Machine Learning Model for Prediction of Development of Cancer Stem Cell Subpopulation in Tumurs Subjected to Polystyrene Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Study
2.2. Cell Cultures and Polystyrene Particles Treatment
2.3. Flow Cytometry Analysis
2.4. Machine Learning Model (ML)—Genetic Algorithm (GA)
2.5. Statistical Analysis
3. Results
3.1. CSC Protein Marker Analyses—Flow Cytometry
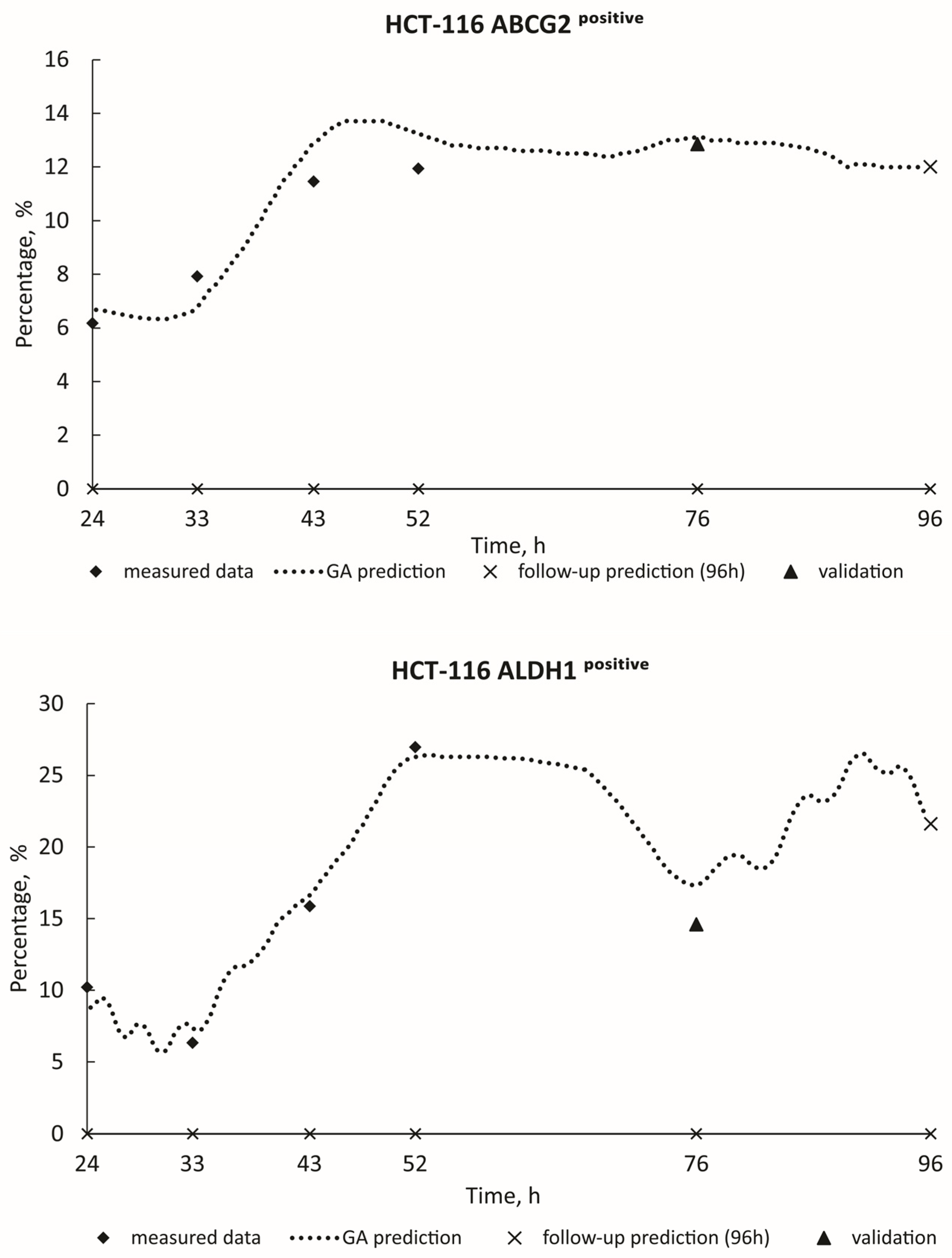
3.2. ML Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Ma, H.; Zhang, J.; Zhu, L.; Wang, C.; Yang, Y. Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem cell markers in tumorigenesis and metastasis. Sci. Rep. 2017, 7, 13856. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Liu, S.; Cong, Y.; Wang, D.; Sun, Y.; Deng, L.; Liu, Y.; Martin-Trevino, R.; Shang, L.; McDermott, S.P.; Landis, M.D.; et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2013, 2, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Tiezzi, D.G.; Sicchieri, R.D.; Mouro, L.R.; Oliveira, T.M.G.; Silveira, W.A.; Antonio Valdair, H.M.R.; Muglia, F.; Moreira de Andrade, J. ABCG2 as a potential cancer stem cell marker in breast cancer. J. Clin. Oncol. 2013, 31, e12007. [Google Scholar] [CrossRef]
- Vishnubalaji, R.; Manikandan, M.; Fahad, M.; Hamam, R.; Alfayez, M.; Kassem, M.; Aldahmash, A.; Alajez, N.M. Molecular profiling of ALDH1+ colorectal cancer stem cells reveal preferential activation of MAPK, FAK, and oxidative stress pro-survival signalling pathways. Oncotarget 2018, 9, 13551–13564. [Google Scholar] [CrossRef]
- Sahlberg, S.H.; Spiegelberg, D.; Glimelius, B.; Stenerlöw, B.; Nestor, M. Evaluation of cancer stem cell markers CD133, CD44, CD24: Association with AKT isoforms and radiation resistance in colon cancer cells. PLoS ONE 2014, 9, e94621. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.M.; Gandhi, S.C.; Wilding, J.L.; Muschel, R.; Bodmer, W.F. Cancer stem cells from colorectal cancer-derived cell lines. Proc. Natl. Acad. Sci. USA 2010, 107, 3722–3727. [Google Scholar] [CrossRef]
- Huang, E.H.; Hynes, M.J.; Zhang, T.; Ginestier, C.; Dontu, G.; Appelman, H.; Fields, J.Z.; Wicha, M.S.; Boman, B.M. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009, 69, 9. [Google Scholar] [CrossRef]
- Nikolic, S.; Gazdic-Jankovic, M.; Rosic, R.; Miletic-Kovacevic, M.; Jovicic, N.; Nestorovic, N.; Stojkovic, P.; Filipovic, N.; Milosevic-Djordjevic, O.; Selakovic, D.; et al. Orally administered fluorescent nanosized polystyrene particles affect cell viability, hormonal and inflammatory profile, and behavior in treated mice. Environ. Pollut. 2022, 305, 119206. [Google Scholar] [CrossRef]
- Vecchiotti, G.; Colafarina, S.; Aloisi, M.; Zarivi, O.; Di Carlo, P.; Poma, A. Genotoxicity and oxidative stress induction by polystyrene nanoparticles in the colorectal cancer cell line HCT116. PLoS ONE 2021, 16, e0255120. [Google Scholar] [CrossRef]
- Chen, G.; Shan, H.; Xiong, S.; Zhao, Y.; van Gestel, C.A.M.; Qiu, H.; Wang, Y. Polystyrene nanoparticle exposure accelerates ovarian cancer development in mice by altering the tumor microenvironment. Sci. Total Environ. 2024, 906, 167592. [Google Scholar] [CrossRef]
- Xu, J.L.; Lin, X.; Wang, J.J.; Gowen, A.A. A review of potential human health impacts of micro- and nanoplastics exposure. Sci. Total Environ. 2022, 851 Pt 1, 158111. [Google Scholar] [CrossRef]
- Domenech, J.; de Britto, M.; Velázquez, A.; Pastor, S.; Hernández, A.; Marcos, R.; Cortés, C. Long-Term Effects of Polystyrene Nanoplastics in Human Intestinal Caco-2 Cells. Biomolecules 2021, 11, 1442. [Google Scholar] [CrossRef]
- Bonanomi, M.; Salmistraro, N.; Porro, D.; Pinsino, A.; Colangelo, A.M.; Gaglio, D. Polystyrene micro and nano-particles induce metabolic rewiring in normal human colon cells: A risk factor for human health. Chemosphere 2022, 303 Pt 1, 134947. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Chen, J.; Miao, X.; Li, G.; He, Q.; Xu, H.; Li, H.; Wei, Y. Cytotoxic effects of polystyrene nanoplastics with different surface functionalization on human HepG2 cells. Sci. Total Environ. 2020, 723, 138180. [Google Scholar] [CrossRef]
- Barguilla, I.; Domenech, J.; Ballesteros, S.; Rubio, L.; Marcos, R.; Hernández, A. Long-term exposure to nanoplastics alters molecular and functional traits related to the carcinogenic process. J. Hazard. Mater. 2022, 438, 129470. [Google Scholar] [CrossRef]
- Kim, H.; Zaheer, J.; Choi, E.J.; Kim, J.S. Enhanced ASGR2 by microplastic exposure leads to resistance to therapy in gastric cancer. Theranostics 2022, 12, 3217–3236. [Google Scholar] [CrossRef]
- Roje, Ž.; Ilić, K.; Galić, E.; Pavičić, I.; Turčić, P.; Stanec, Z.; Vrček, I.V. Synergistic effects of parabens and plastic nanoparticles on proliferation of human breast cancer cells. Arh. Hig. Rada Toksikol. 2019, 70, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Dai, H.; Wang, B.; Xu, J.; Zhang, Y.; Chen, Y.; Ma, Q.; Xu, F.; Cheng, H.; Sun, D.; et al. Nanoplastics Shape Adaptive Anticancer Immunity in the Colon in Mice. Nano Lett. 2023, 23, 3516–3523. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Zhong, Y.; Huang, Y.; Lin, X.; Liu, J.; Lin, L.; Hu, M.; Jiang, J.; Dai, M.; Wang, B.; et al. Underestimated health risks: Polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis. Part. Fibre Toxicol. 2021, 18, 20. [Google Scholar] [CrossRef]
- De Jong, K. Learning with genetic algorithms: An overview. Mach. Learn. 1988, 3, 121–138. [Google Scholar] [CrossRef]
- Banzhaf, W.; Nordin, P.; Keller, R.E.; Francone, F.D. Genetic Programming—An Introduction; Morgan Kaufmann: San Francisco, CA, USA, 1998. [Google Scholar]
- O’Neill, M.; Poli, R. A Field Guide to Genetic Programming. Genet. Program. Evolvable Mach. 2009, 10, 229–230. [Google Scholar] [CrossRef]
- Gad, A.F. Pygad: An intuitive genetic algorithm Python library. In Multimedia Tools and Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–14. [Google Scholar]
- Turney, S. Coefficient of Determination (R2)|Calculation & Interpretation. Scribbr. 14 September 2022. Available online: https://www.scribbr.com/statistics/coefficient-of-determination/ (accessed on 3 May 2022).
- Xanthos, D.; Walker, T.R. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): A review. Mar. Pollut. Bull. 2017, 18, 17–26. [Google Scholar] [CrossRef]
- Andrady, A.; Neal, M. Applications and societal benefits of plastics. Philos. Trans. R. Soc. Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Stojkovic, M.; Ortuño Guzmán, F.M.; Han, D.; Stojkovic, P.; Dopazo, J.; Stankovic, K.M. Polystyrene nanoplastics affect transcriptomic and epigenomic signatures of human fibroblasts and derived induced pluripotent stem cells: Implications for human health. Environ. Pollut. 2023, 320, 120849. [Google Scholar] [CrossRef]
- Sulukan, E.; Şenol, O.; Baran, A.; Kankaynar, M.; Yıldırım, S.; Kızıltan, T.; Bolat, İ.; Ceyhun, S.B. Nano-sized polystyrene plastic particles affect many cancer-related biological processes even in the next generations, zebrafish modeling. Sci. Total Environ. 2022, 838 Pt 3, 156391. [Google Scholar] [CrossRef]
- Prud’homme, G.J.; Glinka, Y.; Toulina, A.; Ace, O.; Subramaniam, V.; Jothy, S. Breast Cancer Stem-Like Cells Are Inhibited by a Non-Toxic Aryl Hydrocarbon Receptor Agonist. PLoS ONE 2010, 11, e13831. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.C.; Huber, S.L.; Herrler, T.; Aicher, A.; Ellwart, J.W.; Guba, M.; Bruns, C.J.; Heeschen, C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007, 1, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Sin, W.C.; Lim, C.L. Breast cancer stem cells-from origins to targeted therapy. Stem Cell Investig. 2017, 4, 96. [Google Scholar] [CrossRef]
- Wanandi, S.I.; Syahrani, R.A.; Arumsari, S.; Wideani, G.; Hardiany, N.S. Profiling of Gene Expression Associated with Stemness and Aggressiveness of ALDH1A1-Expressing Human Breast Cancer Cells. Malays. J. Med. Sci. 2019, 26, 38–52. [Google Scholar] [CrossRef]





| Biological Models | Plastic Particle Source | Polymer Type | Particle Size | Exposure Concentration | Results |
|---|---|---|---|---|---|
| In vivo: epithelial ovarian cancer mice model [12] | Purchased from Huge Biotechnology (Shanghai, China) | polystyrene | 100 nm | 10 mg/L for 27 days | PS-NP exposure accelerated EOC tumor growth in mice |
| In vitro: human colon adenocarcinoma cells (Caco-2) [14] | Commercially obtained (Spherotech, Inc., Chicago, IL, USA) | polystyrene | 50 nm | range of different concentrations: 0, 6.5, 13, 26, and 39 μg/cm2 | Accumulation of PSNPs in exposed cells in a concentration-dependent manner |
| In vitro: normal human intestinal cells (CCD-18Co) [15] | purchased from Sigma–Aldrich (St Louis, MO, USA) | polystyrene | 0.5 μm and 2 μm | range of different concentrations (1–5-10–20 μg/mL) | NPs and MPs exposure cause oxidative stress |
| In vitro: HepG2 cells [16] | obtained from the DK Nano Tech (Beijing, China) | polystyrene | 50 nm | 10 μg/mL for 12 h | reduced the cell viability |
| In vitro: mouse embryonic fibroblasts [17] | purchased from Spherotech (Chicago, IL, USA) | polystyrene | 50 nm | increasing doses of PSNPLs (10, 25, 75, and 100 μg/mL) for 24 h | exacerbated cancer |
| In vivo: BALB/c nude mice In vitro: human gastric cancer cell lines (AGS, MKN1, MKN45, NCI-N87, and KATOIII) [18] | purchased from Cospheric (Somis, CA, USA) | polystyrene | 9.5–11.5 µm | In vivo: 1.72 × 104 particles/mL In vitro: 8.61 × 105 particles/mL | induced resistance to chemo- and monoclonal antibody-therapy |
| In vitro: human breast cancer cell lines: MDA-MB 231, and MCF-7 [19] | purchased from Thermo Fisher Scientific, Waltham, MA, USA | polystyrene | 60 nm | 1, 10, and 100 mg/mL | influence cell viability and proliferation |
| In vivo: C57BL/6 J mice [20] | purchased from Magsphere (Pasadena, CA, USA) | polyethylene | 50.7, 503.6, and 5047.0 nm | 20 mL/kg body weight, for 28 consecutive days | causing severe dysfunction of the intestinal barrier |
| Model System | R2—Score of the Prediction |
|---|---|
| HCT-116 ABCG2positive | 0.99968 |
| HCT-116 ALDH1positive | 0.98868 |
| HCT-116 CD24positive ABCG2positive | 0.95683 |
| HCT-116 CD24positive ALDHpositive | 0.99745 |
| MDA-MB-231 CD24positive ABCG2positive | 0.96353 |
| MDA-MB-231 CD24positive ALDH1positive | 0.95011 |
| MDA-MB-231 ABCG2positive CD24positive | 0.99847 |
| MDA-MB-231 ALDH1positive CD24positive | 0.93221 |
| MDA-MB-231 CD44positive | 0.99055 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramović Hamzagić, A.; Gazdić Janković, M.; Cvetković, D.; Nikolić, D.; Nikolić, S.; Milivojević Dimitrijević, N.; Kastratović, N.; Živanović, M.; Miletić Kovačević, M.; Ljujić, B. Machine Learning Model for Prediction of Development of Cancer Stem Cell Subpopulation in Tumurs Subjected to Polystyrene Nanoparticles. Toxics 2024, 12, 354. https://doi.org/10.3390/toxics12050354
Ramović Hamzagić A, Gazdić Janković M, Cvetković D, Nikolić D, Nikolić S, Milivojević Dimitrijević N, Kastratović N, Živanović M, Miletić Kovačević M, Ljujić B. Machine Learning Model for Prediction of Development of Cancer Stem Cell Subpopulation in Tumurs Subjected to Polystyrene Nanoparticles. Toxics. 2024; 12(5):354. https://doi.org/10.3390/toxics12050354
Chicago/Turabian StyleRamović Hamzagić, Amra, Marina Gazdić Janković, Danijela Cvetković, Dalibor Nikolić, Sandra Nikolić, Nevena Milivojević Dimitrijević, Nikolina Kastratović, Marko Živanović, Marina Miletić Kovačević, and Biljana Ljujić. 2024. "Machine Learning Model for Prediction of Development of Cancer Stem Cell Subpopulation in Tumurs Subjected to Polystyrene Nanoparticles" Toxics 12, no. 5: 354. https://doi.org/10.3390/toxics12050354
APA StyleRamović Hamzagić, A., Gazdić Janković, M., Cvetković, D., Nikolić, D., Nikolić, S., Milivojević Dimitrijević, N., Kastratović, N., Živanović, M., Miletić Kovačević, M., & Ljujić, B. (2024). Machine Learning Model for Prediction of Development of Cancer Stem Cell Subpopulation in Tumurs Subjected to Polystyrene Nanoparticles. Toxics, 12(5), 354. https://doi.org/10.3390/toxics12050354







