Association of Prenatal Dietary Toxicants and Inorganic Arsenic Exposure with Children’s Emotional and Behavioral Problems: ECLIPSES Study
Abstract
1. Introduction
2. Methods
2.1. Design and Participant Selection
2.2. Gathering Maternal Information
2.3. Children’s Data Collection
2.4. Statistical Analyses
3. Results
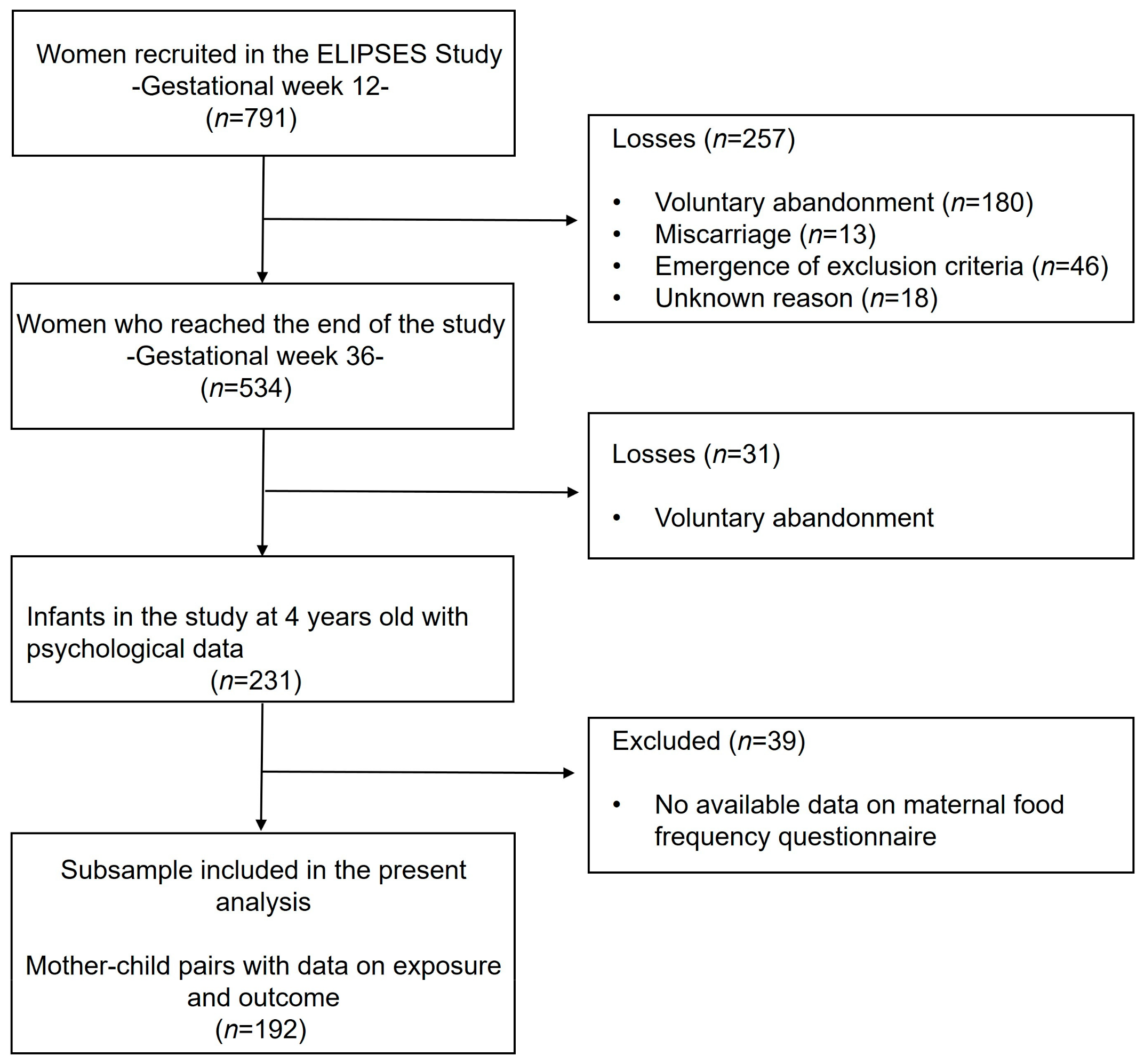
3.1. Study Design
3.2. Characteristics of Pregnant Women
3.3. Psychological Data of Four-Year-Old Children
3.4. Association between Prenatal iAs Intake and Children’s Psychological Problem Scores
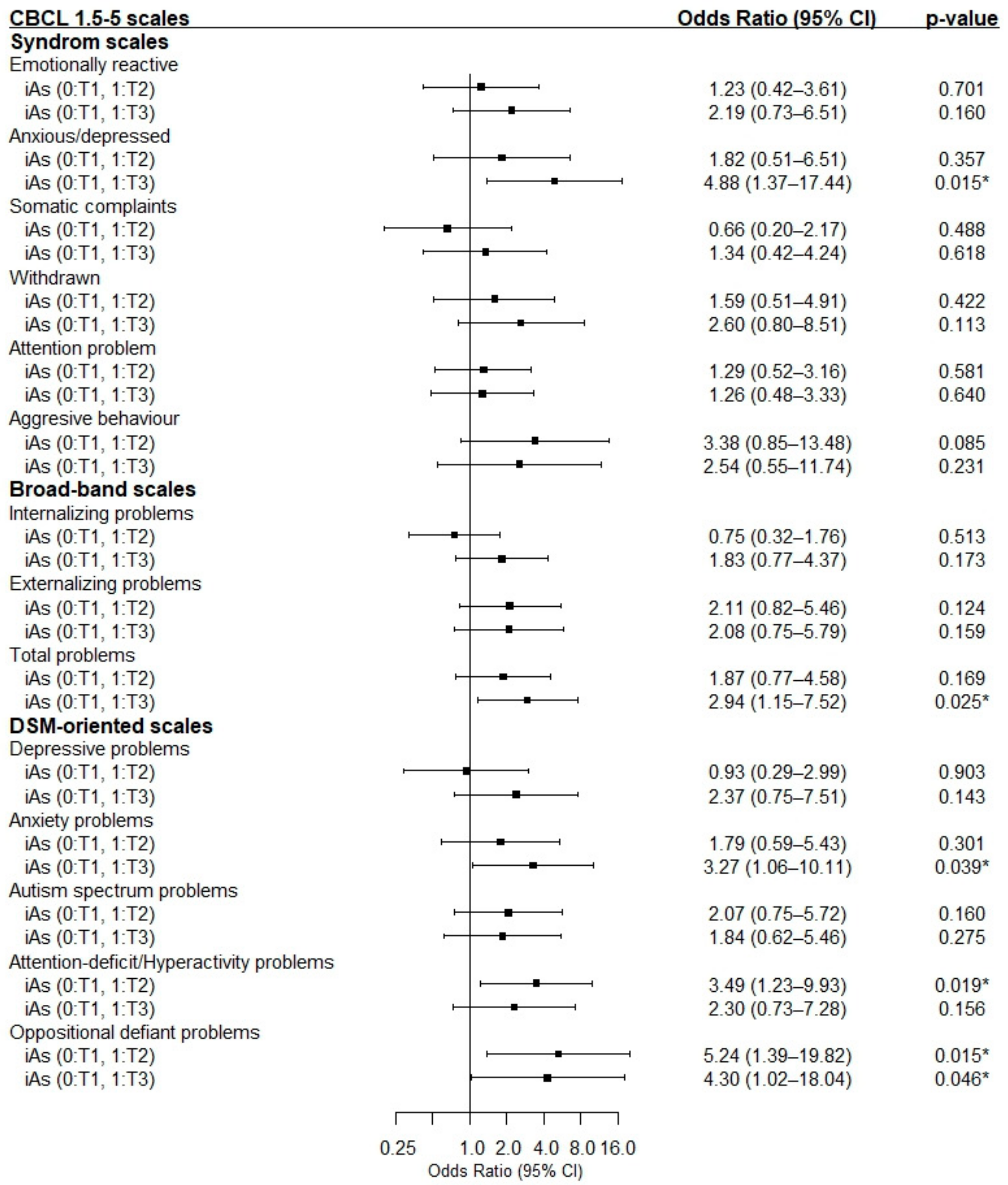
3.5. Association between Prenatal iAs Intake and Risk of Children’s Psychological Problems
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miguel, P.M.; Pereira, L.O.; Silveira, P.P.; Meaney, M.J. Early Environmental Influences on the Development of Children’s Brain Structure and Function. Dev. Med. Child Neurol. 2019, 61, 1127–1133. [Google Scholar] [CrossRef]
- De Asis-Cruz, J.; Andescavage, N.; Limperopoulos, C. Adverse Prenatal Exposures and Fetal Brain Development: Insights from Advanced Fetal Magnetic Resonance Imaging. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Roig, M.D.; Pascal, R.; Cahuana, M.J.; García-Algar, O.; Sebastiani, G.; Andreu-Fernández, V.; Martínez, L.; Rodríguez, G.; Iglesia, I.; Ortiz-Arrabal, O.; et al. Environmental Exposure during Pregnancy: Influence on Prenatal Development and Early Life: A Comprehensive Review. Fetal Diagn. Ther. 2021, 48, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Molina-Mesa, S.; Martínez-Cendán, J.P.; Moyano-Rubiales, D.; Cubillas-Rodríguez, I.; Molina-García, J.; González-Mesa, E. Detection of Relevant Heavy Metal Concentrations in Human Placental Tissue: Relationship between the Concentrations of Hg, As, Pb and Cd and the Diet of the Pregnant Woman. Int. J. Environ. Res. Public Health 2022, 19, 14731. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, I.H.; Haugen, M.; Schjølberg, S.; Vejrup, K.; Knutsen, H.K.; Brantsæter, A.L.; Meltzer, H.M.; Alexander, J.; Magnus, P.; Kvalem, H.E. Maternal Dietary Exposure to Dioxins and Polychlorinated Biphenyls (PCBs) Is Associated with Language Delay in 3year Old Norwegian Children. Environ. Int. 2016, 91, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Vejrup, K.; Schjølberg, S.; Knutsen, H.K.; Kvalem, H.E.; Brantsæter, A.L.; Meltzer, H.M.; Alexander, J.; Magnus, P.; Haugen, M. Prenatal Methylmercury Exposure and Language Delay at Three Years of Age in the Norwegian Mother and Child Cohort Study. Environ. Int. 2016, 92–93, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Vejrup, K.; Brandlistuen, R.E.; Brantsæter, A.L.; Knutsen, H.K.; Caspersen, I.H.; Alexander, J.; Lundh, T.; Meltzer, H.M.; Magnus, P.; Haugen, M. Prenatal Mercury Exposure, Maternal Seafood Consumption and Associations with Child Language at Five Years. Environ. Int. 2018, 110, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Iwai-Shimada, M.; Nakayama, S.F.; Isobe, T.; Kobayashi, Y.; Tatsuta, N.; Taniguchi, Y.; Sekiyama, M.; Michikawa, T.; Yamazaki, S.; et al. Association of Prenatal Exposure to Cadmium with Neurodevelopment in Children at 2 Years of Age: The Japan Environment and Children’s Study. Environ. Int. 2021, 156, 106762. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, C.; Zhang, J.; Qi, X.; Lv, S.; Jiang, S.; Zhou, T.; Lu, D.; Feng, C.; Chang, X.; et al. Prenatal Exposure to Mixture of Heavy Metals, Pesticides and Phenols and IQ in Children at 7 Years of Age: The SMBCS Study. Environ. Int. 2020, 139, 105692. [Google Scholar] [CrossRef] [PubMed]
- Freire, C.; Amaya, E.; Gil, F.; Fernández, M.F.; Murcia, M.; Llop, S.; Andiarena, A.; Aurrekoetxea, J.; Bustamante, M.; Guxens, M.; et al. Prenatal Co-Exposure to Neurotoxic Metals and Neurodevelopment in Preschool Children: The Environment and Childhood (INMA) Project. Sci. Total Environ. 2018, 621, 340–351. [Google Scholar] [CrossRef]
- Doherty, B.T.; Romano, M.E.; Gui, J.; Punshon, T.; Jackson, B.P.; Karagas, M.R.; Korrick, S.A. Periconceptional and Prenatal Exposure to Metal Mixtures in Relation to Behavioral Development at 3 Years of Age. Environ. Epidemiol. 2020, 4, e0106. [Google Scholar] [CrossRef] [PubMed]
- Fruh, V.; Rifas-Shiman, S.L.; Amarasiriwardena, C.; Cardenas, A.; Bellinger, D.C.; Wise, L.A.; White, R.F.; Wright, R.O.; Oken, E.; Claus Henn, B. Prenatal Lead Exposure and Childhood Executive Function and Behavioral Difficulties in Project Viva. Neurotoxicology 2019, 75, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Garí, M.; Grzesiak, M.; Krekora, M.; Kaczmarek, P.; Jankowska, A.; Król, A.; Kaleta, D.; Jerzyńska, J.; Janasik, B.; Kuraś, R.; et al. Prenatal Exposure to Neurotoxic Metals and Micronutrients and Neurodevelopmental Outcomes in Early School Age Children from Poland. Environ. Res. 2022, 204, 112049. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-L.; Hsieh, C.-J.; Wu, M.-T.; Chen, M.-L.; Kuo, P.-H.; Wang, S.-L. Co-Exposure to Toxic Metals and Phthalates in Pregnant Women and Their Children’s Mental Health Problems Aged Four Years—Taiwan Maternal and Infant Cohort Study (TMICS). Environ. Int. 2023, 173, 107804. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.X.; Dou, J.F.; Volk, H.E.; Bakulski, K.M.; Benke, K.; Hertz-Picciotto, I.; Schmidt, R.J.; Newschaffer, C.J.; Feinberg, J.I.; Daniels, J.; et al. Prenatal Metal Exposures and Child Social Responsiveness Scale Scores in 2 Prospective Studies. Environ. Health Insights 2024, 18, 11786302231225313. [Google Scholar] [CrossRef]
- Kornvig, S.; Wielsøe, M.; Long, M.; Bonefeld-Jørgensen, E.C. Prenatal Exposure to Persistent Organic Pollutants and Metals and Problematic Child Behavior at 3-5 Years of Age: A Greenlandic Cohort Study. Sci. Rep. 2021, 11, 22182. [Google Scholar] [CrossRef] [PubMed]
- Coghill, D.; Banaschewski, T.; Cortese, S.; Asherson, P.; Brandeis, D.; Buitelaar, J.; Daley, D.; Danckaerts, M.; Dittmann, R.W.; Doepfner, M.; et al. The Management of ADHD in Children and Adolescents: Bringing Evidence to the Clinic: Perspective from the European ADHD Guidelines Group (EAGG). Eur. Child Adolesc. Psychiatry 2023, 32, 1337–1361. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Eyre, O.; Patel, V.; Brent, D. Depression in Young People. Lancet 2022, 400, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, G.V.; Salum, G.A.; Sugaya, L.S.; Caye, A.; Rohde, L.A. Annual Research Review: A Meta-Analysis of the Worldwide Prevalence of Mental Disorders in Children and Adolescents. J. Child Psychol. Psychiatry 2015, 56, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.A.; Nemeroff, C.B. Early Life Stress, Mood, and Anxiety Disorders. Chronic Stress 2017, 1, 2470547017694461. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Opinion of the Scientific Panel on Contaminants in the Food Chain [CONTAM] Related to the Presence of Non Dioxin-like Polychlorinated Biphenyls (PCB) in Feed and Food. EFSA J. 2005, 3, 284. [Google Scholar] [CrossRef]
- EFSA; Arcella, D.; Cascio, C.; Gómez Ruiz, J.Á. Chronic Dietary Exposure to Inorganic Arsenic. EFSA J. 2021, 19, e06380. [Google Scholar] [CrossRef] [PubMed]
- Perelló, G.; Vicente, E.; Castell, V.; Llobet, J.M.; Nadal, M.; Domingo, J.L. Dietary Intake of Trace Elements by the Population of Catalonia (Spain): Results from a Total Diet Study. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Bulló, M.; Rovira, J.; Díaz-López, A.; Arija, V. Dietary Intake of Metals, Metalloids, and Persistent Organic Pollutants in Spanish Pregnant Women. ECLIPSES Study. Chemosphere 2023, 344, 140319. [Google Scholar] [CrossRef] [PubMed]
- Arija, V.; Fargas, F.; March, G.; Abajo, S.; Basora, J.; Canals, J.; Ribot, B.; Aparicio, E.; Serrat, N.; Hernández-Martínez, C.; et al. Adapting Iron Dose Supplementation in Pregnancy for Greater Effectiveness on Mother and Child Health: Protocol of the ECLIPSES Randomized Clinical Trial. BMC Pregnancy Childbirth 2014, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- de Catalunya, G.; Catalunya, I. d’Estadística de Classificació Catalana d’ocupacions 2011 (CCO-2011); Adaptació de La CNO-2011; Institut d’Estadística de Catalunya: Catalonia, Spain, 2011. [Google Scholar]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Rodríguez, I.T.; Ballart, J.F.; Pastor, G.C.; Jordà, E.B.; Val, V.A. Validation of a short questionnaire on frequency of dietary intake: Reproducibility and validity. Nutr. Hosp. 2008, 23, 242–252. [Google Scholar] [PubMed]
- Javier, A.; Guadalupe, C.B.; Fernández García, J.; Marta, G.A.; Ángel, H.G.; de Emilio, M.V.; Rosa, O.A.; Carmen, R.P.; Joan, Q.I. Guía de La Alimentación Saludable Para Atención Primaria y Colectivos Ciudadanos. Soc. Española Nutr. Comunitaria 2018, 1, 12–32. [Google Scholar]
- Agència Catalana de Seguretat Alimentària [ACSA] Contaminants Químics. V Estudi de Dieta Total a Catalunya Metalls Pesants, Dioxines (PCDD/F) i Bifenils Policlorats (PCB); Agència Catalana de Seguretat Alimentària [ACSA]: Barcelona, Spain, 2017. [Google Scholar]
- Julian, L.J. Measures of Anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res. 2011, 63 (Suppl. S1), S467–S472. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, T.M.; Rescorla, L.A. Manual for the ASEBA Preschool Forms & Profiles; University of Vermont: Burlington, VT, USA, 2000. [Google Scholar]
- de la Osa, N.; Granero, R.; Domenech, J.M.; Shamay-Tsoory, S.; Ezpeleta, L. Cognitive and Affective Components of Theory of Mind in Preschoolers with Oppositional Defiance Disorder: Clinical Evidence. Psychiatry Res. 2016, 241, 128–134. [Google Scholar] [CrossRef] [PubMed]
- De Waal, T.; Haziza, D.; Rao, C.R. Statistical Data Editing and Imputation; Wiley: Hoboken, NJ, USA, 2007; Volume 29, ISBN 9780470542804. [Google Scholar]
- Nishida, C.; Mucavele, P. Monitoring the Rapidly Emerging Public Health Problem of Overweight and Obesity; The WHO Global Database on Body Mass Index: Geneva, Switzerland, 2004. [Google Scholar]
- Kim, W.; Jang, Y.; Lim, Y.-H.; Kim, B.-N.; Shin, C.H.; Lee, Y.A.; Kim, J.I.; Hong, Y.-C. The Effect of Prenatal Cadmium Exposure on Attention-Deficit/Hyperactivity Disorder in 6-Year-Old Children in Korea. J. Prev. Med. Public Health 2020, 53, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, A.H.; Høyer, B.B.; Julvez, J.; Sunyer, J.; Pedersen, H.S.; Lenters, V.; Jönsson, B.A.G.; Bonde, J.P.; Toft, G. Prenatal and Postnatal PCB-153 and p,p’-DDE Exposures and Behavior Scores at 5–9 Years of Age among Children in Greenland and Ukraine. Environ. Health Perspect. 2017, 125, 107002. [Google Scholar] [CrossRef] [PubMed]
- Simeone, R.M.; Howards, P.P.; Anderson, E.; Jusko, T.A.; Drobná, B.; Kočan, A.; Čonka, K.; Fabišiková, A.; Murínová, Ľ.P.; Canfield, R.L.; et al. Pre- and Postnatal Polychlorinated Biphenyl Exposure and Cognitive and Behavioral Development at Age 45 Months in a Cohort of Slovak Children. Chemosphere 2022, 287, 132375. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, I.H.; Aase, H.; Biele, G.; Brantsæter, A.L.; Haugen, M.; Kvalem, H.E.; Skogan, A.H.; Zeiner, P.; Alexander, J.; Meltzer, H.M.; et al. The Influence of Maternal Dietary Exposure to Dioxins and PCBs during Pregnancy on ADHD Symptoms and Cognitive Functions in Norwegian Preschool Children. Environ. Int. 2016, 94, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, H.; Wang, X.; Wu, Y.; Zhang, Y.; Chen, S.; Zhang, W.; Sun, X.; Zheng, T.; Xia, W.; et al. Prenatal Arsenic Exposure, Arsenic Metabolism and Neurocognitive Development of 2-Year-Old Children in Low-Arsenic Areas. Environ. Int. 2023, 174, 107918. [Google Scholar] [CrossRef] [PubMed]
- Tofail, F.; Vahter, M.; Hamadani, J.D.; Nermell, B.; Huda, S.N.; Yunus, M.; Rahman, M.; Grantham-McGregor, S.M. Effect of Arsenic Exposure during Pregnancy on Infant Development at 7 Months in Rural Matlab, Bangladesh. Environ. Health Perspect. 2009, 117, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Hamadani, J.D.; Grantham-McGregor, S.M.; Tofail, F.; Nermell, B.; Fängström, B.; Huda, S.N.; Yesmin, S.; Rahman, M.; Vera-Hernández, M.; Arifeen, S.E.; et al. Pre- and Postnatal Arsenic Exposure and Child Development at 18 Months of Age: A Cohort Study in Rural Bangladesh. Int. J. Epidemiol. 2010, 39, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Signes-Pastor, A.J.; Vioque, J.; Navarrete-Muñoz, E.M.; Carey, M.; García-Villarino, M.; Fernández-Somoano, A.; Tardón, A.; Santa-Marina, L.; Irizar, A.; Casas, M.; et al. Inorganic Arsenic Exposure and Neuropsychological Development of Children of 4-5 Years of Age Living in Spain. Environ. Res. 2019, 174, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Soler-Blasco, R.; Murcia, M.; Lozano, M.; Sarzo, B.; Esplugues, A.; Riutort-Mayol, G.; Vioque, J.; Lertxundi, N.; Santa Marina, L.; Lertxundi, A.; et al. Prenatal Arsenic Exposure, Arsenic Methylation Efficiency, and Neuropsychological Development among Preschool Children in a Spanish Birth Cohort. Environ. Res. 2022, 207, 112208. [Google Scholar] [CrossRef] [PubMed]
- Meakin, C.J.; Szilagyi, J.T.; Avula, V.; Fry, R.C. Inorganic Arsenic and Its Methylated Metabolites as Endocrine Disruptors in the Placenta: Mechanisms Underpinning Glucocorticoid Receptor (GR) Pathway Perturbations. Toxicol. Appl. Pharmacol. 2020, 409, 115305. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.S.; Chatterjee, D.; Das, N.; Giri, A.K. Substantial Evidences Indicate That Inorganic Arsenic Is a Genotoxic Carcinogen: A Review. Toxicol. Res. 2018, 34, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-C.; Lin, J.-W.; Liu, J.-M.; Liu, S.-H.; Fang, K.-M.; Su, C.-C.; Hsu, R.-J.; Wu, C.-C.; Huang, C.-F.; Lee, K.-I.; et al. Arsenic Induces Autophagy-Dependent Apoptosis via Akt Inactivation and AMPK Activation Signaling Pathways Leading to Neuronal Cell Death. Neurotoxicology 2021, 85, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Gera, R.; Kushwaha, R.; Sharma, A.K.; Patnaik, S.; Ghosh, D. Hijacking Microglial Glutathione by Inorganic Arsenic Impels Bystander Death of Immature Neurons through Extracellular Cystine/Glutamate Imbalance. Sci. Rep. 2016, 6, 30601. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Rachamalla, M.; Niyogi, S.; Datusalia, A.K.; Flora, S.J.S. Molecular Mechanism of Arsenic-Induced Neurotoxicity Including Neuronal Dysfunctions. Int. J. Mol. Sci. 2021, 22, 10077. [Google Scholar] [CrossRef] [PubMed]
- Silva-Adaya, D.; Ramos-Chávez, L.A.; Petrosyan, P.; González-Alfonso, W.L.; Pérez-Acosta, A.; Gonsebatt, M.E. Early Neurotoxic Effects of Inorganic Arsenic Modulate Cortical GSH Levels Associated with the Activation of the Nrf2 and NFκB Pathways, Expression of Amino Acid Transporters and NMDA Receptors and the Production of Hydrogen Sulfide. Front. Cell. Neurosci. 2020, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Escudero-Lourdes, C. Toxicity Mechanisms of Arsenic That Are Shared with Neurodegenerative Diseases and Cognitive Impairment: Role of Oxidative Stress and Inflammatory Responses. Neurotoxicology 2016, 53, 223–235. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.-C.; et al. Update of the Risk Assessment of Inorganic Arsenic in Food. EFSA J. 2024, 22, e8488. [Google Scholar] [CrossRef]
- Espejo-Herrera, N.; Kogevinas, M.; Castaño-Vinyals, G.; Aragonés, N.; Boldo, E.; Ardanaz, E.; Azpiroz, L.; Ulibarrena, E.; Tardón, A.; Molina, A.J.; et al. Nitrate and Trace Elements in Municipal and Bottled Water in Spain. Gac. Sanit. 2013, 27, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Palau, M.; Guevara, E. Calidad Del Agua de Consumo Humano En España. Informe Técnico. Año 2013; Minist. Sanidad, Serv. Soc. e Igual: Madrid, Spain, 2014. [Google Scholar]
- Cubadda, F.; Jackson, B.P.; Cottingham, K.L.; Van Horne, Y.O.; Kurzius-Spencer, M. Human Exposure to Dietary Inorganic Arsenic and Other Arsenic Species: State of Knowledge, Gaps and Uncertainties. Sci. Total Environ. 2017, 579, 1228–1239. [Google Scholar] [CrossRef]
- Kollander, B.; Sand, S.; Almerud, P.; Ankarberg, E.H.; Concha, G.; Barregård, L.; Darnerud, P.O. Inorganic Arsenic in Food Products on the Swedish Market and a Risk-Based Intake Assessment. Sci. Total Environ. 2019, 672, 525–535. [Google Scholar] [CrossRef] [PubMed]
| Dietary iAs Intake | |||||
|---|---|---|---|---|---|
| Characteristics | Total (n = 192) | Tertile 1 <3.04 μg/day (n = 64) | Tertile 2 3.04–4.16 μg/day (n = 64) | Tertile 3 >4.16 μg/day (n = 64) | |
| Maternal Characteristics | Summary Statistics | p | |||
| Age (years), mean ± SD | 31.88 ± 4.44 | 32.45± 4.17 | 31.72 ± 4.67 | 31.47 ± 4.50 | 0.430 |
| BMI (kg/m2), n (%) | 0.404 | ||||
| <25 (normal weight) | 110 (57.3%) | 33 (51.6%) | 39 (60.9%) | 38 (59.4%) | |
| 25–29 (overweight) | 56 (29.2%) | 18 (28.1%) | 18 (28.1%) | 20 (31.3%) | |
| ≥30 (obesity) | 26 (13.5%) | 13 (20.3%) | 7 (11.0%) | 6 (9.3%) | |
| Social class, n (%) | 0.148 | ||||
| Low | 16 (8.3%) | 2 (3.1%) | 6 (9.4%) | 8 (12.5%) | |
| Middle/High | 176 (91.7%) | 62 (96.9%) | 58 (90.6%) | 56 (87.5%) | |
| Smoking status, n (%) | 0.930 | ||||
| Never | 132 (68.8%) | 44 (68.8%) | 43 (67.2%) | 45 (70.3%) | |
| Ex-smoker/Smoker | 60 (31.3%) | 20 (31.3%) | 21 (32.8%) | 19 (29.7%) | |
| MedDiet during pregnancy (score), mean ± SD | 9.83 ± 2.45 | 10.02 ± 2.48 | 9.53 ± 2.27 | 9.92± 2.59 | 0.495 |
| Energy intake during pregnancy (kcal/d), mean ± SD | 1956.17 ± 521.15 | 1696.30 ± 390.73 ac | 1972.12 ± 442.64 ab | 2200.10 ± 587.79 bc | <0.001 |
| State-trait anxiety inventory score, mean ± SD | 14.80 ± 6.80 | 14.58 ± 7.35 | 14.76 ± 5.94 | 15.07 ± 7.13 | 0.920 |
| Dietary iAs Intake | |||||||
|---|---|---|---|---|---|---|---|
| Emotional and Behavior Scale | Tertile 1 <3.04 μg/day | Tertile 2 3.04–4.16 μg/day | Tertile 3 >4.16 μg/day | p | |||
| Mean | SD | Mean | SD | Mean | SD | ||
| Syndrome Scales | |||||||
| Emotionally reactive | 56.33 | 9.14 | 56.28 | 8.21 | 58.73 | 9.66 | 0.216 |
| % * | 15.6% | 15.6% | 21.9% | 0.564 | |||
| Anxious/depressed | 54.73 | 7.04 | 56.06 | 7.59 | 57.59 | 7.73 | 0.098 |
| % * | 7.8% | 12.5% | 23.4% | 0.037 | |||
| Somatic complaints | 54.91 | 6.95 | 54.14 | 5.75 | 56.95 | 7.11 | 0.048 b |
| % * | 12.5% | 10.9% | 20.3% | 0.274 | |||
| Withdrawn | 55.95 a | 6.79 | 57.63 | 7.05 | 59.92 a | 8.13 | 0.010 |
| % * | 12.5% | 17.2% | 20.3% | 0.490 | |||
| Attention problems | 55.97 a | 6.69 | 58.23 | 7.84 | 59.08 a | 7.13 | 0.045 |
| % * | 21.9% | 26.6% | 26.6% | 0.779 | |||
| Aggressive behavior | 54.02 | 5.53 | 55.97 | 7.92 | 56.66 | 8.66 | 0.120 |
| % * | 6.3% | 15.6% | 12.5% | 0.238 | |||
| Broad-band scales | |||||||
| Internalizing problems | 52.27 a | 12.38 | 53.55 | 11.99 | 58.27 a | 11.00 | 0.011 |
| % * | 34.4% | 28.1% | 43.8% | 0.177 | |||
| Externalizing problems | 51.36 | 9.31 | 53.25 | 12.69 | 55.66 | 11.10 | 0.093 |
| % * | 18.8% | 29.7% | 26.6% | 0.338 | |||
| Total problems | 51.98 a | 11.17 | 53.61 | 13.21 | 57.61 a | 11.81 | 0.027 |
| % * | 21.9% | 29.7% | 35.9% | 0.215 | |||
| DSM-Oriented Scales | |||||||
| Depressive problems | 55.27 | 6.70 | 55.98 | 7.03 | 58.22 | 7.42 | 0.050 |
| % * | 14.1% | 12.5% | 21.9% | 0.303 | |||
| Anxiety problems | 55.86 | 7.42 | 57.25 | 8.11 | 59.25 | 8.62 | 0.060 |
| % * | 10.9% | 17.2% | 26.6% | 0.070 | |||
| Autism spectrum problems | 56.19 | 6.83 | 57.11 | 7.27 | 58.55 | 7.38 | 0.174 |
| % * | 15.6% | 23.4% | 21.9% | 0.509 | |||
| Attention-deficit/Hyperactivity problems | 55.16 | 6.14 | 58.36 | 8.75 | 58.38 | 8.39 | 0.030 b |
| % * | 10.9% | 28.1% | 21.9% | 0.050 | |||
| Oppositional defiant problems | 53.59 | 5.28 | 55.63 | 7.74 | 55.80 | 7.45 | 0.137 |
| % * | 6.3% | 18.8% | 14.1% | 0.105 | |||
| Dietary iAs Intake | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 (Ref.) | Tertile 2 | Tertile 3 | Per 10 Increment Unit | |||||||
| β | 95%CI | p | β | 95%CI | p | β | 95%CI | p | ||
| Syndrome scale | ||||||||||
| Emotionally reactive | ||||||||||
| Unadjusted model | −0.05 | (−3.19–3.10) | 0.977 | 2.41 | (−0.07–5.55) | 0.133 | 0.07 | (−0.01–0.14) | 0.078 | |
| Adjusted model | 0.44 | (−2.70–3.59) | 0.781 | 3.30 | (−0.03–6.64) | 0.052 | 0.08 | (0.00–0.15) | 0.056 | |
| Anxious/depressed | ||||||||||
| Unadjusted model | 1.33 | (−1.27–3.93) | 0.315 | 2.86 | (0.26–5.46) | 0.031 | 0.06 | (0.00–0.12) | 0.047 | |
| Adjusted model | 1.68 | (−0.92–4.27) | 0.204 | 3.18 | (0.44–5.93) | 0.023 | 0.07 | (0.00–0.13) | 0.036 | |
| Somatic complaints | ||||||||||
| Unadjusted model | −0.77 | (−3.08–1.55) | 0.514 | 2.05 | (−0.26–4.36) | 0.082 | 0.08 | (0.03–0.14) | 0.002 | |
| Adjusted model | −1.12 | (−3.45–1.22) | 0.347 | 1.54 | (−0.93–4.02) | 0.219 | 0.08 | (0.02–0.14) | 0.007 | |
| Withdrawn | ||||||||||
| Unadjusted model | 1.67 | (−0.89–4.23) | 0.200 | 3.97 | (1.41–6.53) | 0.003 | 0.06 | (0.00–0.12) | 0.043 | |
| Adjusted model | 1.86 | (−0.75–4.46 | 0.161 | 4.41 | (1.65–7.18) | 0.002 | 0.07 | (0.00–0.13) | 0.038 | |
| Attention problems | ||||||||||
| Unadjusted model | 2.27 | (−0.26–4.79) | 0.078 | 3.11 | (0.59–5.63) | 0.016 | 0.05 | (0.00–0.12) | 0.067 | |
| Adjusted model | 2.64 | (0.15–5.13) | 0.037 | 3.59 | (0.95–6.22) | 0.008 | 0.06 | (0.00–0.12) | 0.059 | |
| Aggressive behavior | ||||||||||
| Unadjusted model | 1.95 | (−0.66–4.57) | 0.142 | 2.64 | (0.03–5.25) | 0.048 | 0.05 | (−0.01–0.11) | 0.121 | |
| Adjusted model | 2.37 | (−0.18–4.92) | 0.068 | 3.21 | (0.51–5.92) | 0.020 | 0.06 | (−0.01–0.12) | 0.088 | |
| Broad-band scales | ||||||||||
| Internalizing problems | ||||||||||
| Unadjusted model | 1.28 | (−2.84–5.40) | 0.540 | 6.00 | (1.88–10.12) | 0.005 | 0.14 | (0.04–0.23) | 0.006 | |
| Adjusted model | 1.89 | (−2.29–6.07) | 0.374 | 6.92 | (2.48–11.35) | 0.002 | 0.15 | (0.04–0.25) | 0.006 | |
| Externalizing problems | ||||||||||
| Unadjusted model | 1.89 | (−1.99–5.77) | 0.337 | 4.30 | (0.42–8.17) | 0.030 | 0.09 | (0.00–0.18) | 0.055 | |
| Adjusted model | 2.79 | (−1.03–6.60) | 0.151 | 5.39 | (1.35–9.44) | 0.009 | 0.10 | (0.00–0.19) | 0.041 | |
| Total problems | ||||||||||
| Unadjusted model | 1.62 | (−2.59–5.84) | 0.448 | 5.62 | (1.41–9.84) | 0.009 | 0.13 | (0.03–0.23) | 0.012 | |
| Adjusted model | 2.42 | (−1.75–6.59) | 0.253 | 6.77 | (2.35–11.19) | 0.003 | 0.14 | (0.04–0.24) | 0.007 | |
| DSM-Oriented Scales | ||||||||||
| DSM Depressive problems | ||||||||||
| Unadjusted model | 0.72 | (−1.74–3.18) | 0.565 | 2.95 | (0.49–5.41) | 0.019 | 0.08 | 0.03–0.14 | 0.005 | |
| Adjusted model | 0.92 | (−1.56–3.41) | 0.465 | 3.25 | (0.62–5.89) | 0.016 | 0.09 | 0.03–0.15 | 0.005 | |
| DSM Anxiety problems | ||||||||||
| Unadjusted model | 1.39 | (−1.42–4.20) | 0.331 | 3.39 | (0.58–6.20) | 0.018 | 0.08 | 0.01–0.14 | 0.022 | |
| Adjusted model | 1.48 | (−1.33–4.30) | 0.300 | 3.41 | (0.42–6.39) | 0.026 | 0.09 | 0.01–0.15 | 0.018 | |
| DSM Autism spectrum problems | ||||||||||
| Unadjusted model | 0.92 | (−1.58–3.42) | 0.468 | 2.36 | (−0.14–4.86) | 0.064 | 0.04 | −0.02–0.10 | 0.148 | |
| Adjusted model | 1.37 | (−1.11–3.86) | 0.277 | 2.88 | (0.25–5.52) | 0.032 | 0.05 | −0.02–0.11 | 0.141 | |
| DSM Attention-deficit/Hyperactivity problems | ||||||||||
| Unadjusted model | 3.20 | (0.47–5.94) | 0.022 | 3.22 | (0.48–5.95) | 0.021 | 0.06 | 0.00–0.13 | 0.055 | |
| Adjusted model | 3.31 | (0.59–6.03) | 0.017 | 3.59 | (0.70–6.48) | 0.015 | 0.07 | 0.00–0.14 | 0.046 | |
| DSM Oppositional defiant problems | ||||||||||
| Unadjusted model | 2.03 | (−0.38–4.44) | 0.098 | 2.20 | (−0.21–4.61) | 0.073 | 0.05 | 0.00–0.11 | 0.062 | |
| Adjusted model | 2.56 | (0.11–5.01) | 0.040 | 3.12 | (0.52–5.72) | 0.019 | 0.07 | 0.01–0.13 | 0.028 | |
| Dietary iAs Intake | |||
|---|---|---|---|
| Maternal Characteristics | β * | (95% CI) | p |
| Age (years) | 0.11 | (−0.26–0.49) | 0.554 |
| BMI (kg/m2) | |||
| <25 (normal weight) (ref.) | |||
| 25–29 (overweight) | 1.06 | (−2.55–4.68) | 0.562 |
| ≥30 (obesity) | 4.19 | (−0.55–8.94) | 0.083 |
| Social class | |||
| Low (ref.) | |||
| Middle/High | 0.50 | (−5.19–6.20) | 0.861 |
| Smoking status | |||
| Never (ref.) | |||
| Ex-smoker/Smoker | −0.39 | (−3.86–3.08) | 0.825 |
| MedDiet during pregnancy (score) | −0.64 | (−1.54–0.25) | 0.156 |
| Iron supplement (mg/day) | 0.02 | (−0.05–0.09) | 0.552 |
| State-trait anxiety inventory score | −0.03 | (−0.25–0.19) | 0.796 |
| Milk | 0.01 | (0.00–0.02) | 0.179 |
| Cheese | −0.12 | (−0.28–0.04) | 0.148 |
| White/processed meat | −0.01 | (−0.09–0.06) | 0.691 |
| Red meat | 0.14 | (0.02–0.25) | 0.019 |
| White fish | 0.02 | (−0.13–0.17) | 0.772 |
| Blue fish | −0.12 | (−0.25–0.01) | 0.067 |
| Seafood | 0.29 | (−0.07–0.65) | 0.117 |
| Eggs | 0.30 | (0.11–0.48) | 0.002 |
| Sweet cereal | −0.05 | (−0.12–0.03) | 0.204 |
| Cereal and tubers | 0.33 | (0.28–0.39) | <0.001 |
| Vegetables | −0.03 | (−0.08–0.03) | 0.319 |
| Fruits | 0.03 | (0.01–0.05) | 0.001 |
| Pulses | 0.27 | (0.05–0.48) | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kou, X.; Canals, J.; Bulló, M.; Becerra-Tomás, N.; Jardí, C.; Arija, V. Association of Prenatal Dietary Toxicants and Inorganic Arsenic Exposure with Children’s Emotional and Behavioral Problems: ECLIPSES Study. Toxics 2024, 12, 398. https://doi.org/10.3390/toxics12060398
Kou X, Canals J, Bulló M, Becerra-Tomás N, Jardí C, Arija V. Association of Prenatal Dietary Toxicants and Inorganic Arsenic Exposure with Children’s Emotional and Behavioral Problems: ECLIPSES Study. Toxics. 2024; 12(6):398. https://doi.org/10.3390/toxics12060398
Chicago/Turabian StyleKou, Xiruo, Josefa Canals, Monica Bulló, Nerea Becerra-Tomás, Cristina Jardí, and Victoria Arija. 2024. "Association of Prenatal Dietary Toxicants and Inorganic Arsenic Exposure with Children’s Emotional and Behavioral Problems: ECLIPSES Study" Toxics 12, no. 6: 398. https://doi.org/10.3390/toxics12060398
APA StyleKou, X., Canals, J., Bulló, M., Becerra-Tomás, N., Jardí, C., & Arija, V. (2024). Association of Prenatal Dietary Toxicants and Inorganic Arsenic Exposure with Children’s Emotional and Behavioral Problems: ECLIPSES Study. Toxics, 12(6), 398. https://doi.org/10.3390/toxics12060398










