A Rapid In Vivo Toxicity Assessment Method for Antimicrobial Peptides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Antimicrobial Activity of the Peptides
2.3. Haemolytic Activity of the Peptides
2.4. Cytotoxicity of the Peptides
2.5. Mouse Acute Lung Injury (ALI) Model
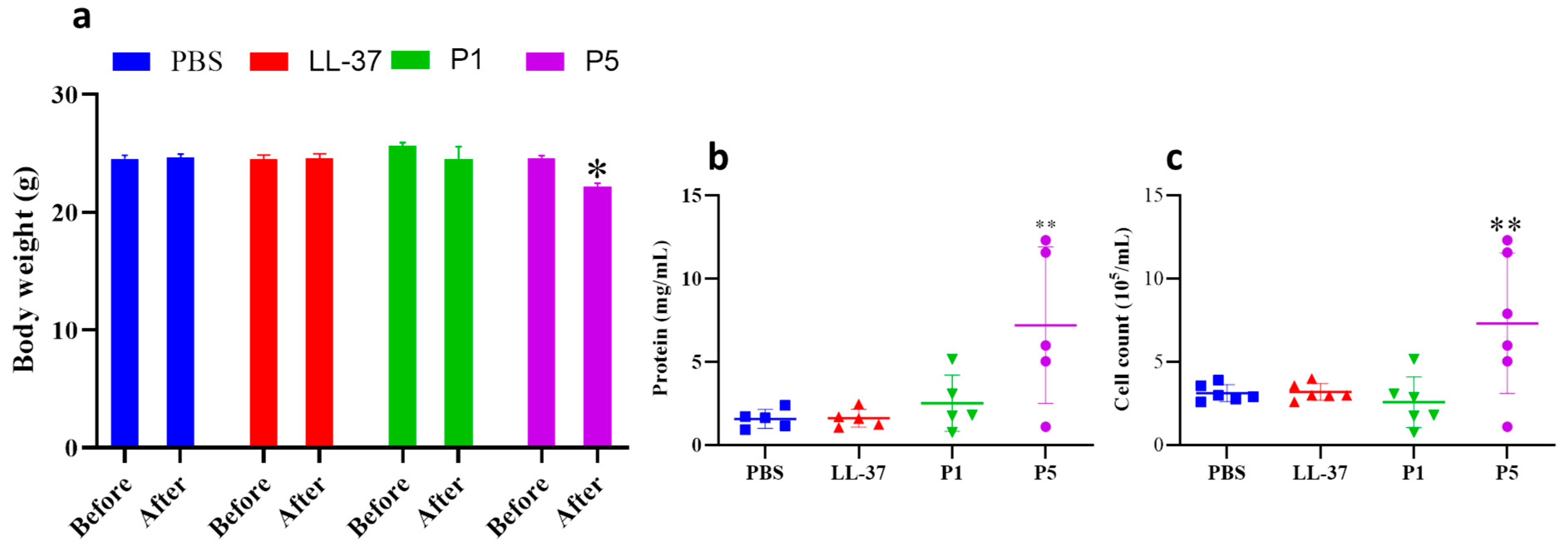
2.6. Bronchoalveolar Lavage
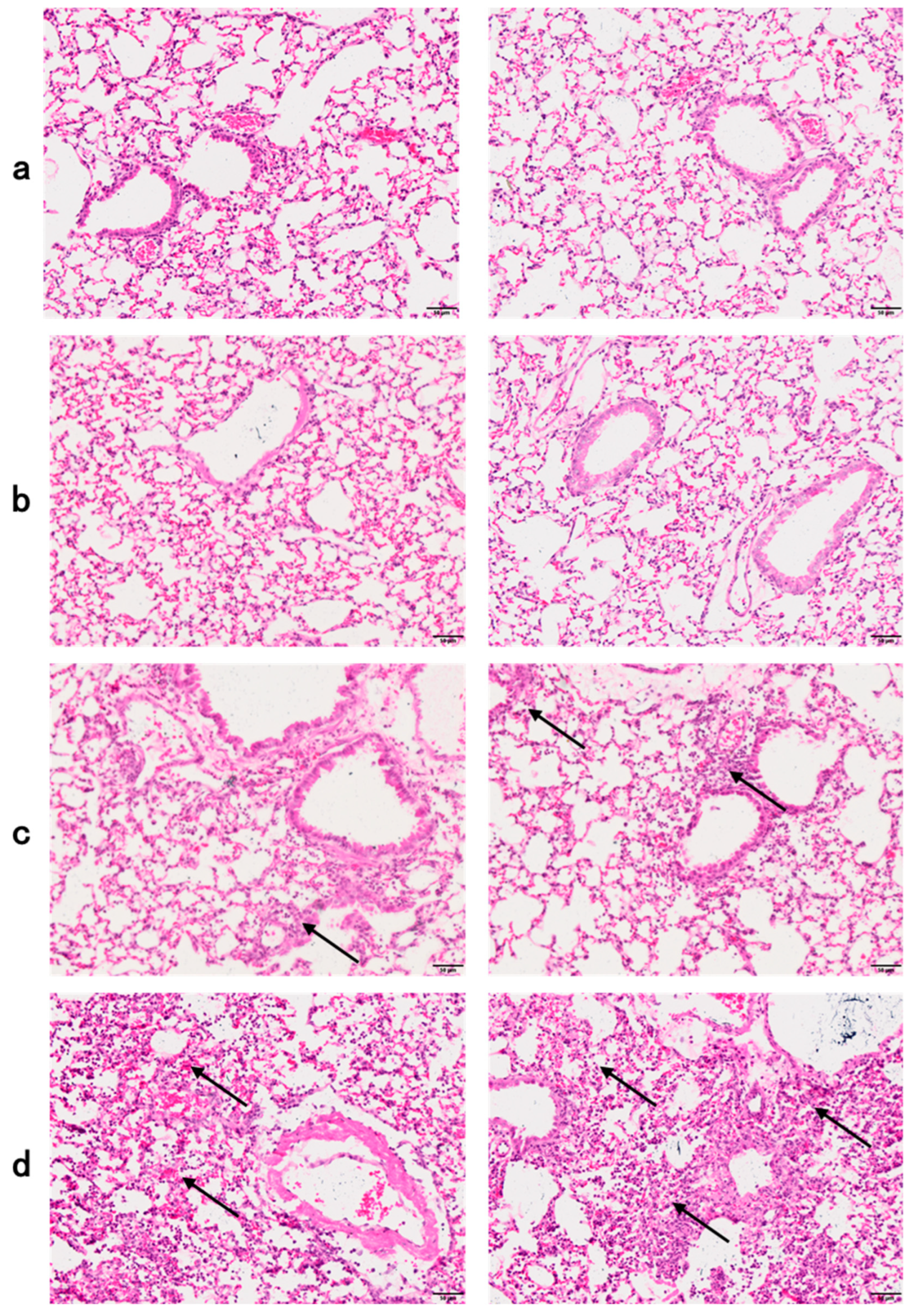
2.7. Histology
2.8. Statistical Analysis
3. Results
3.1. Biophysical Properties of Peptides
3.2. Antimicrobial Activity of Peptides
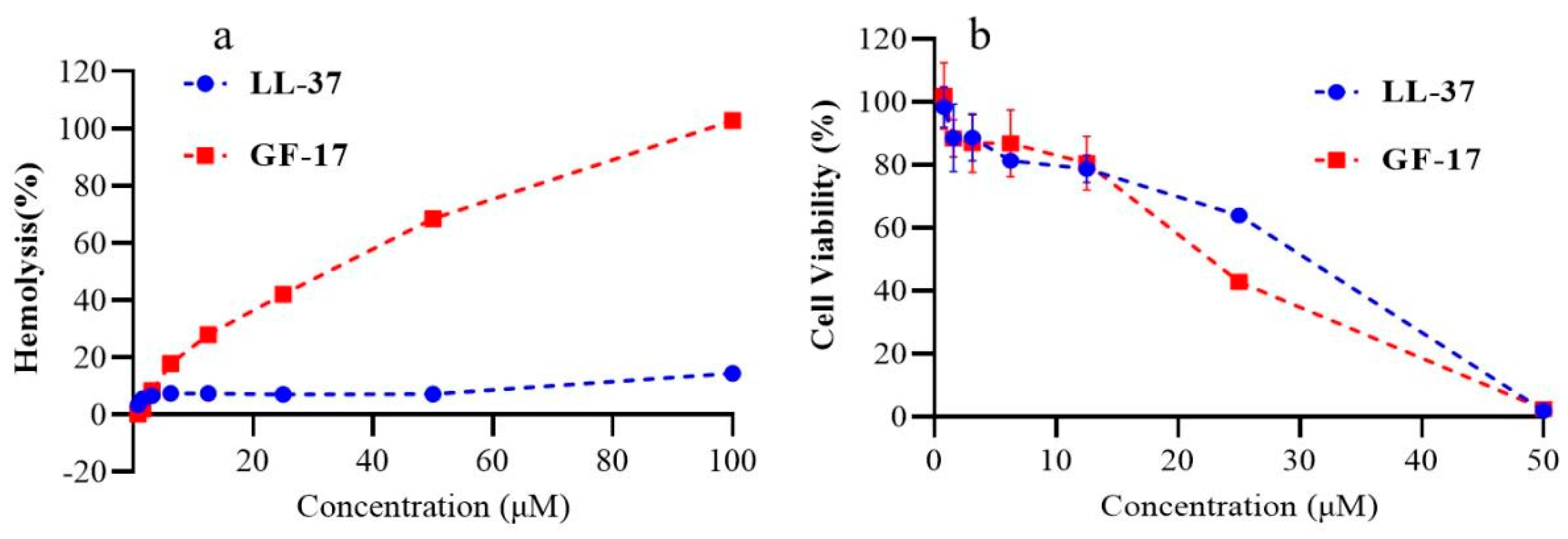
3.3. Haemolytic Activity and Cytotoxicity of Peptides
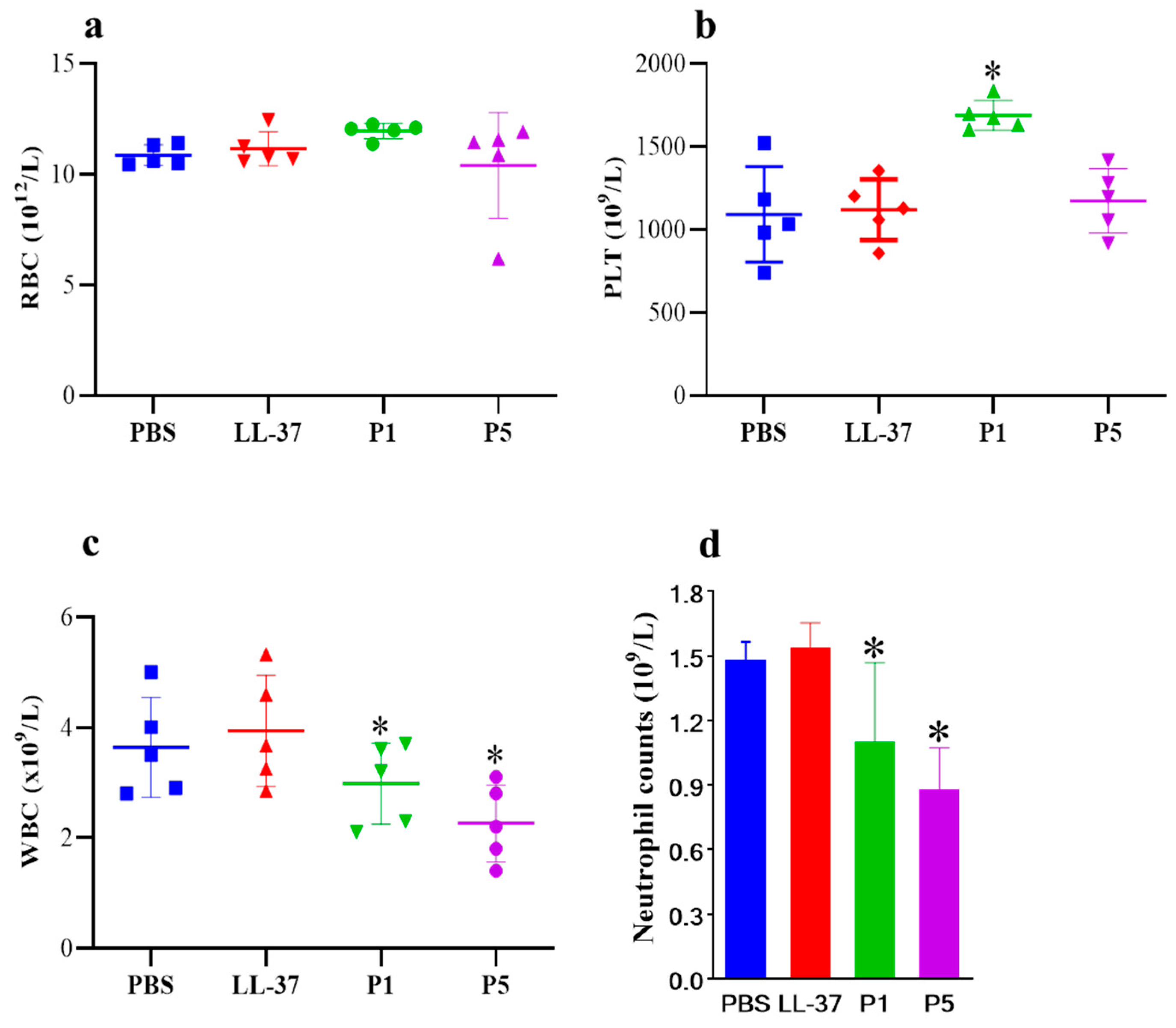
3.4. In Vivo Studies
3.5. Histology of Lung Sections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, Potent Antimicrobial Peptides and the Fight against Multi Drug Resistant Species: Resistance Is Futile? Antibiotics 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, M. Antimicrobial Peptides: From Design to Clinical Application. Antibiotics 2022, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance: Tackling a Crisis for the Future Health and Wealth of Nations. 2014. Available online: http://amr-review.org/ (accessed on 11 March 2015).
- WHO. No Time to Wait: Securing the Future from Drug-Resistant Infections, Report to the Secretary-General of the United Nations. 2019. Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/final-report/en/ (accessed on 5 June 2020).
- Coque, T.M.; Cantón, R.; Pérez-Cobas, A.E.; Fernández-De-Bobadilla, M.D.; Baquero, F. Antimicrobial Resistance in the Global Health Network: Known Unknowns and Challenges for Efficient Responses in the 21st Century. Microorganisms 2023, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Luther, A.; Urfer, M.; Zahn, M.; Müller, M.; Wang, S.Y.; Mondal, M.; Vitale, A.; Hartmann, J.B.; Sharpe, T.; Monte, F.L.; et al. Chimeric peptidomimetic antibiotics against Gram-negative bacteria. Nature 2019, 576, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.; Rastogi, A.; Pandey, S.; Gupta, S.; Sohal, J.S. Multidrug-Resistant Bacteria: Their Mechanism of Action and Prophylaxis. BioMed Res. Int. 2022, 2022, 5419874. [Google Scholar] [CrossRef]
- Gilbert, R.J.C. Peptide-based pore formation and cell membrane deformation: European Biophysics Journal Prizes at EBSA 2023. Eur. Biophys. J. 2023, 52, 619–623. [Google Scholar] [CrossRef]
- Bui Thi Phuong, H.; Doan Ngan, H.; Le Huy, B.; Vu Dinh, H.; Luong Xuan, H. The amphipathic design in helical antimicrobial peptides. ChemMedChem 2024, 19, e202300480. [Google Scholar] [CrossRef]
- Florin, T.; Maracci, C.; Graf, M.; Karki, P.; Klepacki, D.; Berninghausen, O.; Beckmann, R.; Vázquez-Laslop, N.; Wilson, D.N.; Rodnina, M.V.; et al. An antimicrobial peptide that inhibits translation by trapping release factors on the ribosome. Nat. Struct. Mol. Biol. 2017, 24, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.G.; Roy, R.N.; Lomakin, I.B.; Florin, T.; Mankin, A.S.; Steitz, T.A. Structures of proline-rich peptides bound to the ribosome reveal a common mechanism of protein synthesis inhibition. Nucleic Acids Res. 2016, 44, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Separovic, F.; O’Brien-Simpson, N.M.; Wade, J.D. Chemically modified and conjugated antimicrobial peptides against superbugs. Chem. Soc. Rev. 2021, 50, 4932–4973. [Google Scholar] [CrossRef] [PubMed]
- Malins, L.R. Peptide modification and cyclization via transition-metal catalysis. Curr. Opin. Chem. Biol. 2018, 46, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Jayaraman, A.; Van Deventer, J.A.; Lee, K. Engineering Selectively Targeting Antimicrobial Peptides. Annu. Rev. Biomed. Eng. 2021, 23, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Kang, X.; Dong, F.; Liu, Y.; Zhu, N.; Hu, Y.; Xu, H.; Lao, X.; Zheng, H. DRAMP 3.0: An enhanced comprehensive data repository of antimicrobial peptides. Nucleic Acids Res. 2022, 50, D488–D496. Available online: http://dramp.cpu-bioinfor.org (accessed on 20 May 2024). [CrossRef] [PubMed]
- Brook, I.; Wexler, H.M.; Goldstein, E.J.C. Antianaerobic antimicrobials: Spectrum and susceptibility testing. Clin. Microbiol. Rev. 2013, 26, 526–546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-K.; Ma, Q.; Li, S.-B.; Gao, H.-W.; Tan, Y.-X.; Gong, F.; Ji, S.-P. RV-23, a Melittin-Related Peptide with Cell-Selective Antibacterial Activity and High Hemocompatibility. J. Microbiol. Biotechnol. 2016, 26, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef]
- Aoki, N.; Tateda, K.; Kikuchi, Y.; Kimura, S.; Miyazaki, C.; Ishii, Y.; Tanabe, Y.; Gejyo, F.; Yamaguchi, K. Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2009, 63, 534–542. [Google Scholar] [CrossRef]
- Meijer, M.T.; de Vos, A.F.; Sengers, H.P.; Scicluna, B.P.; Roelofs, J.J.; Fayçal, C.A.; Uhel, F.; Orend, G.; van der Poll, T. Tenascin C Has a Modest Protective Effect on Acute Lung Pathology during Methicillin-Resistant Staphylococcus aureus-Induced Pneumonia in Mice. Microbiol. Spectr. 2021, 9, e0020721. [Google Scholar] [CrossRef]
- Wang, X.; Mishra, B.; Lushnikova, T.; Narayana, J.L.; Wang, G. Amino Acid Composition Determines Peptide Activity Spectrum and Hot-Spot-Based Design of Merecidin. Adv. Biosyst. 2018, 2, 1700259. [Google Scholar] [CrossRef]
- Wang, X.; Junior, J.C.B.; Mishra, B.; Lushnikova, T.; Epand, R.M.; Wang, G. Arginine-lysine positional swap of the LL-37 peptides reveals evolutional advantages of the native sequence and leads to bacterial probes. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mant, C.T.; Farmer, S.W.; Hancock, R.E.W.; Vasil, M.L.; Hodges, R.S. Rational design of alpha-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index. J. Biol. Chem. 2005, 280, 12316–12329. [Google Scholar] [CrossRef]
- Zhang, S.-K.; Song, J.-W.; Gong, F.; Li, S.-B.; Chang, H.-Y.; Xie, H.-M.; Gao, H.-W.; Tan, Y.-X.; Ji, S.-P. Design of an α-helical antimicrobial peptide with improved cell-selective and potent anti-biofilm activity. Sci. Rep. 2016, 6, 27394. [Google Scholar] [CrossRef] [PubMed]
- Marquette, A.; Bechinger, B. Biophysical Investigations Elucidating the Mechanisms of Action of Antimicrobial Peptides and Their Synergism. Biomolecules 2018, 8, 18. [Google Scholar] [CrossRef]
- Gaiser, R.A.; Mangado, J.A.; Mechkarska, M.; Kaman, W.E.; van Baarlen, P.; Conlon, J.M.; Wells, J.M. Selection of antimicrobial frog peptides and temporin-1DRa analogues for treatment of bacterial infections based on their cytotoxicity and differential activity against pathogens. Chem. Biol. Drug Des. 2020, 96, 1103–1113. [Google Scholar] [CrossRef]
- Park, H.J.; Kang, H.K.; Park, E.; Kim, M.K.; Park, Y. Bactericidal activities and action mechanism of the novel antimicrobial peptide Hylin a1 and its analog peptides against Acinetobacter baumannii infection. Eur. J. Pharm. Sci. 2022, 175, 106205. [Google Scholar] [CrossRef]
- Matos, G.M.; Garcia-Teodoro, B.; Martins, C.P.; Schmitt, P.; Guzmán, F.; de Freitas, A.C.O.; Stoco, P.H.; Ferreira, F.A.; Stadnik, M.J.; Robl, D.; et al. Antimicrobial Spectrum of Activity and Mechanism of Action of Linear Alpha-Helical Peptides Inspired by Shrimp Anti-Lipopolysaccharide Factors. Biomolecules 2023, 13, 150. [Google Scholar] [CrossRef]
- Chen, Y.; Mant, C.T.; Hodges, R.S. Determination of stereochemistry stability coefficients of amino acid side-chains in an amphipathic alpha-helix. J. Pept. Res. 2002, 59, 18–33. [Google Scholar] [CrossRef]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, L.; Huang, Y.; Zhu, G. LL-37 and its analog FF/CAP18 attenuate neutrophil migration in sepsis-induced acute lung injury. J. Cell. Biochem. 2019, 120, 4863–4871. [Google Scholar]
- Yu, P.; Lin, B.; Li, J.; Luo, Y.; Zhang, D.; Sun, J.; Meng, X.; Hu, Y.; Xiang, L. Noninvasive Intratracheal Lipopolysaccharide Instillation in Mice. J. Vis. Exp. 2023, 193, e65151. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Rodrigues, S.; de Almeida, M.A.P.; Castro-Faria-Neto, H.C.; Silva, A.R.; Gonçalves-De-Albuquerque, C.F. Mouse Model of Oleic Acid-Induced Acute Respiratory Distress Syndrome. J. Vis. Exp. 2022, 184, e63566. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, J.; Park, Y.; Kim, J.H.; Eickelberg, O.; Yang, S.-R. WKYMVm ameliorates acute lung injury via neutrophil antimicrobial peptide derived STAT1/IRF1 pathway. Biochem. Biophys. Res. Commun. 2020, 533, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Millar, F.R.; Summers, C.; Griffiths, M.J.; Toshner, M.R.; Proudfoot, A.G. The pulmonary endothelium in acute respiratory distress syndrome: Insights and therapeutic opportunities. Thorax 2016, 71, 462–473. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Yang, T.; Zhang, X.X.; Xu, M.X. Bergamottin alleviates LPS-induced acute lung injury by inducing SIRT1 and suppressing NF-κB. Innate Immun. 2021, 27, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Tsai, Y.-F.; Pan, Y.-L.; Hwang, T.-L. Understanding the role of neutrophils in acute respiratory distress syndrome. Biomed. J. 2021, 44, 439–446. [Google Scholar] [CrossRef]
- Scozzi, D.; Liao, F.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The role of neutrophil extracellular traps in acute lung injury. Front. Immunol. 2022, 13, 953195. [Google Scholar] [CrossRef]
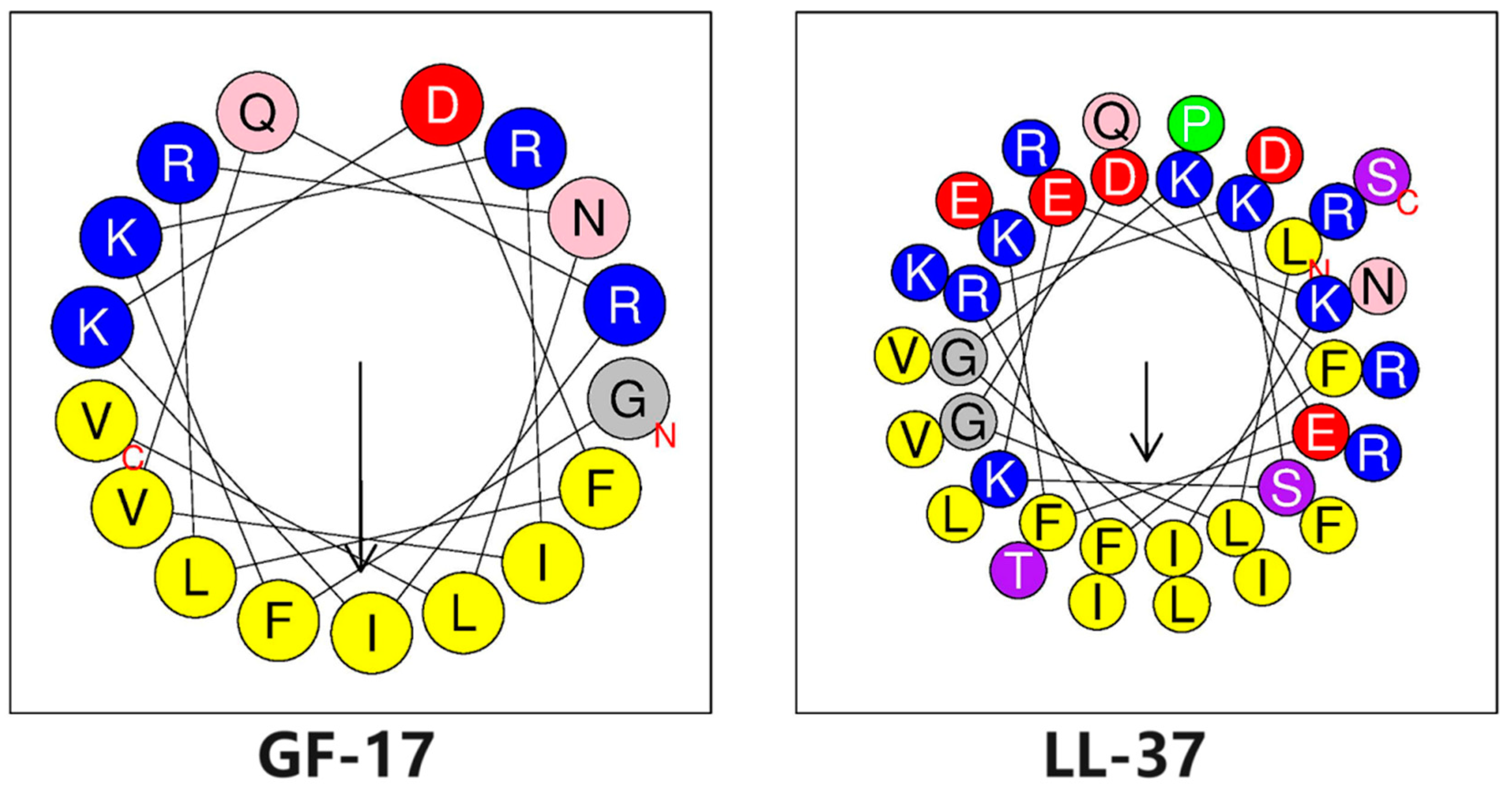
| LL-37 | GF-17 | |
|---|---|---|
| Sequence | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES.NH2 | GFKRIVQRIKDFLRNLV.NH2 |
| Net charge | 4 | 6 |
| FreqPolar | 0.622 | 0.529 |
| FreqNoPola | 0.378 | 0.471 |
| Val_angle M | 4.044 | 4.845 |
| Calculated mass MW | 4493.32 | 2101.54 |
| Observed mass MW a | 4493.3 | 2101.5 |
| Hydrophobicity (H) | 0.201 | 0.378 |
| Hyd.Moment (µH) b | 0.521 | 0.771 |
| tR (min) c | 11.125 | 20.727 |
| Peptide | MIC (μmol/L) a | ||||
|---|---|---|---|---|---|
| E. coli | P. aeruginosa | K. pneumoniae | S. aureus | S. epidermidis | |
| LL-37 | 1.56 | 12.5 | 100 | 100 | 100 |
| GF-17 | 6.25 | 6.25 | 6.25 | 6.25 | 3.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, Y.; Peng, Y.; Zhang, S.; Tang, S.; Zhang, W.; Dai, C.; Ji, S. A Rapid In Vivo Toxicity Assessment Method for Antimicrobial Peptides. Toxics 2024, 12, 387. https://doi.org/10.3390/toxics12060387
Chi Y, Peng Y, Zhang S, Tang S, Zhang W, Dai C, Ji S. A Rapid In Vivo Toxicity Assessment Method for Antimicrobial Peptides. Toxics. 2024; 12(6):387. https://doi.org/10.3390/toxics12060387
Chicago/Turabian StyleChi, Yulang, Yunhui Peng, Shikun Zhang, Sijia Tang, Wenzhou Zhang, Congjie Dai, and Shouping Ji. 2024. "A Rapid In Vivo Toxicity Assessment Method for Antimicrobial Peptides" Toxics 12, no. 6: 387. https://doi.org/10.3390/toxics12060387





