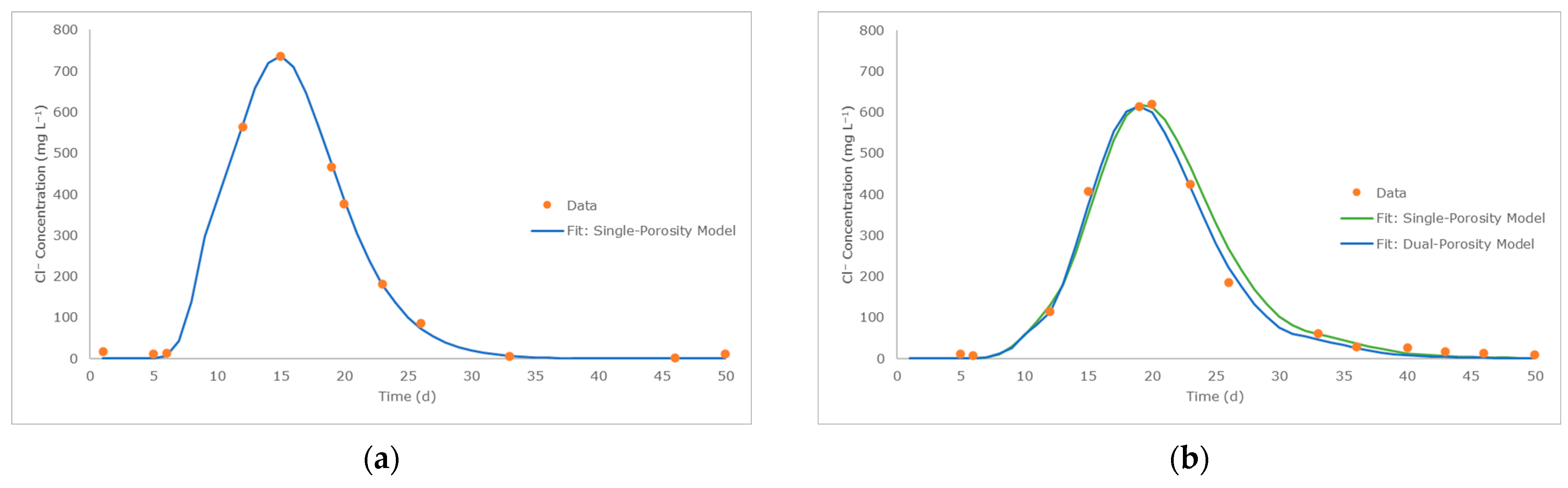
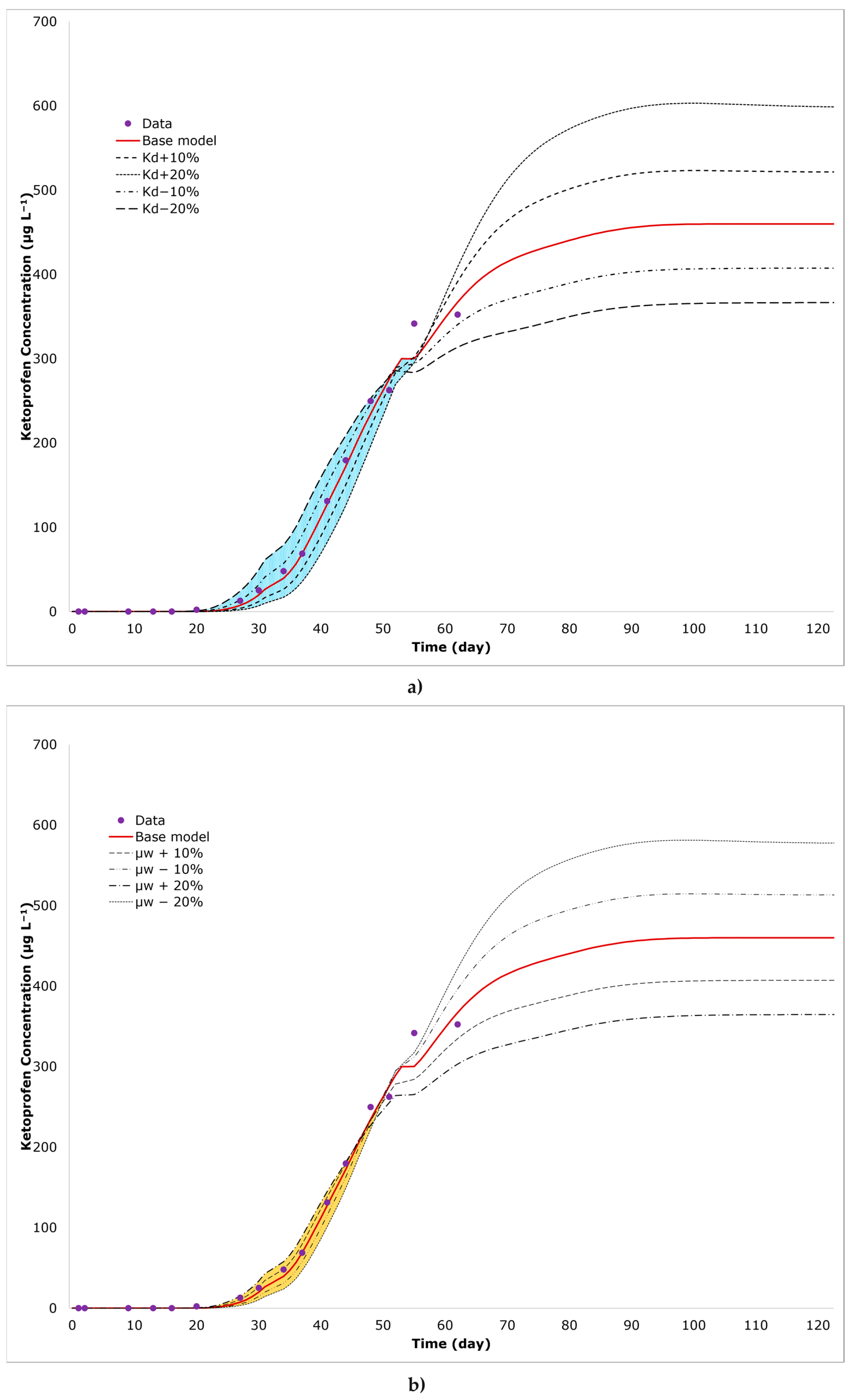
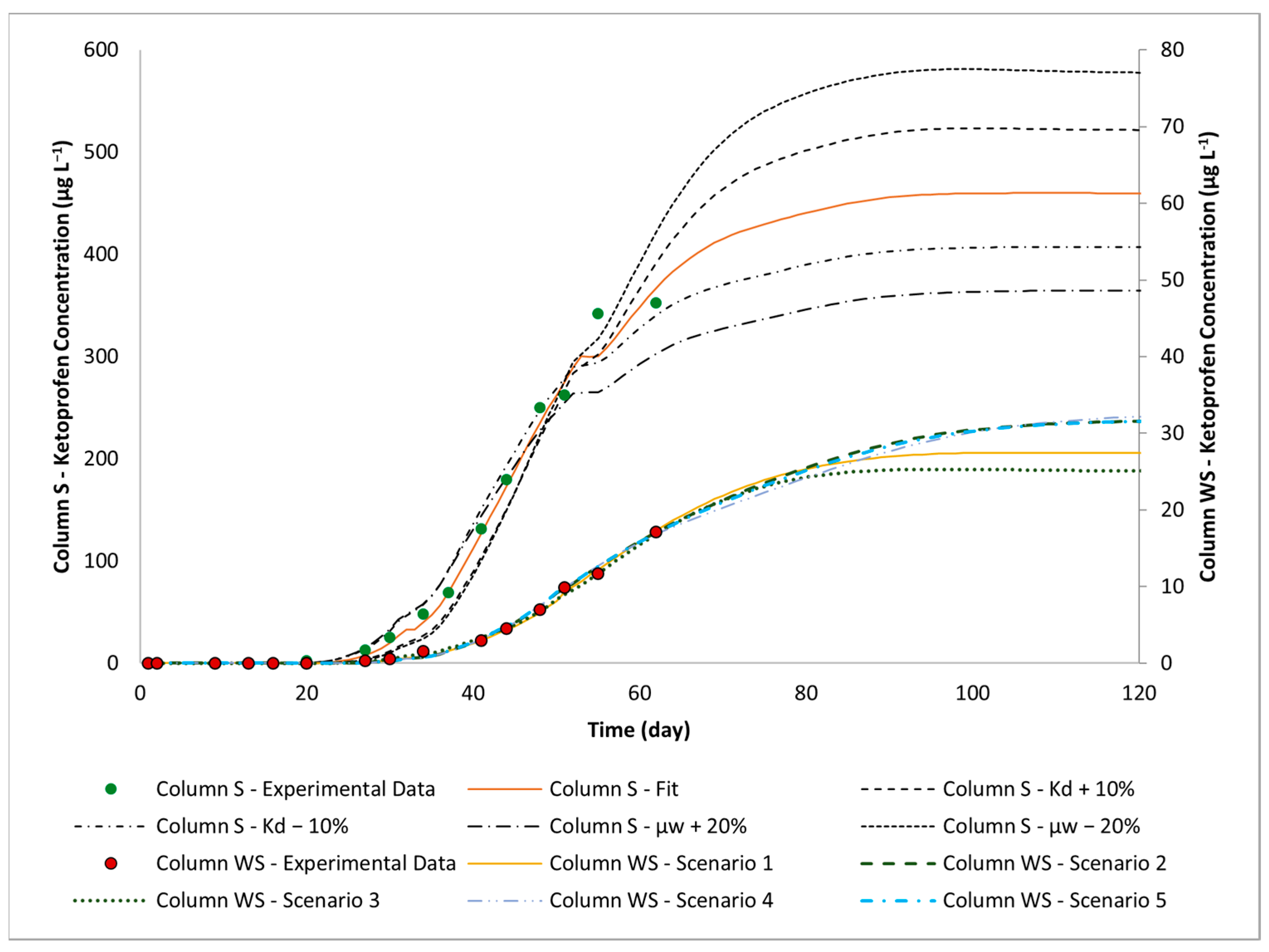

| Parameter | Values | Unit | ||||
|---|---|---|---|---|---|---|
| Column S | Column WS | |||||
| Soil Layer | Woodchip Layer | |||||
| Model domain | Column characteristics | Profile length | 18.00 | 28.00 | cm | |
| Number of materials | 1 | 2 | - | |||
| Layer thickness | 18.00 | 18.00 | 10.00 | cm | ||
| Grid discretization | Number of nodes | 1001 | 1001 | - | ||
| Number of fixed points for mesh density | 3 | 3 | ||||
| Location of fixed points | 1/557/1001 | 1/427/1001 | ||||
| Mesh upper density/lower density applied at fixed points | 1:1/1 557:10/10 1001:1/ | 1:1/0.2 427:0.1/0.1 1001:0.3/1 | - | |||
| Number of observation points | 3 | 3 | - | |||
| Location of observation points | 1/220/644 | 1/736/1001 | - | |||
| Time domain | Time discretization | Initial time step | 0.1 | 0.1 | s | |
| Minimum time step | 0.01 | 0.01 | s | |||
| Maximum time step | 1.728 × 107 | 1.728 × 107 | s | |||
| Simulation time | 200.00 | 200.00 | d | |||
| Hydraulic properties | Measured | Bulk density (ρ) | 1.40 | 1.40 | 0.12 | g cm−3 |
| Sand | 55.00 | 55.00 | - | % | ||
| Silt | 26.70 | 26.70 | - | % | ||
| Clay | 18.30 | 18.30 | - | % | ||
| Saturated water content (θs) | 0.4207 | 0.4207 | 0.85 | cm3 cm−3 | ||
| Rosetta estimation | Residual water content (θr) | 0.058 | 0.058 | - | - | |
| Hydraulic properties | Rosetta estimation | Empiric parameter in the soil water retention function (α) | 0.0173 | 0.0173 | - | cm−1 |
| Empiric parameter in the soil water retention function (n) | 1.45 | 1.45 | - | - | ||
| Tortuosity parameter in the conductivity function (l) (a) | 0.50 | 0.50 | - | - | ||
| Initial conditions | Flow (b) | Upper pressure head | −18.00 | −28.00 | cm | |
| Lower pressure head | 0.00 | 0.00 | cm | |||
| Transport | NaCl concentration | 0.00 | 0.00 | cm s−1 | ||
| Pharmaceutical concentration | 0.00 | 0.00 | cm s−1 | |||
| Boundary conditions | Flow | Tracer flux (1 s single pulse) | 0.6367 | 0.6367 | cm s−1 | |
| Pharmaceutical flux (pulse: 1 s every 24 h) | 0.6367 | 0.6367 | cm s−1 | |||
| Maximum h at the soil surface | 0.65 | 0.65 | cm | |||
| Transport | NaCl concentration | 0.1711 | 0.1711 | mmol cm−3 | ||
| Daily pharmaceutical concentration | 1.00 | 1.00 | mg L−1 | |||
| Calibration parameters | Woodchip hydraulic parameters | Mobile residual water content (θrmo) | X | X | X | cm3 cm−3 |
| Mobile saturated water content (θsmo) | X | cm3 cm−3 | ||||
| Empiric parameter in the soil water retention function (α) | X | X | X | cm−1 | ||
| Empiric parameter in the soil water retention function (n) | X | X | X | - | ||
| Immobile residual water content (θrim) | X | cm3 cm−3 | ||||
| Immobile saturated water content (θsim) | X | cm3 cm−3 | ||||
| Calibration parameters | Woodchip hydraulic parameters | Mass transfer coefficient (ω) | X | s−1 | ||
| Tracer transport | Saturated hydraulic conductivity (Ks) | X | X | X | cm s−1 | |
| Longitudinal dispersivity (αL) | X | X | X | cm | ||
| Tracer molecular diffusion coefficient in free water (Dw) | X | X | X | cm2 s−1 | ||
| Pharmaceutical transport | Distribution coefficient (Kd) | X | X | X | cm3 g−1 | |
| First-order kinetic removal rate (μw) | X | X | X | s−1 | ||
| Pharmaceutical molecular diffusion coefficient in free water (Dw) | X | X | X | cm2 s−1 | ||
| Parameter | Column S | Column WS | |
|---|---|---|---|
| Single-Porosity Model (mL) | Single- Porosity Model (mL) | Dual- Porosity Model (mL) | |
| Cumulative experimental water volume | 2032.34 | 2016.97 | 2016.97 |
| Cumulative simulated water volume | 2033.61 | 1937.87 | 1995.72 |
| Root Mean Squared Error (RMSE) | 4.59 | 9.40 | 4.95 |
| Parameter | Soil Layer | Woodchip Layer |
|---|---|---|
| Ks (cm s−1) | 3.11 × 10−5 | 0.0218 |
| αL (cm) | 0.0696 | 0.748 |
| Parameter | Value |
|---|---|
| Woodchip Layer | |
| θrmo (cm3 cm−3) | 1.52 × 10−4 |
| θrIm (cm3 cm−3) | 0.00 |
| θsmo (cm3 cm−3) | 0.273 |
| θsIm (cm3 cm−3) | 0.577 |
| α (cm−1) | 0.02 |
| n (-) | 1.50 |
| ω (s−1) | 3.54 × 10−7 |
| Scenario | Kd (L kg−1) | µw (d−1) | R2 | RMSE (μg L−1) | Parameter Variation (%) | |
|---|---|---|---|---|---|---|
| Kd | µw | |||||
| Best fit model | 0.482 | 0.0729 | 0.991 | 12.64 | - | - |
| Kd + 10% | 0.530 | 0.0647 | 0.979 | 20.49 | 10.0% | −11.3% |
| Kd + 20% | 0.579 | 0.0563 | 0.953 | 31.75 | 20.0% | −22.8% |
| Kd − 10% | 0.434 | 0.0802 | 0.986 | 15.67 | −10.0% | 9.9% |
| Kd − 20% | 0.386 | 0.0867 | 0.960 | 26.87 | −20.0% | 18.9% |
| µw + 10% | 0.452 | 0.0802 | 0.990 | 16.02 | −6.4% | 10.0% |
| µw + 20% | 0.423 | 0.0875 | 0.980 | 24.54 | −12.2% | 20.0% |
| µw − 10% | 0.514 | 0.0657 | 0.986 | 16.93 | 6.6% | −10.0% |
| µw − 20% | 0.547 | 0.0584 | 0.970 | 24.51 | 13.5% | −20.0% |
| Model | Parameter | Soil Layer | Woodchip Layer | R2 | RMSE |
|---|---|---|---|---|---|
| Scenario 1 | Kd | 0.482 | 13.110 | 0.997 | 0.494 |
| μw | 0.255 | 0.255 | |||
| Scenario 2 | Kd | 0.482 | 15.260 | 0.997 | 0.506 |
| μw | 0.092 | 0.709 | |||
| Scenario 3 | Kd | 0.482 | 11.200 | 0.997 | 0.567 |
| μw | 0.379 | 0.000 | |||
| Scenario 4 | Kd | 0.482 | 15.410 | 0.995 | 0.526 |
| μw | 0.000 | 1.090 | |||
| Scenario 5 | Kd | 0.482 | 15.250 | 0.996 | 0.523 |
| μw | 0.073 | 0.783 |
| Layer | Parameter | % Change in Parameter | Sensitivity Coefficient |
|---|---|---|---|
| Woodchips | θsIm | −25% | 0.122 |
| −15% | 0.111 | ||
| 15% | 0.125 | ||
| 25% | 0.043 | ||
| θsmo | −25% | 1.242 | |
| −15% | 0.973 | ||
| 15% | 22.682 | ||
| 25% | 2.334 | ||
| θrmo | −25% | 0.034 | |
| −15% | 0.102 | ||
| 15% | 0.033 | ||
| 25% | 0.055 | ||
| α | −25% | 0.110 | |
| −15% | 0.198 | ||
| 15% | 5.625 | ||
| 25% | 5.054 | ||
| n | −25% | 1.404 | |
| −15% | 3.019 | ||
| 15% | 1.679 | ||
| 25% | 1.911 | ||
| l | −25% | 0.014 | |
| −15% | 0.169 | ||
| 15% | 0.094 | ||
| 25% | 0.035 | ||
| ω | −25% | 0.125 | |
| −15% | 0.080 | ||
| 15% | 0.061 | ||
| 25% | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvi-Taga, R.G.; Meffe, R.; Martínez-Hernández, V.; De Miguel Garcia, A.; De Bustamante, I. Amended Vegetation Filters as Nature-Based Solutions for the Treatment of Pharmaceuticals: Infiltration Experiments Coupled to Reactive Transport Modelling. Toxics 2024, 12, 334. https://doi.org/10.3390/toxics12050334
Salvi-Taga RG, Meffe R, Martínez-Hernández V, De Miguel Garcia A, De Bustamante I. Amended Vegetation Filters as Nature-Based Solutions for the Treatment of Pharmaceuticals: Infiltration Experiments Coupled to Reactive Transport Modelling. Toxics. 2024; 12(5):334. https://doi.org/10.3390/toxics12050334
Chicago/Turabian StyleSalvi-Taga, Raisa Gabriela, Raffaella Meffe, Virtudes Martínez-Hernández, Angel De Miguel Garcia, and Irene De Bustamante. 2024. "Amended Vegetation Filters as Nature-Based Solutions for the Treatment of Pharmaceuticals: Infiltration Experiments Coupled to Reactive Transport Modelling" Toxics 12, no. 5: 334. https://doi.org/10.3390/toxics12050334
APA StyleSalvi-Taga, R. G., Meffe, R., Martínez-Hernández, V., De Miguel Garcia, A., & De Bustamante, I. (2024). Amended Vegetation Filters as Nature-Based Solutions for the Treatment of Pharmaceuticals: Infiltration Experiments Coupled to Reactive Transport Modelling. Toxics, 12(5), 334. https://doi.org/10.3390/toxics12050334






