Associations between PM2.5 Components and Mortality of Ischemic Stroke, Chronic Obstructive Pulmonary Disease and Diabetes in Beijing, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Data Collection
2.2.1. Exposure Measurement
2.2.2. Outcome
2.2.3. Covariates
2.3. Statistical Analysis
2.3.1. Calculation of Attributable Fractions and Numbers
2.3.2. Sensitivity Analysis
3. Results
3.1. Descriptive Statistics
3.2. Associations between PM2.5 Components and Cause-Specific Mortality
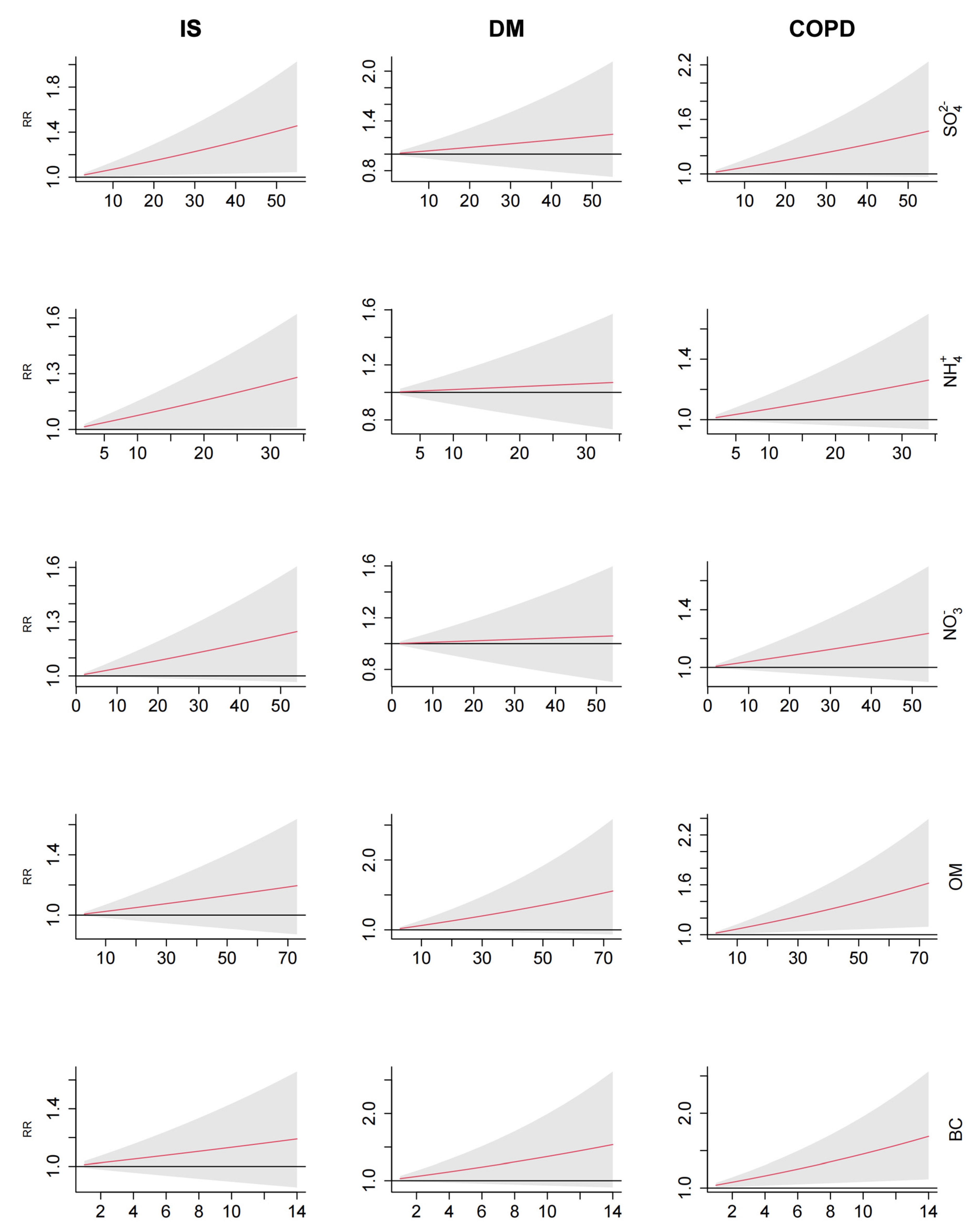
3.3. Exposure–response Relationships
3.4. Attributable Fractions (AF) and Numbers (AN)
3.5. Stratified Analysis
3.6. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 12 December 2023).
- Su, B.; Guo, S.; Zheng, X. Transitions in Chronic Disease Mortality in China: Evidence and Implications. China CDC Wkly 2023, 5, 1131–1134. [Google Scholar] [CrossRef]
- Liu, L.; Wang, D.; Wong, K.S.; Wang, Y. Stroke and stroke care in China: Huge burden, significant workload, and a national priority. Stroke 2011, 42, 3651–3654. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Advancing COPD care in China through a comprehensive approach. Available online: https://www.who.int/news-room/feature-stories/detail/advancing-copd-care-in-china-through-a-comprehensive-approach (accessed on 12 January 2024).
- Wang, L.; Peng, W.; Zhao, Z.; Zhang, M.; Shi, Z.; Song, Z.; Zhang, X.; Li, C.; Huang, Z.; Sun, X.; et al. Prevalence and Treatment of Diabetes in China, 2013–2018. JAMA 2021, 326, 2498–2506. [Google Scholar] [CrossRef]
- Wang, F.; Wang, W.; Yin, P.; Liu, Y.; Liu, J.; Wang, L.; Qi, J.; You, J.; Lin, L.; Zhou, M. Mortality and Years of Life Lost in Diabetes Mellitus and Its Subcategories in China and Its Provinces, 2005–2020. J Diabetes Res 2022, 2022, 1609267. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.; Landrigan, P.J.; Balakrishnan, K.; Bathan, G.; Bose-O’Reilly, S.; Brauer, M.; Caravanos, J.; Chiles, T.; Cohen, A.; Corra, L.; et al. Pollution and health: A progress update. Lancet Planet Health 2022, 6, e535–e547. [Google Scholar] [CrossRef]
- Li, B.; Ma, Y.; Zhou, Y.; Chai, E. Research progress of different components of PM2.5 and ischemic stroke. Sci Rep 2023, 13, 15965. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Yan, Y.; Al-Aly, Z. Burden of Cause-Specific Mortality Associated With PM2.5 Air Pollution in the United States. JAMA Netw Open 2019, 2, e1915834. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.T.; Lang, C.F.; Chen, C.; Harry, A.M.; Fang, Y.; Zhang, R.D.; Jiang, L.Q.; Fang, X.; Chen, Y.; He, Y.S.; et al. Association between air pollution exposure and coronary heart disease hospitalization in a humid sub-tropical region of China: A time-series study. Front Public Health 2022, 10, 1090443. [Google Scholar] [CrossRef]
- Luo, K.; Li, R.; Li, W.; Wang, Z.; Ma, X.; Zhang, R.; Fang, X.; Wu, Z.; Cao, Y.; Xu, Q. Acute Effects of Nitrogen Dioxide on Cardiovascular Mortality in Beijing: An Exploration of Spatial Heterogeneity and the District-specific Predictors. Sci. Rep. 2016, 6, 38328. [Google Scholar] [CrossRef]
- Wang, H.T.; Zhang, H.; Xue, F.Z.; Zhao, L.; Cao, W.C. Associations of air pollutants with pneumonia hospital admissions in Qingdao, China: A prospective cohort study. Environ. Sci. Pollut. Res. Int. 2022, 29, 27779–27787. [Google Scholar] [CrossRef]
- Wang, B.; Eum, K.D.; Kazemiparkouhi, F.; Li, C.; Manjourides, J.; Pavlu, V.; Suh, H. The impact of long-term PM2.5 exposure on specific causes of death: Exposure-response curves and effect modification among 53 million U.S. Medicare beneficiaries. Environ. Health 2020, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, S.; Kioumourtzoglou, M.A.; Wu, C.D.; Schwartz, J.D.; Koutrakis, P.; Papatheodorou, S.I. Acute effects of fine particulate matter constituents on mortality: A systematic review and meta-regression analysis. Environ. Int. 2017, 109, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Chen, Y.; Su, S.; Zhang, X.; Lin, X. Acute effect of fine particulate matter and respiratory mortality in Changsha, China: A time-series analysis. BMC Pulm. Med. 2022, 22, 416. [Google Scholar] [CrossRef] [PubMed]
- McDuffie, E.; Martin, R.; Yin, H.; Brauer, M. Global Burden of Disease from Major Air Pollution Sources (GBD MAPS): A Global Approach. Res.Rep. Health Eff. Inst. 2021, 210, 1–45. [Google Scholar]
- Lang, J.; Zhang, Y.; Zhou, Y.; Cheng, S.; Chen, D.; Guo, X.; Chen, S.; Li, X.; Xing, X.; Wang, H. Trends of PM2.5 and Chemical Composition in Beijing, 2000–2015. Aerosol Air Qual. Res. 2017, 17, 412–425. [Google Scholar] [CrossRef]
- Yang, Y.; Ruan, Z.; Wang, X.; Yang, Y.; Mason, T.G.; Lin, H.; Tian, L. Short-term and long-term exposures to fine particulate matter constituents and health: A systematic review and meta-analysis. Environ. Pollut. 2019, 247, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, B.; Jiang, Y.; Wang, X.; Chen, L.; Wang, X.; Chen, T.; Yang, L.; Silang, Y.; Hong, F.; et al. Long-term Exposure to Ambient PM2.5 and Its Components Associated With Diabetes: Evidence From a Large Population-Based Cohort From China. Diabetes Care 2023, 46, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S. The Effects and Pathogenesis of PM2.5 and Its Components on Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2023, 18, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Wyzga, R.E.; Rohr, A.C. Long-term particulate matter exposure: Attributing health effects to individual PM components. J. Air Waste Manag. Assoc. 2015, 65, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Sacks, J.; Buckley, B.; Deflorio-Barker, S.; Jenkins, S.; Kirrane, E.; Krajewski, A.; Luben, T.; McDow, S.; Stewart, M.; Dubois, J.J.; et al. Supplement to the 2019 Integrated Science Assessment for Particulate Matter; U.S. Environmental Protection Agency: Research Triangle Park, NC, USA, 2022. [Google Scholar]
- Liu, S.; Geng, G.; Xiao, Q.; Zheng, Y.; Liu, X.; Cheng, J.; Zhang, Q. Tracking Daily Concentrations of PM2.5 Chemical Composition in China since 2000. Environ. Sci. Technol. 2022, 56, 16517–16527. [Google Scholar] [CrossRef]
- Geng, G.; Zhang, Q.; Tong, D.; Li, M.; Zheng, Y.; Wang, S.; He, K. Chemical composition of ambient PM2. 5 over China and relationship to precursor emissions during 2005–2012. Atmos Chem. Phys. 2017, 17, 9187–9203. [Google Scholar] [CrossRef]
- Gou, A.; Tan, G.; Ding, X.; Wang, J.; Lv, X.; Gou, C.; Tan, Q. Urban-rural difference in the lagged effects of PM2.5 and PM10 on COPD mortality in Chongqing, China. BMC Public Health 2023, 23, 1270. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yan, Y.; Chen, X.; Gong, J.; Guo, Y.; Zhao, Y.; Yang, N.; Dai, J.; Zhang, F.; Xiang, H. Short-term exposure to ambient air pollution and type 2 diabetes mortality: A population-based time series study. Environ. Pollut. 2021, 289, 117886. [Google Scholar] [CrossRef] [PubMed]
- Di, Q.; Dai, L.; Wang, Y.; Zanobetti, A.; Choirat, C.; Schwartz, J.D.; Dominici, F. Association of Short-term Exposure to Air Pollution With Mortality in Older Adults. JAMA 2017, 318, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, K.K.; Ethan, C.J.; Yu, Y.; Shale, K.; Liu, F. Ambient air pollution and respiratory mortality in Xi’an, China: A time-series analysis. Respir. Res. 2019, 20, 139. [Google Scholar] [CrossRef]
- Gasparrini, A.; Guo, Y.; Hashizume, M.; Lavigne, E.; Zanobetti, A.; Schwartz, J.; Tobias, A.; Tong, S.; Rocklov, J.; Forsberg, B.; et al. Mortality risk attributable to high and low ambient temperature: A multicountry observational study. Lancet 2015, 386, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.; Armstrong, B.; Kenward, M.G. Distributed lag non-linear models. Stat. Med. 2010, 29, 2224–2234. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.; Leone, M. Attributable risk from distributed lag models. BMC Med. Res. Methodol. 2014, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Chen, R.; Yin, P.; van Donkelaar, A.; Martin, R.V.; Burnett, R.; Cohen, A.J.; Brauer, M.; Liu, C.; Wang, W.; et al. Associations of long-term exposure to fine particulate matter and its constituents with cardiovascular mortality: A prospective cohort study in China. Environ. Int. 2022, 162, 107156. [Google Scholar] [CrossRef]
- Rodins, V.; Lucht, S.; Ohlwein, S.; Hennig, F.; Soppa, V.; Erbel, R.; Jockel, K.H.; Weimar, C.; Hermann, D.M.; Schramm, S.; et al. Long-term exposure to ambient source-specific particulate matter and its components and incidence of cardiovascular events-The Heinz Nixdorf Recall study. Environ. Int. 2020, 142, 105854. [Google Scholar] [CrossRef]
- Cai, M.; Lin, X.; Wang, X.; Zhang, S.; Wang, C.; Zhang, Z.; Pan, J.; Lin, H. Long-term exposure to ambient fine particulate matter chemical composition and in-hospital case fatality among patients with stroke in China. Lancet Reg. Health West. Pac. 2023, 32, 100679. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, C.W.; Fu, Y.Y.; Li, Y.Z.; Chen, L.; Zhang, Q.W.; Chen, Y.F. Global, regional, and national burden of chronic respiratory diseases and associated risk factors, 1990-2019: Results from the Global Burden of Disease Study 2019. Front. Med. 2023, 10, 1066804. [Google Scholar] [CrossRef]
- Safiri, S.; Carson-Chahhoud, K.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Ahmadian Heris, J.; Ansarin, K.; Mansournia, M.A.; Collins, G.S.; Kolahi, A.A.; et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. BMJ 2022, 378, e69679. [Google Scholar] [CrossRef]
- Atkinson, R.W.; Fuller, G.W.; Anderson, H.R.; Harrison, R.M.; Armstrong, B. Urban ambient particle metrics and health: A time-series analysis. Epidemiology 2010, 21, 501–511. [Google Scholar] [CrossRef]
- Brook, R.D.; Cakmak, S.; Turner, M.C.; Brook, J.R.; Crouse, D.L.; Peters, P.A.; van Donkelaar, A.; Villeneuve, P.J.; Brion, O.; Jerrett, M.; et al. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care 2013, 36, 3313–3320. [Google Scholar] [CrossRef]
- Strak, M.; Weinmayr, G.; Rodopoulou, S.; Chen, J.; de Hoogh, K.; Andersen, Z.J.; Atkinson, R.; Bauwelinck, M.; Bekkevold, T.; Bellander, T.; et al. Long term exposure to low level air pollution and mortality in eight European cohorts within the ELAPSE project: Pooled analysis. BMJ 2021, 374, n1904. [Google Scholar] [CrossRef]
- Rohr, A.C.; Wyzga, R.E. Attributing health effects to individual particulate matter constituents. Atmos. Environ. 2012, 62, 130–152. [Google Scholar] [CrossRef]
- Ringbaek, T.; Seersholm, N.; Viskum, K. Standardised mortality rates in females and males with COPD and asthma. Eur. Respir J. 2005, 25, 891–895. [Google Scholar] [CrossRef]
- Yang, Y.; Qi, J.; Ruan, Z.; Yin, P.; Zhang, S.; Liu, J.; Liu, Y.; Li, R.; Wang, L.; Lin, H. Changes in Life Expectancy of Respiratory Diseases from Attaining Daily PM2.5 Standard in China: A Nationwide Observational Study. Innovation 2020, 1, 100064. [Google Scholar] [CrossRef]
- Orellano, P.; Reynoso, J.; Quaranta, N.; Bardach, A.; Ciapponi, A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ. Int. 2020, 142, 105876. [Google Scholar] [CrossRef]
- Samoli, E.; Analitis, A.; Touloumi, G.; Schwartz, J.; Anderson, H.R.; Sunyer, J.; Bisanti, L.; Zmirou, D.; Vonk, J.M.; Pekkanen, J.; et al. Estimating the exposure-response relationships between particulate matter and mortality within the APHEA multicity project. Environ. Health Perspect. 2005, 113, 88–95. [Google Scholar] [CrossRef] [PubMed]
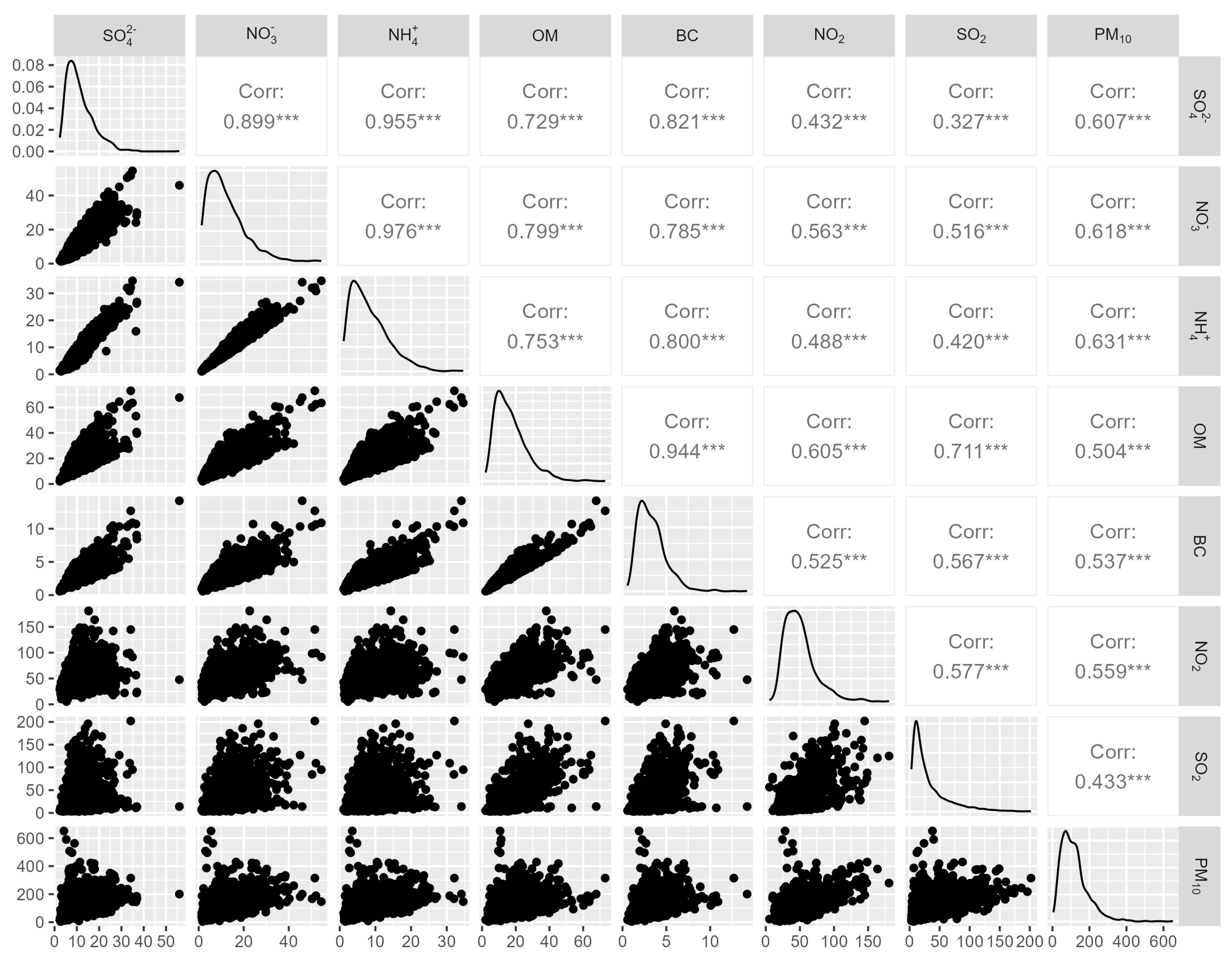

| Variable | Mean | SD | MAD | Min | P25 | P50 | P75 | IQR | Max |
|---|---|---|---|---|---|---|---|---|---|
| Daily death counts | |||||||||
| IS | 15 | 5 | 4 | 4 | 11 | 14 | 18 | 7 | 36 |
| COPD | 9 | 4 | 3 | 1 | 6 | 8 | 11 | 5 | 27 |
| DM | 5 | 2 | 3 | 0 | 4 | 5 | 7 | 3 | 14 |
| PM2.5 components (μg/m3) | |||||||||
| SO42− | 11.24 | 6.02 | 5.28 | 2.34 | 6.73 | 9.79 | 14.26 | 7.53 | 55.98 |
| NH4+ | 8.37 | 5.30 | 5.03 | 1.10 | 4.18 | 7.19 | 11.31 | 7.13 | 34.62 |
| NO3− | 12.00 | 8.00 | 7.11 | 1.34 | 6.01 | 10.08 | 16.24 | 10.23 | 54.44 |
| OM | 17.34 | 9.90 | 8.87 | 2.09 | 10.02 | 15.31 | 22.35 | 12.33 | 73.06 |
| BC | 3.32 | 1.60 | 1.50 | 0.58 | 2.12 | 3.06 | 4.18 | 2.06 | 14.21 |
| Other air pollutants (μg/m3) | |||||||||
| NO2 | 49.82 | 23.13 | 19.42 | 5.37 | 33.17 | 45.90 | 60.00 | 26.83 | 180.67 |
| SO2 | 32.27 | 31.66 | 16.31 | 3.00 | 10.96 | 20.00 | 42.00 | 31.04 | 202.00 |
| PM10 | 117.75 | 74.14 | 60.31 | 4.91 | 65.09 | 104.00 | 146.55 | 81.46 | 651.18 |
| Meteorological factors | |||||||||
| Temp (°C) | 13.21 | 11.34 | 14.97 | −12.50 | 2.20 | 14.90 | 24.00 | 21.8 | 34.50 |
| Humd (%) | 50.87 | 19.97 | 25.20 | 9.00 | 34.00 | 52.00 | 67.00 | 33 | 95.00 |
| Pressure (hPa) | 1012.42 | 10.28 | 12.16 | 989.70 | 1004.10 | 1011.80 | 1020.50 | 16.4 | 1039.30 |
| Air Pollution | IS | COPD | DM | |||
|---|---|---|---|---|---|---|
| AN | AF (95% CI) | AN | AF (95% CI) | AN | AF (95% CI) | |
| SO42− | 1597 | 7.36 (1.03, 13.59) | 1004 | 7.48 (−0.94, 14.63) | 345 | 4.34 (−6.93, 15.02) |
| NH4+ | 1279 | 5.90 (0.29, 11.52) | 741 | 5.53 (−1.78, 12.04) | 142 | 1.79 (−8.09, 10.19) |
| NO3− | 1047 | 4.83 (−0.52, 9.97) | 625 | 4.66 (−1.94, 10.65) | 111 | 1.40 (−8.77, 9.64) |
| OM | 938 | 4.32 (−2.58, 10.91) | 1528 | 11.39 (1.80, 19.65) | 814 | 10.24 (−1.63, 20.69) |
| BC | 900 | 4.15 (−3.95, 12.11) | 1611 | 12.01 (2.16, 20.42) | 783 | 9.85 (−2.78, 20.50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, H.; Yang, Y.; Ye, H.; Xu, J.; Zhao, M.; Jin, Y.; Zhang, S. Associations between PM2.5 Components and Mortality of Ischemic Stroke, Chronic Obstructive Pulmonary Disease and Diabetes in Beijing, China. Toxics 2024, 12, 381. https://doi.org/10.3390/toxics12060381
Feng H, Yang Y, Ye H, Xu J, Zhao M, Jin Y, Zhang S. Associations between PM2.5 Components and Mortality of Ischemic Stroke, Chronic Obstructive Pulmonary Disease and Diabetes in Beijing, China. Toxics. 2024; 12(6):381. https://doi.org/10.3390/toxics12060381
Chicago/Turabian StyleFeng, Hao, Yisen Yang, Hong Ye, Jing Xu, Meiduo Zhao, Ye Jin, and Shuyang Zhang. 2024. "Associations between PM2.5 Components and Mortality of Ischemic Stroke, Chronic Obstructive Pulmonary Disease and Diabetes in Beijing, China" Toxics 12, no. 6: 381. https://doi.org/10.3390/toxics12060381





