Utilization of Peptidoglycans from Lactic Acid Bacterial Cell Walls for the Mitigation of Acrylamide and 5-Hydroxymethylfurfural
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bacterial Strains and Culture Cultivation
2.3. Preparation of LAB-Based PGN
2.4. AA/HMF Binding Assay
2.5. Removal of AA/HMF by the PGN of Strain B. lactis B1-04
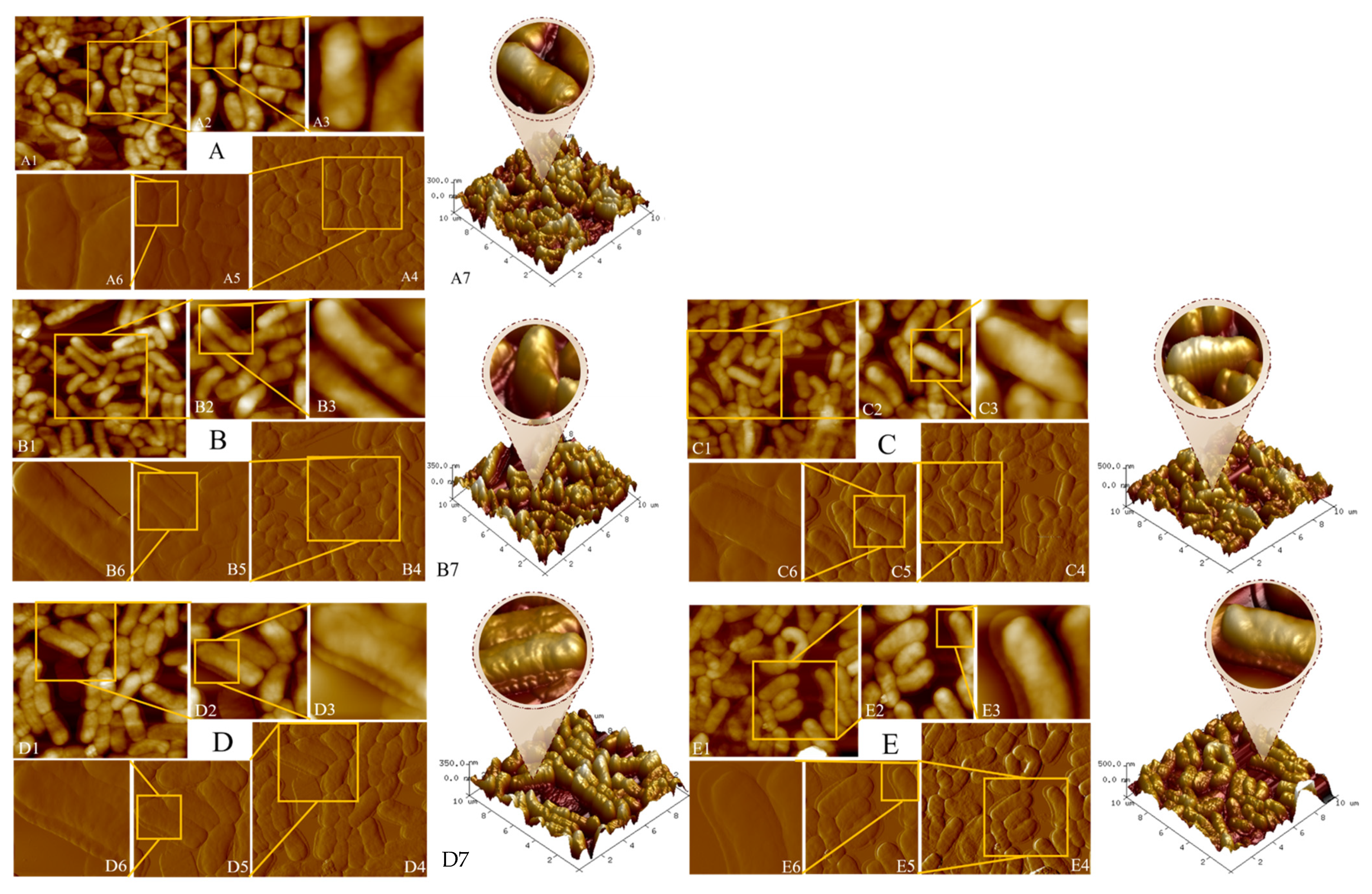
2.5.1. Morphological Observation of B. lactis B1-04 Binding AA/HMF
2.5.2. XRD Analysis of the PGN of Strain B1-04 Binding AA/HMF
2.5.3. FTIR Analysis of the PGN of Strain B1-04 Binding AA/HMF
2.6. Adsorption Action of the PGNs from Selected LAB Strains to AA/HMF
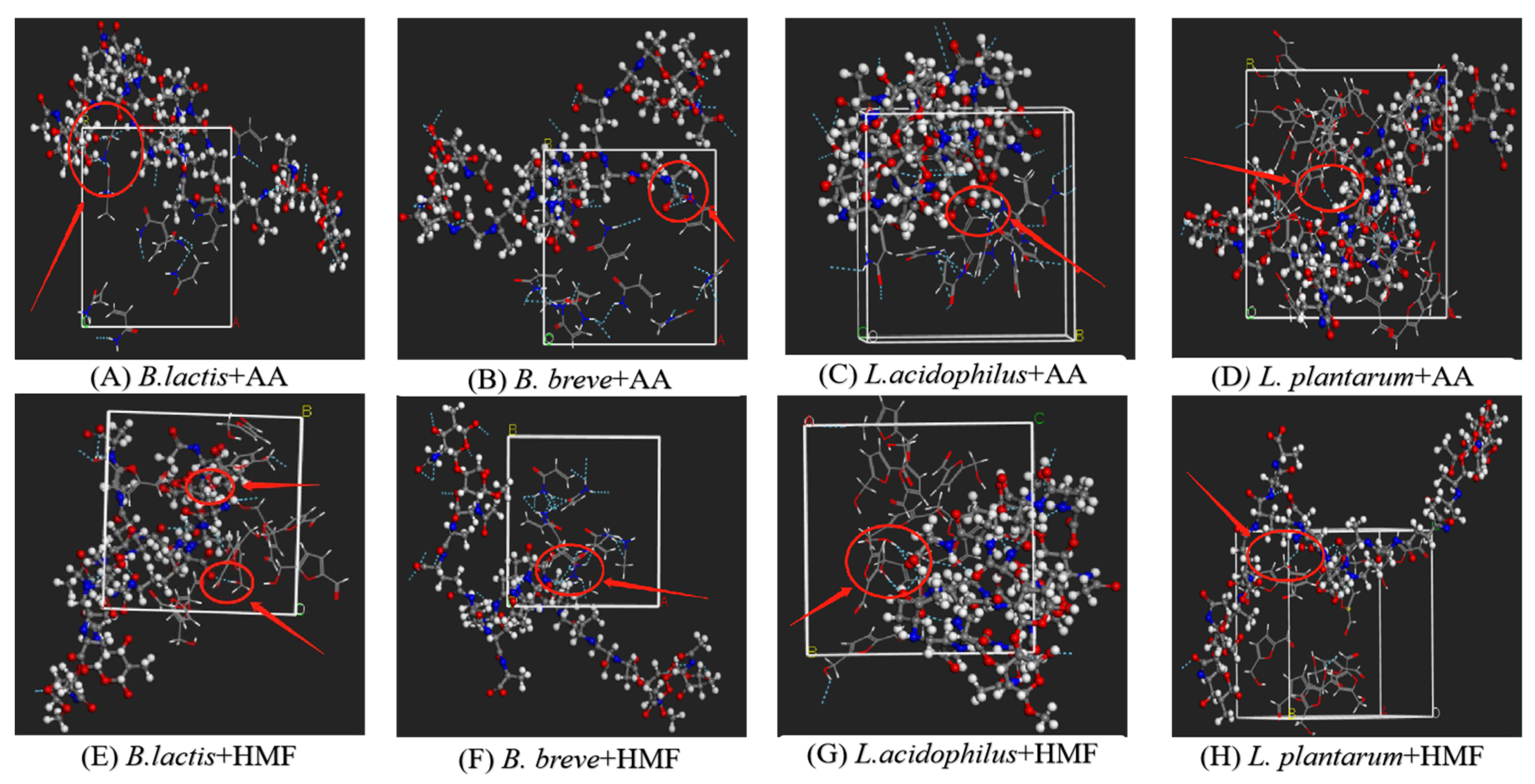
2.6.1. Construction of the LAB-Based PGN Molecules and AA/HMF Molecular Models
2.6.2. Calculation of the Interaction Energy
2.6.3. Calculation of Fractional Free Volume (FFV)
2.6.4. Calculation of Radial Distribution Function (RDF)
2.7. Correlation between the Specific Groups of LAB-Based PGN and AA/HMF Binding
2.8. Statistical Analysis
3. Results
3.1. AA/HMF Binding Assay
3.2. Morphology and Specific Groups of B. lactis B1-04 Strain Binding AA/HMF
3.2.1. Visualization of the Adsorption of B. lactis B1-04 Cells to AA/HMF
3.2.2. Specific Groups Responsible for the Binding of B. lactis B1-04 PGN to AA/HMF
3.3. Adsorption Action of the PGNs from the Selected LAB Strains to AA/HMF
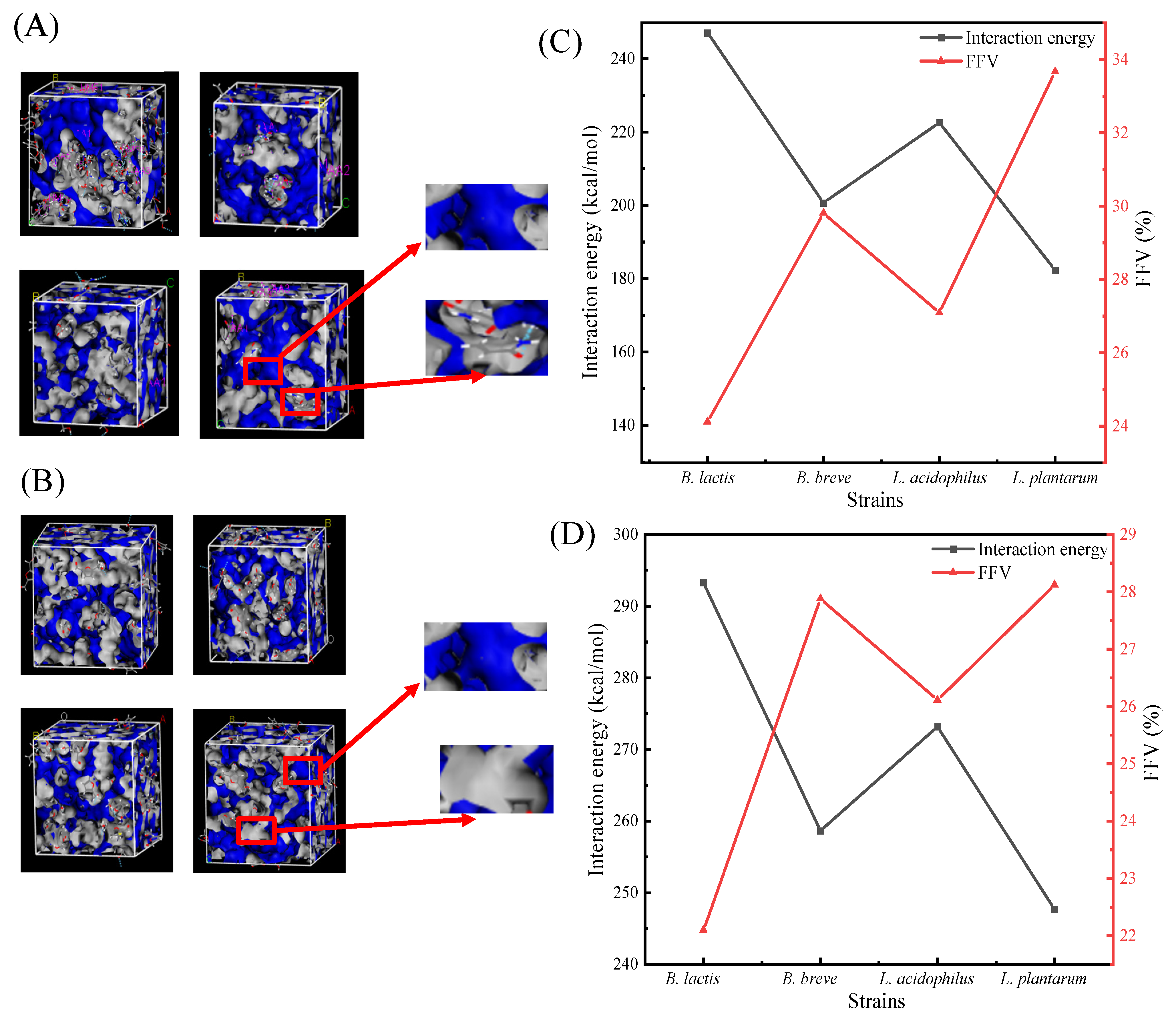
3.3.1. Interaction Energy Analysis of Adsorbed AA/HMF
3.3.2. FFV Analysis of Adsorbed AA/HMF
3.4. Effect of Relative Content of Specific Groups on LAB-Based PGN on AA/HMF Binding
3.4.1. The Full Spectrum Analysis of XPS
3.4.2. C Spectrum
3.4.3. N Spectrum
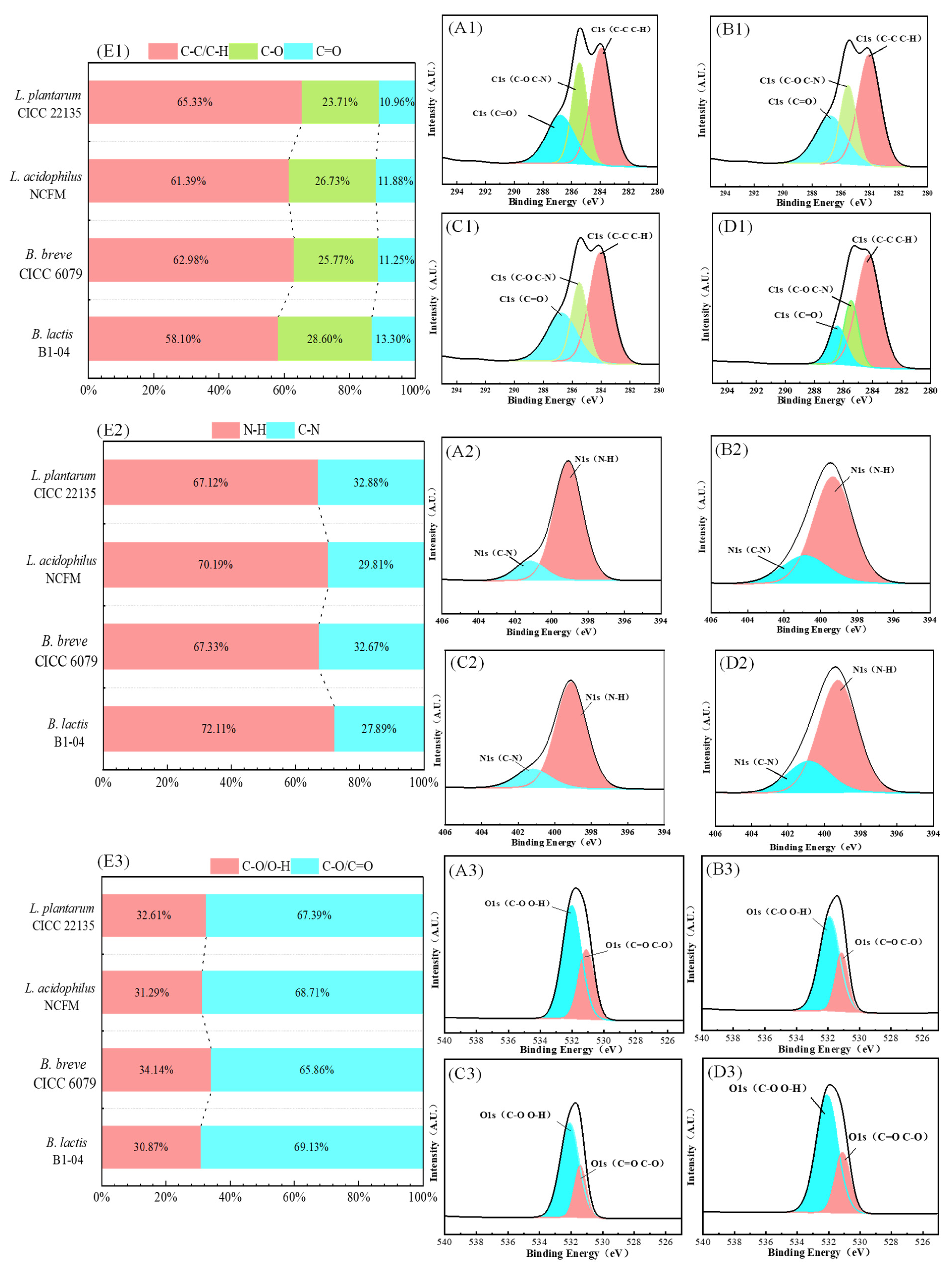
3.4.4. O Spectrum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arvanitoyannis, I.S.; Dionisopoulou, N. Acrylamide: Formation, occurrence in food products, detection methods, and legislation. Crit. Rev. Food Sci. 2014, 54, 708–733. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, B.P.; Bora, P.K.; Kemprai, P.; Borah, G.; Lal, M.; Haldar, S. Thermolabile essential oils, aromas and flavours: Degradation pathways, effect of thermal processing and alteration of sensory quality. Food Res. Int. 2021, 145, 110404. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, V.; Palazoglu, T.K. Acrylamide formation in foods during thermal processing with a focus on frying. Food Bioprocess Technol. 2008, 1, 35–42. [Google Scholar] [CrossRef]
- Jebasingh, S.E.J.; Lakshmikandan, M.; Rajesh, R.P.; Raja, P. Biodegradation of acrylamide and purification of acrylamidase from newly isolated bacterium Moraxella osloensis MSU11. Int. Biodeterior. Biodegrad. 2013, 85, 120–125. [Google Scholar] [CrossRef]
- Maan, A.A.; Anjum, M.A.; Khan, M.K.I.; Nazir, A.; Saeed, F.; Afzaal, M.; Aadil, R.M. Acrylamide formation and different mitigation strategies during food processing—A Review. Food Rev. Int. 2022, 38, 70–87. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef]
- Friedman, M.; Levin, C.E. Review of methods for the reduction of dietary content and toxicity of acrylamide. J. Agric. Food Chem. 2008, 56, 6113–6140. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Acrylamide: Inhibition of formation in processed food and mitigation of toxicity in cells, animals, and humans. Food Funct. 2015, 6, 1752–1772. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.; Gurtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and risk assessment of 5-Hydroxymethylfurfural in food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef]
- Husoy, T.; Haugen, M.; Murkovic, M.; Jobstl, D.; Stolen, L.H.; Bjellaas, T.; Ronningborg, C.; Glatt, H.; Alexander, J. Dietary exposure to 5-hydroxymethylfurfural from Norwegian food and correlations with urine metabolites of short-term exposure. Food Chem. Toxicol. 2008, 46, 3697–3702. [Google Scholar] [CrossRef]
- Capuano, E.; Fogliano, V. Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies. LWT-Food Sci. Technol. 2011, 44, 793–810. [Google Scholar] [CrossRef]
- Zhang, L.L.; Kong, Y.; Yang, X.; Zhang, Y.Y.; Sun, B.G.; Chen, H.T.; Sun, Y. Kinetics of 5-hydroxymethylfurfural formation in the sugar-amino acid model of Maillard reaction. J. Sci. Food Agric. 2019, 99, 2340–2347. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Van der Fels-Klerx, H.J.; Peters, R.J.B.; Van Boekel, M.A.J.S. Acrylamide and 5-hydroxymethylfurfural formation during baking of biscuits: Part I: Effects of sugar type. Food Chem. 2016, 192, 575–585. [Google Scholar] [CrossRef]
- Nguyen, H.T.; van der Fels-Klerx, H.J.; van Boekel, M.A.J.S. Acrylamide and 5-hydroxymethylfurfural formation during biscuit baking. Part II: Effect of the ratio of reducing sugars and asparagine. Food Chem. 2017, 230, 14–23. [Google Scholar] [CrossRef]
- Bartkiene, E.; Jakobsone, I.; Pugajeva, I.; Bartkevics, V.; Vidmantiene, D.; Juodeikiene, G. Influence of the addition of Helianthus tuberosus L. fermented with different lactobacilli on acrylamide content in biscuits. Int. J. Food Sci. Technol. 2015, 50, 431–439. [Google Scholar] [CrossRef]
- Fashandi, H.M.; Abbasi, R.; Khaneghah, A.M. The detoxification of aflatoxin M-1 by Lactobacillus acidophilus and Bifidobacterium spp.: A review. J. Food Process Pres. 2018, 42, e13704. [Google Scholar] [CrossRef]
- Zhang, X.B.; Ohta, Y. Binding of mutagens by fractions of the cell wall skeleton of lactic acid bacteria on mutagens. J. Dairy Sci. 1991, 74, 1477–1481. [Google Scholar] [CrossRef]
- Lahtinen, S.J.; Haskard, C.A.; Ouwehand, A.C.; Salminen, S.J.; Ahokas, J.T. Binding of aflatoxin B1 to cell wall components of Lactobacillus rhamnosus strain GG. Food Addit. Contam. 2004, 21, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.L.; Wei, J.Y.; Pan, X.; Jie, Y.; Zhu, B.Q.; Zhao, H.F.; Zhang, B.L. Critical analysis of peptidoglycan structure of Lactobacillus acidophilus for phthalate removal. Chemosphere 2021, 282, 130982. [Google Scholar] [CrossRef]
- Goyal, S.P.; Agarwal, T.; Mishra, V.; Kumar, A.; Saravanan, C. Adsorption characterization of Lactobacillus sp. for Di-(2-ethylhexyl) phthalate. Probiotics Antimicro. Prot. 2023, 16, 519–530. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, D.D.; Guo, Y.X.; Sun, Y.Y.; Zeng, X.Q. Peptidoglycan diversity and anti-inflammatory capacity in Lactobacillus strains. Carbohydr. Polym. 2015, 128, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhao, S.J.; Zhao, X.D.; Sun, H.Y.; Shao, M.L.; Xu, H.H. In vitro adsorption mechanism of acrylamide by lactic acid bacteria. LWT-Food Sci. Technol. 2019, 100, 119–125. [Google Scholar] [CrossRef]
- Albedwawi, A.S.; Al Sakkaf, R.; Osaili, T.M.; Yusuf, A.; Olaimat, A.; Liu, S.Q.; Palmisano, G.; Shah, N.P.; Ayyash, M.M. Investigating acrylamide mitigation by potential probiotics Bifidobacterium breve and: Optimization, in vitro gastrointestinal conditions, and mechanism. LWT-Food Sci. Technol. 2022, 163, 113553. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, W.; Li, L.; Zhao, H.Y.; Sun, H.Y.; Meng, M.H.; Zhang, S.; Shao, M.L. Key role of peptidoglycan on acrylamide binding by lactic acid bacteria. Food Sci. Biotechnol. 2017, 26, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Albedwawi, A.S.; Al Sakkaf, R.; Yusuf, A.; Osaili, T.M.; Al-Nabulsi, A.; Liu, S.Q.; Palmisano, G.; Ayyash, M.M. Acrylamide elimination by lactic acid bacteria: Screening, optimization, in vitro digestion and mechanism. Microorganisms 2022, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Pan, D.D.; Guo, Y.X.; Zeng, X.Q. Structure and anti-inflammatory capacity of peptidoglycan from Lactobacillus acidophilus in RAW-264.7 cells. Carbohydr. Polym. 2013, 96, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Vemula, H.; Ayon, N.J.; Gutheil, W.G. Cytoplasmic peptidoglycan intermediate levels in Staphylococcus aureus. Biochimie 2016, 121, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ge, N.; Xu, J.J.; Peng, B.Z.; Pan, S.Y. Adsorption mechanism of tenuazonic acid using inactivated lactic acid bacteria. Food Control 2017, 82, 274–282. [Google Scholar] [CrossRef]
- Meroueh, S.O.; Bencze, K.Z.; Hesek, D.; Lee, M.; Fisher, J.F.; Stemmler, T.L.; Mobashery, S. Three-dimensional structure of the bacterial cell wall peptidoglycan. Proc. Natl. Acad. Sci. USA 2006, 103, 4404–4409. [Google Scholar] [CrossRef]
- Koch, D.; Schleifer, K.H.; Kandler, O. The amino acid sequence of the serine and aspartic acid containing mureins of Bifidobacterium bifidum Orla Jensen. Z. Für Naturforschung B 1970, 25, 1294–1301. [Google Scholar] [CrossRef]
- Plapp, R.; Kandler, O. Isolation of an ornithine-containing cell wall precursor of Lactobacillus cellobiosus. Biochem. Biophys. Res. Commun. 1967, 28, 141–145. [Google Scholar] [CrossRef]
- Schleifer, K.H.; Kandler, O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Coyette, J.; Ghuysen, J.M. Structure of the walls of Lactobacillus acidophilus strain 63 AM Gasser. Biochemistry 1970, 9, 2935–2943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Xiao, Y.C.; Chung, T.S.; Jiang, J.W. Mechanistic understanding of CO2-induced plasticization of a polyimide membrane: A combination of experiment and simulation study. Polymer 2010, 51, 4439–4447. [Google Scholar] [CrossRef]
- Tang, X.M.; Koenig, P.H.; Larson, R.G. Molecular dynamics simulations of sodium dodecyl sulfate micelles in water-the effect of the force field. J. Phys. Chem. B 2014, 118, 3864–3880. [Google Scholar] [CrossRef] [PubMed]
- Ramstedt, M.; Leone, L.; Persson, P.; Shchukarev, A. Cell wall composition of Bacillus subtilis changes as a function of pH and Zn2+ exposure: Insights from Cryo-XPS measurements. Langmuir 2014, 30, 4367–4374. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.X.; Feng, C.H.; Zhou, W.J.; Yu, H. Municipal sludge-derived carbon anode with nitrogen- and oxygen-containing functional groups for high-performance microbial fuel cells. J. Power Sources 2016, 307, 105–111. [Google Scholar] [CrossRef]
- Sun, G.L.; Yu, P.; Ruan, Q.; Li, D.T.; Cheng, C.G. Studies on the Herba Plantaginis of different kinds and habitats by FTIR reflection spectroscopy-chemometrics analysis. Spectrosc. Spectr. Anal. 2009, 29, 1822–1825. [Google Scholar]
- Jain, M.; Garg, V.K.; Kadirvelu, K. Chromium(VI) removal from aqueous system using Helianthus annuus (sunflower) stem waste. J. Hazard. Mater. 2009, 162, 365–372. [Google Scholar] [CrossRef]
- Yin, H.; He, B.; Peng, H.; Ye, J.; Yang, F.; Zhang, N. Removal of Cr(VI) and Ni(II) from aqueous solution by fused yeast: Study of cations release and biosorption mechanism. J. Hazard. Mater. 2008, 158, 568–576. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.L. Removal of Pb2+, Ag+, Cs+ and Sr2+ from aqueous solution by brewery’s waste biomass. J. Hazard. Mater. 2008, 151, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Jimenez, L.; Ramirez-Ortiz, K.; Gonzalez-Cordova, A.F.; Vallejo-Cordoba, B.; Garcia, H.S.; Hernandez-Mendoza, A. Evaluation of acrylamide-removing properties of two Lactobacillus strains under simulated gastrointestinal conditions using a dynamic system. Microbiol. Res. 2016, 190, 19–26. [Google Scholar] [CrossRef]
- Frateur, I.; Lecoeur, J.; Zanna, S.; Olsson, C.O.A.; Landolt, D.; Marcus, P. Adsorption of BSA on passivated chromium studied by a flow-cell EQCM and XPS. Electrochim. Acta 2007, 52, 7660–7669. [Google Scholar] [CrossRef]
- Szczes, A.; Czemierska, M.; Jarosz-Wilkolazka, A. Calcium carbonate formation on mica supported extracellular polymeric substance produced by Rhodococcus opacus. J. Solid State Chem. 2016, 242, 212–221. [Google Scholar] [CrossRef]
- Bautista, B.E.T.; Wikiel, A.J.; Datsenko, I.; Vera, M.; Sand, W.; Seyeux, A.; Zanna, S.; Frateur, I.; Marcus, P. Influence of extracellular polymeric substances (EPS) from Pseudomonas NCIMB 2021 on the corrosion behaviour of 70Cu-30Ni alloy in seawater. J. Electroanal. Chem. 2015, 737, 184–197. [Google Scholar] [CrossRef]
- Feng, L.J.; Wang, J.J.; Liu, S.C.; Sun, X.D.; Yuan, X.Z.; Wang, S.G. Role of extracellular polymeric substances in the acute inhibition of activated sludge by polystyrene nanoparticles. Environ. Pollut. 2018, 238, 859–865. [Google Scholar] [CrossRef]
- Sun, X.F.; Wang, S.G.; Zhang, X.M.; Chen, J.P.; Li, X.M.; Gao, B.Y.; Ma, Y. Spectroscopic study of Zn2+ and Co2+ binding to extracellular polymeric substances (EPS) from aerobic granules. J. Colloid Interface Sci. 2009, 335, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.P.; Zhang, Y.; Chen, Y.H.; Han, Z.J.; Chen, X.M.; Peng, Y.; Chen, L.; Chen, S. Molecular dynamics simulation of the adsorption properties of graphene oxide/graphene composite for alkali metal ions. J. Mol. Graph. Model. 2022, 114, 108184. [Google Scholar] [CrossRef]
- Zhao, L.L.; Wei, J.Y.; Zhao, H.F.; Zhu, B.Q.; Zhang, B.L. Detoxification of cancerogenic compounds by lactic acid bacteria strains. Crit. Rev. Food Sci. 2018, 58, 2727–2742. [Google Scholar] [CrossRef]
- Song, M.; Zhao, X.Y.; Li, Y.; Hu, S.K.; Zhang, L.Q.; Wu, S.Z. Molecular dynamics simulations and microscopic analysis of the damping performance of hindered phenol AO-60/nitrile-butadiene rubber composites. RSC Adv. 2014, 4, 6719–6729. [Google Scholar] [CrossRef]
- Lai, J.H.; Zhu, Y.T.; Liao, X.D.; Zhang, M.M.; Li, J.L.; Li, Q.; Liu, A.P.; He, L.; Liu, S.L. Cell components, interaction types and functional groups involved in the in vitro binding of bisphenol A by Lactiplantibacillus plantarum RS20D and DL7X. J. Appl. Microbiol. 2022, 132, 2968–2979. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Bertsche, U. Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochim. Biophys. Acta–Biomembr. 2008, 1778, 1714–1734. [Google Scholar] [CrossRef] [PubMed]
- Ramstedt, M.; Nakao, R.; Wai, S.N.; Uhlin, B.E.; Boily, J.F. Monitoring surface chemical changes in the bacterial cell wall multivariate analysis of cryo-x-ray photoelectron spectroscopy data. J. Biol. Chem. 2011, 286, 12389–12396. [Google Scholar] [CrossRef] [PubMed]
 B. lactis B1-04;
B. lactis B1-04;  B. breve CICC 6079;
B. breve CICC 6079;  L. acidophilus NCFM;
L. acidophilus NCFM;  L. plantarum CICC 22135. (C,D) The binding rate of the four selected strains and their PGNs to AA/HMF under the condition of co-culture for 6 h; bars with different letters are statistically different from each other (p < 0.05).
L. plantarum CICC 22135. (C,D) The binding rate of the four selected strains and their PGNs to AA/HMF under the condition of co-culture for 6 h; bars with different letters are statistically different from each other (p < 0.05).  the binding rate of strains;
the binding rate of strains;  the binding rate of PGNs.
the binding rate of PGNs.
 B. lactis B1-04;
B. lactis B1-04;  B. breve CICC 6079;
B. breve CICC 6079;  L. acidophilus NCFM;
L. acidophilus NCFM;  L. plantarum CICC 22135. (C,D) The binding rate of the four selected strains and their PGNs to AA/HMF under the condition of co-culture for 6 h; bars with different letters are statistically different from each other (p < 0.05).
L. plantarum CICC 22135. (C,D) The binding rate of the four selected strains and their PGNs to AA/HMF under the condition of co-culture for 6 h; bars with different letters are statistically different from each other (p < 0.05).  the binding rate of strains;
the binding rate of strains;  the binding rate of PGNs.
the binding rate of PGNs.

| Strains | Simulation System | Total Energy (kcal/mol) |
|---|---|---|
| EAA | 24.613 | |
| EHMF | 28.193 | |
| B. lactis | EPGN1 | 571.464 |
| EPGN1+AA | 843.164 | |
| EPGN1+HMF | 892.950 | |
| △EA1 | 247.087 | |
| △EH1 | 293.293 | |
| B. breve | EPGN2 | 482.068 |
| EPGN2+AA | 707.387 | |
| EPGN2+HMF | 768.930 | |
| △EA2 | 200.706 | |
| △EH2 | 258.669 | |
| L. acidophilus | EPGN3 | 496.291 |
| EPGN3+AA | 743.481 | |
| EPGN3+HMF | 828.534 | |
| △EA3 | 222.577 | |
| △EH3 | 273.210 | |
| L. plantarum | EPGN4 | 530.694 |
| EPGN4+AA | 737.666 | |
| EPGN4+HMF | 768.596 | |
| △EA4 | 182.359 | |
| △EH4 | 247.675 |
| Bacterial Species | Interaction Energy I (1) (kcal/mol) | FFVⅠ(%) | Interaction Energy II (2) (kcal/mol) | FFV II (%) |
|---|---|---|---|---|
| B. lactis B1-04 | 247.09 | 24.12 | 293.29 | 22.10 |
| B. breve CICC 6079 | 200.71 | 29.81 | 258.67 | 27.88 |
| L. acidophilus NCFM | 222.57 | 27.09 | 273.21 | 26.11 |
| L. plantarum CICC 22135 | 182.36 | 33.67 | 247.68 | 28.12 |
| Peak | Binding Energy (eV) | Proportion of Fitted Peak Area (%) | Group | |||
|---|---|---|---|---|---|---|
| B. lactis B1-04 | B. breve CICC 6079 | L. acidophilus NCFM | L. plantarum CICC 22135 | |||
| C1 | 284.28–284.45 | 1,935,134 (58.1%) | 20,984,085 (62.98%) | 20,454,318 (61.39%) | 217,670,731 (65.33%) | C-C/C-H |
| C2 | 286.75–286.92 | 9,529,133 (28.6%) | 8,586,216 (25.77%) | 8,906,075 (26.73%) | 7,899,851 (23.71%) | C-O |
| C3 | 287.56–287.86 | 4,431,380 (13.3%) | 3,748,348 (11.25%) | 3,958,255 (11.88%) | 3,651,724 (10.96%) | C=O |
| Peak | Binding Energy (eV) | Proportion of Fitted Peak Area (%) | Group | |||
|---|---|---|---|---|---|---|
| B. lactis B1-04 | B. breve CICC 6079 | L. acidophilus NCFM | L. plantarum CICC 22135 | |||
| N1 | 399.27–399.34 | 41,658 (72.11%) | 38,897 (67.33%) | 40,549 (70.19%) | 38,776 (67.12%) | N-H |
| N2 | 400.01–401.05 | 116,112 (27.89%) | 18,874 (32.67%) | 17,221 (29.81%) | 18,995 (32.88%) | C-N |
| Peak | Binding Energy (eV) | Proportion of Fitted Peak Area (%) | Group | |||
|---|---|---|---|---|---|---|
| B. lactis B1-04 | B. Breve CICC 6079 | L. acidophilus NCFM | L. plantarum CICC 22135 | |||
| O1 | 532.15–532.37 | 77,819 (30.87%) | 86,062 (34.14%) | 78,878 (31.29%) | 82,205 (32.61%) | -O/O-H |
| O2 | 531.18–531.56 | 174,267 (69.13%) | 166,024 (65.86%) | 173,209 (68.71%) | 169,881 (67.39%) | C-O/C=O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, X.; Zhu, Y.; Zhang, B.; Fan, J.; Zhao, H.; Zhang, B. Utilization of Peptidoglycans from Lactic Acid Bacterial Cell Walls for the Mitigation of Acrylamide and 5-Hydroxymethylfurfural. Toxics 2024, 12, 380. https://doi.org/10.3390/toxics12060380
Yang H, Zhang X, Zhu Y, Zhang B, Fan J, Zhao H, Zhang B. Utilization of Peptidoglycans from Lactic Acid Bacterial Cell Walls for the Mitigation of Acrylamide and 5-Hydroxymethylfurfural. Toxics. 2024; 12(6):380. https://doi.org/10.3390/toxics12060380
Chicago/Turabian StyleYang, Hui, Xue Zhang, Yadong Zhu, Bo Zhang, Junfeng Fan, Hongfei Zhao, and Bolin Zhang. 2024. "Utilization of Peptidoglycans from Lactic Acid Bacterial Cell Walls for the Mitigation of Acrylamide and 5-Hydroxymethylfurfural" Toxics 12, no. 6: 380. https://doi.org/10.3390/toxics12060380





