The Easily Overlooked Effect of Global Warming: Diffusion of Heavy Metals
Abstract
:1. Introduction
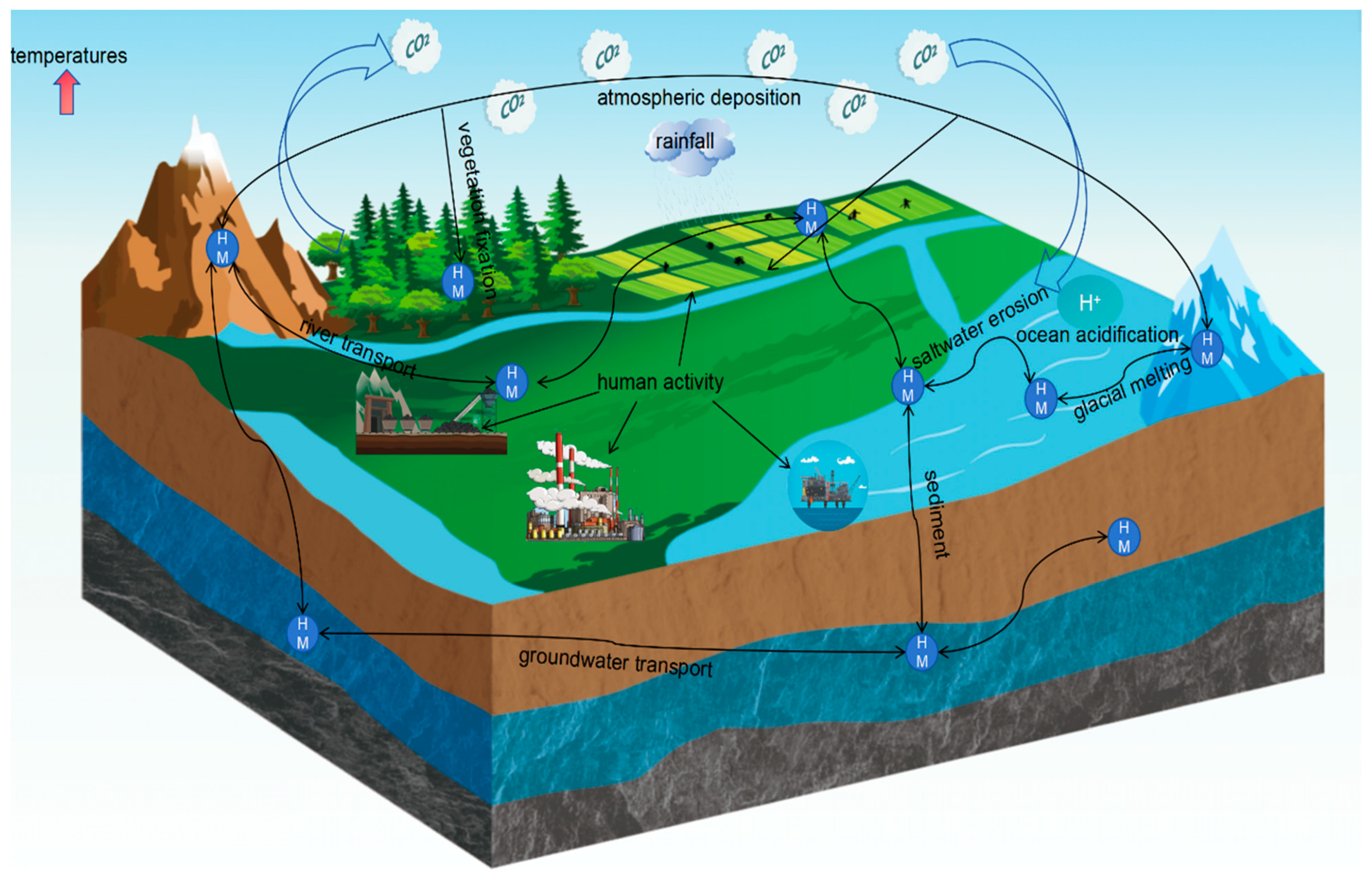
2. Transport of Heavy Metals in the Environment under Global Warming
2.1. Air
2.2. Aquatic Environment
2.3. Soil
2.4. Environmental Metal Risks
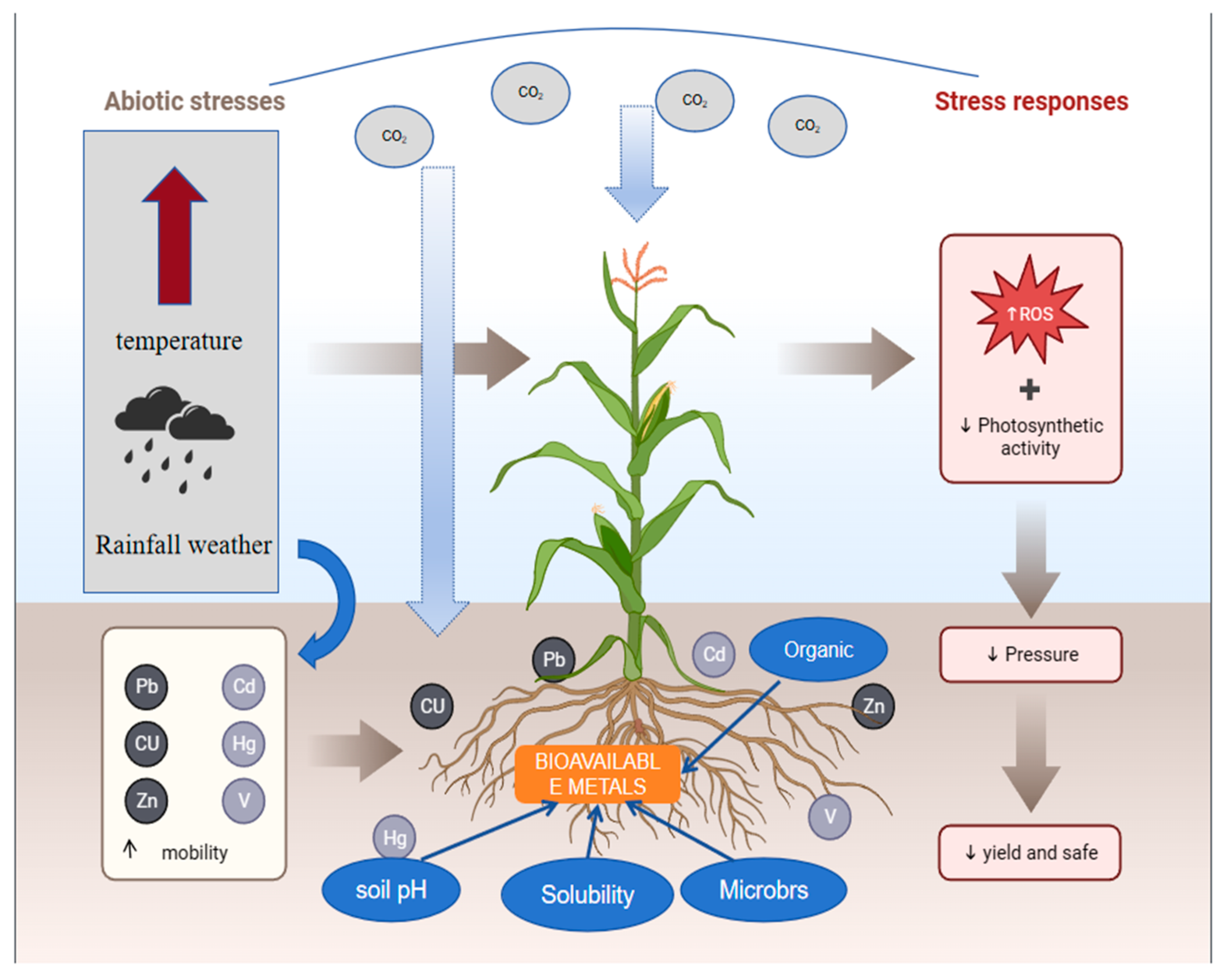
3. Heavy Metal Bioavailability under Global Warming
3.1. Temperature
3.2. CO2
3.3. Organic Matter
3.4. Soil Moisture
4. Effects of Heavy Metals on Organisms under Global Warming
4.1. Aquatic Life
4.2. Soil Organisms
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Wang, S.P.; Ma, X.B.; Gong, J.L. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef] [PubMed]
- Armstrong McKay, D.I.; Staal, A.; Abrams, J.F.; Winkelmann, R.; Sakschewski, B.; Loriani, S.; Fetzer, I.; Cornell, S.E.; Rockström, J.; Lenton, T.M. Exceeding 1.5 °C global warming could trigger multiple climate tipping points. Science 2022, 377, eabn7950. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.S.; von Wuehlisch, G. Mitigation of global warming through renewable biomass. Biomass Bioenergy 2013, 48, 75–89. [Google Scholar] [CrossRef]
- Farlex, I. Definition: Environment, the Free Dictionary; Farlex Inc. Publishing: Huntingdon Valley, PA, USA, 2005; Available online: http://www.thefreedictionary.com/ (accessed on 4 August 2023).
- Stocker, T.; Qin, D.; Plattner, G.; Tignor, M.; Allen, S.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P. The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Climate Change; Cambridge University Press: Cambridge, UK, 2013; p. 2014. [Google Scholar]
- Ding, Y.-j.; Zhang, S.; Zhao, L.; Li, Z.-Q.; Kang, S.-C. Global warming weakening the inherent stability of glaciers and permafrost. Sci. Bull. 2019, 64, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Liu, Y.; Jevrejeva, S.; Jackson, L.P. Future sea level rise along the coast of China and adjacent region under 1.5 °C and 2.0 °C global warming. Adv. Clim. Chang. Res. 2020, 11, 227–238. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Chen, H.Y.H.; Liu, Q.; Ge, B.; Tang, B. A global meta-analysis on the responses of C and N concentrations to warming in terrestrial ecosystems. Catena 2022, 208, 105762. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.Y.; Kim, J.I.; Park, Y.J.; Park, C.M. Plant Thermomorphogenic Adaptation to Global Warming. J. Plant Biol. 2020, 63, 1–9. [Google Scholar] [CrossRef]
- Hayashi, M.; Shiogama, H.; Ogura, T. The Contribution of Climate Change to Increasing Extreme Ocean Warming Around Japan. Geophys. Res. Lett. 2022, 49, e2022GL100785. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Yuan, C.; Zhu, G.; Yang, S.; Xu, G.; Li, Y.; Gong, H.; Wu, C. Soil warming increases soil temperature sensitivity in subtropical forests of SW China. PeerJ 2019, 7, e7721. [Google Scholar] [CrossRef]
- Wang, M.; Yan, L.; Dou, S.; Yang, L.; Zhang, Y.; Huang, W.; Li, S.; Lu, P.; Guo, Y. Blood multiple heavy metals exposure and lung function in young adults: A prospective Cohort study in China. J. Hazard. Mater. 2023, 459, 132064. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Lenntech. Water Treatment and Air Purification: Water Treatment; Lenntech: Rotterdamseweg, The Netherlands, 2004; p. 10. [Google Scholar]
- Yang, S.Y.; Zhao, J.; Chang, S.X.; Collins, C.; Xu, J.M.; Liu, X.M. Status assessment and probabilistic health risk modeling of metals accumulation in agriculture soils across China: A synthesis. Environ. Int. 2019, 128, 165–174. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Ruan, C.J.; Li, H.; Guo, D.G.; Li, W.X. Understanding molecular mechanisms for improving phytoremediation of heavy metal-contaminated soils. Crit. Rev. Biotechnol. 2010, 30, 23–30. [Google Scholar] [CrossRef]
- Ma, J.-Y.; Li, W.-Y.; Yang, Z.-Y.; Su, J.-Z.; Li, L.; Deng, Y.-R.; Tuo, Y.-F.; Niu, Y.-Y.; Xiang, P. The spatial distribution, health risk, and cytotoxicity of metal(loid)s in contaminated field soils: The role of Cd in human gastric cells damage. Sci. Total Environ. 2023, 878, 162942. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, Y.; Chen, X.; Sha, A.; Xiong, Z.; Luo, Y.; Peng, L.; Zou, L.; Zhao, C.; Li, Q. The Diversity and Community Composition of Three Plants’ Rhizosphere Fungi in Kaolin Mining Areas. J. Fungi. 2024, 10, 306. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Sha, A.; Li, N.; Ran, Y.; Xiang, P.; Zhou, L.; Zhang, T.; Wu, Q.; Zou, L.; Chen, Z.; et al. The characteristics of fungal responses to uranium mining activities and analysis of their tolerance to uranium. Ecotoxicol Environ Saf. 2024, 277, 116362. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Zhang, Y.; Chen, X.; Sha, A.; Xiao, W.; Luo, Y.; Peng, L.; Zou, L.; Li, Q. Impact of Vanadium-Titanium-Magnetite Mining Activities on Endophytic Bacterial Communities and Functions in the Root Systems of Local Plants. Genes 2024, 15, 526. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xiong, Z.; Xiang, P.; Zhou, L.; Zhang, T.; Wu, Q.; Zhao, C. Effects of uranium mining on soil bacterial communities and functions in the Qinghai-Tibet plateau. Chemosphere 2024, 347, 140715. [Google Scholar] [CrossRef]
- Rajkumar, M.; Prasad, M.N.V.; Swaminathan, S.; Freitas, H. Climate change driven plant–metal–microbe interactions. Environ. Int. 2013, 53, 74–86. [Google Scholar] [CrossRef]
- Noyes, P.D.; McElwee, M.K.; Miller, H.D.; Clark, B.W.; Van Tiem, L.A.; Walcott, K.C.; Erwin, K.N.; Levin, E.D. The toxicology of climate change: Environmental contaminants in a warming world. Environ. Int. 2009, 35, 971–986. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Liao, X.; Xiao, R.; Liu, K.; Bai, J.; Li, B.; He, Q. Heavy metal pollution in coastal wetlands: A systematic review of studies globally over the past three decades. J. Hazard. Mater. 2022, 424, 127312. [Google Scholar] [CrossRef]
- Biswas, K.; Chatterjee, A.; Chakraborty, J. Comparison of Air Pollutants Between Kolkata and Siliguri, India, and Its Relationship to Temperature Change. J. Geovis. Spat. Anal. 2020, 4, 25. [Google Scholar] [CrossRef]
- Zapletal, M.; Cudlín, P.; Khadka, C.; Křůmal, K.; Mikuška, P.; Cigánková, H.; Polášek, M. Characteristics and Sources of PAHs, Hopanes, and Elements in PM10 Aerosol in Tulsipur and Charikot (Nepal). Water Air Soil Pollut. 2022, 233, 486. [Google Scholar] [CrossRef]
- Marina-Montes, C.; Pérez-Arribas, L.V.; Escudero, M.; Anzano, J.; Cáceres, J.O. Heavy metal transport and evolution of atmospheric aerosols in the Antarctic region. Sci. Total Environ. 2020, 721, 137702. [Google Scholar] [CrossRef]
- Rippey, B.; Rose, N.; Yang, H.; Harrad, S.; Robson, M.; Travers, S. An assessment of toxicity in profundal lake sediment due to deposition of heavy metals and persistent organic pollutants from the atmosphere. Environ. Int. 2008, 34, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zheng, J.; Yang, M.; Meng, Y.; Yu, X.; Zhou, H.; Tong, L.; Wang, K.; Li, Y.-F.; Wang, X.; et al. Atmospheric wet deposition of trace metal elements: Monitoring and modelling. Sci. Total Environ. 2023, 893, 164880. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Guo, Z.; Peng, C.; Xiao, X.; Shi, L.; Zeng, P.; Ran, H.; Xue, Q. Atmospheric bulk deposition of heavy metal(loid)s in central south China: Fluxes, influencing factors and implication for paddy soils. J. Hazard. Mater. 2019, 371, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Collett, J.L., Jr.; Chen, J.; Zhang, X.; Wang, Z.; Wang, W. Microscopic Evaluation of Trace Metals in Cloud Droplets in an Acid Precipitation Region. Environ. Sci. Technol. 2013, 47, 4172–4180. [Google Scholar] [CrossRef]
- Liu, H.-L.; Zhou, J.; Li, M.; Hu, Y.-m.; Liu, X.; Zhou, J. Study of the bioavailability of heavy metals from atmospheric deposition on the soil-pakchoi (Brassica chinensis L.) system. J. Hazard. Mater. 2019, 362, 9–16. [Google Scholar] [CrossRef]
- Falkenberg, L.J.; Dupont, S. Chapter 10—Climate change and the ocean. In Oceans and Human Health, 2nd ed.; Fleming, L.E., Alcantara Creencia, L.B., Gerwick, W.H., Goh, H.C., Gribble, M.O., Maycock, B., Solo-Gabriele, H., Eds.; Academic Press: San Diego, CA, USA, 2023; pp. 265–288. [Google Scholar] [CrossRef]
- Mohan, M.; Sreelakshmi, U.; Vishnu Sagar, G.; Pandit, G.; Sahu, S.; Tiwari, M.; Ajmal, P.; Kannan, V.; Abdul Shukur, M.; Krishnan, K. Metal contamination profile and sediment accumulation rate of Arctic fjords: Implications from a sediment core. Kongsfjorden Svalbard. Mar. Pollut. Bull. 2018, 42, 131. [Google Scholar]
- Singh, N.; Sivaramakrishnan, R.; Choudhary, S.; Chikkamadaiah, K. Spatial distribution and environmental assessment of heavy metals in the surface sediments of Kongsfjorden, Svalbard. Czech Polar Rep. 2018, 8, 1–23. [Google Scholar] [CrossRef]
- Rudnicka-Kępa, P.; Bełdowska, M.; Zaborska, A. Enhanced heavy metal discharges to marine deposits in glacial bays of two Arctic fjords (Hornsund and Kongsfjorden). J. Mar. Syst. 2024, 241, 103915. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, X.; Lin, H.; Ren, J.; Wang, C.; Gong, P. Accumulation of pollutants in proglacial lake sediments: Impacts of glacial meltwater and anthropogenic activities. Environ. Sci. Technol. 2020, 54, 7901–7910. [Google Scholar] [CrossRef]
- Kumar, P.; Meena, N.K.; Diwate, P.; Mahajan, A.K.; Bhushan, R. The heavy metal contamination history during ca 1839–2003 AD from Renuka Lake of Lesser Himalaya, Himachal Pradesh, India. Environ. Earth Sci. 2019, 78, 549. [Google Scholar] [CrossRef]
- Yuan, G.-L.; Liu, C.; Chen, L.; Yang, Z. Inputting history of heavy metals into the inland lake recorded in sediment profiles: Poyang Lake in China. J. Hazard. Mater. 2011, 185, 336–345. [Google Scholar] [CrossRef]
- Omer, I.; Mateescu, R.; Dimache, A. Heavy Metal Pollution of the Romanian Coastal Area. Rev. Chim. 2016, 67, 553–556. [Google Scholar]
- Jiao, X.; Dong, Z.; Kang, S.; Li, Y.; Jiang, C.; Rostami, M. New insights into heavy metal elements deposition in the snowpacks of mountain glaciers in the eastern Tibetan Plateau. Ecotoxicol. Environ. Saf. 2021, 207, 111228. [Google Scholar] [CrossRef]
- Wang, X.; Luo, J.; Yuan, W.; Lin, C.J.; Wang, F.; Liu, C.; Wang, G.; Feng, X. Global warming accelerates uptake of atmospheric mercury in regions experiencing glacier retreat. Proc. Natl. Acad. Sci. USA 2020, 117, 2049–2055. [Google Scholar] [CrossRef]
- Radić, V.; Hock, R. Regionally differentiated contribution of mountain glaciers and ice caps to future sea-level rise. Nat. Geosci. 2011, 4, 91–94. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Q.; Kang, S.; Guo, J.; Li, X.; Yu, Z.; Zhang, G.; Qu, D.; Huang, J.; Cong, Z.; et al. Mercury speciation and distribution in a glacierized mountain environment and their relevance to environmental risks in the inland Tibetan Plateau. Sci. Total Environ. 2018, 631–632, 270–278. [Google Scholar] [CrossRef]
- Pedersen, K.B.; Lejon, T.; Jensen, P.E.; Ottosen, L.M.; Frantzen, M.; Evenset, A. Impacts of climate change on metal leaching and partitioning for submarine mine tailings disposal. Mar. Pollut. Bull. 2022, 184, 114197. [Google Scholar] [CrossRef]
- Superville, P.-J.; Prygiel, E.; Magnier, A.; Lesven, L.; Gao, Y.; Baeyens, W.; Ouddane, B.; Dumoulin, D.; Billon, G. Daily variations of Zn and Pb concentrations in the Deûle River in relation to the resuspension of heavily polluted sediments. Sci. Total Environ. 2014, 470–471, 600–607. [Google Scholar] [CrossRef]
- Ruiz-Fernández, A.C.; Sanchez-Cabeza, J.A.; Pérez-Bernal, L.H.; Gracia, A. Spatial and temporal distribution of heavy metal concentrations and enrichment in the southern Gulf of Mexico. Sci. Total Environ. 2019, 651, 3174–3186. [Google Scholar] [CrossRef]
- Ramirez-Llodra, E.; Trannum, H.C.; Evenset, A.; Levin, L.A.; Andersson, M.; Finne, T.E.; Hilario, A.; Flem, B.; Christensen, G.; Schaanning, M.; et al. Submarine and deep-sea mine tailing placements: A review of current practices, environmental issues, natural analogs and knowledge gaps in Norway and internationally. Mar. Pollut. Bull. 2015, 97, 13–35. [Google Scholar] [CrossRef]
- Oelsner, G.P.; Stets, E.G. Recent trends in nutrient and sediment loading to coastal areas of the conterminous U.S.: Insights and global context. Sci. Total Environ. 2019, 654, 1225–1240. [Google Scholar] [CrossRef]
- Gao, W.; Qu, B.; Yuan, H.; Song, J.; Li, W. Heavy metal mobility in contaminated sediments under seawater acidification. Mar. Pollut. Bull. 2023, 192, 115062. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, F.; Chen, C.; Sun, X.; Shi, Y.; Zhao, H.; Chen, F. Spatial distribution and correlation characteristics of heavy metals in the seawater, suspended particulate matter and sediments in Zhanjiang Bay, China. PLoS ONE 2018, 13, e0201414. [Google Scholar] [CrossRef]
- Yu, Z.-G.; Zhang, L.; Wu, Y.; Jin, B. Efflux behavior of inorganic mercury and methylmercury in the marine copepod Tigriopus japonicus. Sci. Total Environ. 2020, 703, 135655. [Google Scholar] [CrossRef]
- Kløve, B.; Ala-Aho, P.; Bertrand, G.; Gurdak, J.J.; Kupfersberger, H.; Kværner, J.; Muotka, T.; Mykrä, H.; Preda, E.; Rossi, P.; et al. Climate change impacts on groundwater and dependent ecosystems. J. Hydrol. 2014, 518, 250–266. [Google Scholar] [CrossRef]
- Chen, M.; Qin, X.; Zeng, G.; Li, J. Impacts of human activity modes and climate on heavy metal “spread” in groundwater are biased. Chemosphere 2016, 152, 439–445. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Z.-G.; Zeng, G.-M.; Jiang, M.; Yang, Z.-Z.; Cui, F.; Zhu, M.-Y.; Shen, L.-Q.; Hu, L. Effects of sediment geochemical properties on heavy metal bioavailability. Environ. Int. 2014, 73, 270–281. [Google Scholar] [CrossRef]
- Nizoli, E.C.; Luiz-Silva, W. Seasonal AVS–SEM relationship in sediments and potential bioavailability of metals in industrialized estuary, southeastern Brazil. Environ. Geochem. Health 2012, 34, 263–272. [Google Scholar] [CrossRef]
- Fernandes, L.; Nayak, G.N.; Ilangovan, D.; Borole, D.V. Accumulation of sediment, organic matter and trace metals with space and time, in a creek along Mumbai coast, India. Estuar. Coast. Shelf Sci. 2011, 91, 388–399. [Google Scholar] [CrossRef]
- Cornu, J.-Y.; Denaix, L.; Lacoste, J.; Sappin-Didier, V.; Nguyen, C.; Schneider, A. Impact of temperature on the dynamics of organic matter and on the soil-to-plant transfer of Cd, Zn and Pb in a contaminated agricultural soil. Environ. Sci. Pollut. Res. 2016, 23, 2997–3007. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468–469, 843–853. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Zhou, Y.; Dou, L.; Cai, L.; Mo, L.; You, J. Bioavailability and soil-to-crop transfer of heavy metals in farmland soils: A case study in the Pearl River Delta, South China. Environ. Pollut. 2018, 235, 710–719. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Wu, H.; Zhang, T.; Liu, X.; Li, W. Temporal-spatial variation and partitioning of dissolved and particulate heavy metal(loid)s in a river affected by mining activities in Southern China. Environ. Sci. Pollut. Res. 2018, 25, 9828–9839. [Google Scholar] [CrossRef]
- Lu, S.; Teng, Y.; Wang, Y.; Wu, J.; Wang, J. Research on the ecological risk of heavy metals in the soil around a Pb–Zn mine in the Huize County, China. Chin. J. Geochem. 2015, 34, 540–549. [Google Scholar] [CrossRef]
- Bell, J.E.; Sherry, R.; Luo, Y. Changes in soil water dynamics due to variation in precipitation and temperature: An ecohydrological analysis in a tallgrass prairie. Water Resour. Res. 2010, 46, W03523. [Google Scholar] [CrossRef]
- Nedrich, S.M.; Burton, G.A. Indirect effects of climate change on zinc cycling in sediments: The role of changing water levels. Environ. Toxicol. Chem. 2017, 36, 2456–2464. [Google Scholar] [CrossRef]
- Liu, L.; Ouyang, W.; Wang, Y.; Tysklind, M.; Hao, F.; Liu, H.; Hao, X.; Xu, Y.; Lin, C.; Su, L. Heavy metal accumulation, geochemical fractions, and loadings in two agricultural watersheds with distinct climate conditions. J. Hazard. Mater. 2020, 389, 122125. [Google Scholar] [CrossRef]
- Lüders, K.; Dahmke, A.; Fiedler, M.; Köber, R. Temperature influence on mobilisation and (re)fixation of trace elements and heavy metals in column tests with aquifer sediments from 10 to 70 °C. Water Res. 2020, 169, 115266. [Google Scholar] [CrossRef]
- Wang, Z.; Shan, J.; Liu, C.; Wang, K.; Teng, H.; Jiang, Q. Effects of winter warming on the migration characteristics and pollution risk assessment of Zn, Cu, and Pb in the snow-soil continuum in seasonal snow cover area. Environ. Technol. Innov. 2023, 31, 103159. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Wang, P.; Wang, Z.; Li, M.; Zhao, F.-J. Dietary cadmium intake from rice and vegetables and potential health risk: A case study in Xiangtan, southern China. Sci. Total Environ. 2018, 639, 271–277. [Google Scholar] [CrossRef]
- Bhagat, C.; Kumar, M.; Mahlknecht, J.; Hdeib, R.; Mohapatra, P.K. Seawater intrusion decreases the metal toxicity but increases the ecological risk and degree of treatment for coastal groundwater: An Indian perspective. Environ. Pollut. 2022, 310, 119771. [Google Scholar] [CrossRef]
- Karthikeyan1, S.; Arumugam, S.; Muthumanickam, J.; Kulandaisamy, P.; Subramanian, M.; Annadurai, R.; Senapathi, V.; Sekar, S. Causes of heavy metal contamination in groundwater of Tuticorin industrial block, Tamil Nadu, India. Environ. Sci. Pollut. Res. 2021, 28, 18651–18666. [Google Scholar] [CrossRef]
- Cesur, A.; Zeren Cetin, I.; Abo Aisha, A.E.S.; Alrabiti, O.B.M.; Aljama, A.M.O.; Jawed, A.A.; Cetin, M.; Sevik, H.; Ozel, H.B. The usability of Cupressus arizonica annual rings in monitoring the changes in heavy metal concentration in air. Environ. Sci. Pollut. Res. 2021, 28, 35642–35648. [Google Scholar] [CrossRef]
- Mahapatra, B.; Dhal, N.K.; Dash, A.K.; Panda, B.P.; Panigrahi, K.C.S.; Pradhan, A. Perspective of mitigating atmospheric heavy metal pollution: Using mosses as biomonitoring and indicator organism. Environ. Sci. Pollut. Res. 2019, 26, 29620–29638. [Google Scholar] [CrossRef]
- Turkyilmaz, A.; Sevik, H.; Isinkaralar, K.; Cetin, M. Using Acer platanoides annual rings to monitor the amount of heavy metals accumulated in air. Environ. Monit. Assess. 2018, 190, 578. [Google Scholar] [CrossRef] [PubMed]
- Schlutow, A.; Schröder, W.; Scheuschner, T. Assessing the relevance of atmospheric heavy metal deposition with regard to ecosystem integrity and human health in Germany. Environ. Sci. Eur. 2021, 33, 7. [Google Scholar] [CrossRef]
- Malunguja, G.K.; Thakur, B.; Devi, A. Heavy Metal Contamination of Forest Soils by Vehicular Emissions: Ecological Risks and Effects on Tree Productivity. Environ. Process. 2022, 9, 11. [Google Scholar] [CrossRef]
- Vetrimurugan, E.; Shruti, V.C.; Jonathan, M.P.; Roy, P.D.; Sarkar, S.K.; Rawlins, B.K.; Villegas, L.E.C. Comprehensive study on metal contents and their ecological risks in beach sediments of KwaZulu-Natal province, South Africa. Mar. Pollut. Bull. 2019, 149, 110555. [Google Scholar] [CrossRef] [PubMed]
- Vetrimurugan, E.; Shruti, V.; Jonathan, M.; Roy, P.D.; Rawlins, B.; Rivera-Rivera, D. Metals and their ecological impact on beach sediments near the marine protected sites of Sodwana Bay and St. Lucia, South Africa. Mar. Pollut. Bull. 2018, 127, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Nemr, A.E.; Khaled, A.; Sikaily, A.E. Distribution and statistical analysis of leachable and total heavy metals in the sediments of the Suez Gulf. Environ. Monit. Assess. 2006, 118, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Owens, G. Metal distributions in seawater, sediment and marine benthic macroalgae from the South Australian coastline. Int. J. Environ. Sci. Technol. 2014, 11, 1259–1270. [Google Scholar] [CrossRef]
- Alkan, N.; Alkan, A.; Akbaş, U.; Fisher, A. Metal pollution assessment in sediments of the southeastern Black Sea Coast of Turkey. Soil Sediment Contam. Int. J. 2015, 24, 290–305. [Google Scholar] [CrossRef]
- Gutiérrez-Mosquera, H.; Shruti, V.; Jonathan, M.; Roy, P.D.; Rivera-Rivera, D. Metal concentrations in the beach sediments of Bahia Solano and Nuquí along the Pacific coast of Chocó, Colombia: A baseline study. Mar. Pollut. Bull. 2018, 135, 1–8. [Google Scholar] [CrossRef]
- Jonathan, M.; Roy, P.; Thangadurai, N.; Srinivasalu, S.; Rodríguez-Espinosa, P.; Sarkar, S.; Lakshumanan, C.; Navarrete-López, M.; Muñoz-Sevilla, N.J.M.p.b. Metal concentrations in water and sediments from tourist beaches of Acapulco, Mexico. Mar. Pollut. Bull. 2011, 62, 845–850. [Google Scholar] [CrossRef]
- Topcuoğlu, S.; Kirbaşoğlu, Ç.; Yilmaz, Y.Z.J.E.M. Heavy metal levels in biota and sediments in the northern coast of the Marmara Sea. Environ. Monit. Assess. 2004, 96, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.C.; Devi, N.L.; Singh, V.K.; Li, J.; Zhang, G. Spatial distribution, source analysis, and health risk assessment of heavy metals contamination in house dust and surface soil from four major cities of Nepal. Chemosphere 2019, 218, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Štrbac, S.; Ranđelović, D.; Gajica, G.; Hukić, E.; Stojadinović, S.; Veselinović, G.; Orlić, J.; Tognetti, R.; Kašanin-Grubin, M. Spatial distribution and source identification of heavy metals in European mountain beech forests soils. Chemosphere 2022, 309, 136662. [Google Scholar] [CrossRef] [PubMed]
- Sattarova, V.V.; Aksentov, K.I. Geochemistry of mercury in surface sediments of the Kuril Basin of the Sea of Okhotsk, Kuril-Kamchatka Trench and adjacent abyssal plain and northwest part of the Bering Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 154, 24–31. [Google Scholar] [CrossRef]
- Ma, J.; Ullah, S.; Niu, A.; Liao, Z.; Qin, Q.; Xu, S.; Lin, C. Heavy metal pollution increases CH4 and decreases CO2 emissions due to soil microbial changes in a mangrove wetland: Microcosm experiment and field examination. Chemosphere 2021, 269, 128735. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, E.D.; Nwachukwu, E.D. Heavy metal content and health risk assessment of a South-eastern Nigeria River. Appl. Water Sci. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Zeng, S.; Li, X.; Yang, L.; Wang, D. Understanding heavy metal distribution in timberline vegetations: A case from the Gongga Mountain, eastern Tibetan Plateau. Sci. Total Environ. 2023, 874, 162523. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, P.R.S.; Fernandes, G.J.T.; Moraes, E.P.; Moreira, L.F.F. Tropical climate effect on the toxic heavy metal pollutant course of road-deposited sediments. Environ. Pollut. 2019, 251, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Qi, J.; Xia, X. Long-term variations in sediment heavy metals of a reservoir with changing trophic states: Implications for the impact of climate change. Sci. Total Environ. 2017, 609, 242–250. [Google Scholar] [CrossRef]
- Zhang, H.; Huo, S.; Yeager, K.M.; Xi, B.; Zhang, J.; He, Z.; Ma, C.; Wu, F. Accumulation of arsenic, mercury and heavy metals in lacustrine sediment in relation to eutrophication: Impacts of sources and climate change. Ecol. Indic. 2018, 93, 771–780. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, Z.; Zhan, C.; Liu, S.; Zhang, J.; Liu, H.; Liu, Z.; Liu, X. Pollution Characteristics and Associated Risk Assessment of Heavy Metals in Farmland Soils From a Typical County of Hubei Province, Central China. Bull. Environ. Contam. Toxicol. Mech. Methods 2021, 107, 327–335. [Google Scholar] [CrossRef]
- Oubane, M.; Khadra, A.; Ezzariai, A.; Kouisni, L.; Hafidi, M. Heavy metal accumulation and genotoxic effect of long-term wastewater irrigated peri-urban agricultural soils in semiarid climate. Sci. Total Environ. 2021, 794, 148611. [Google Scholar] [CrossRef] [PubMed]
- Zaida, F.; Chadrame, S.; Sedki, A.; Lekouch, N.; Bureau, F.; Arhan, P.; Bouglé, D. Lead and aluminium levels in infants’ hair, diet, and the local environment in the Moroccan city of Marrakech. Sci. Total Environ. 2007, 377, 152–158. [Google Scholar] [CrossRef]
- Nayak, S.K.; Nandimandalam, J.R. Impacts of climate change and coastal salinization on the environmental risk of heavy metal contamination along the odisha coast, India. Environ. Res. 2023, 238, 117175. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Shahid, M.; Natasha;Shah, A.H.; Saeed, F.; Ali, M.; Qaisrani, S.A.; Dumat, C. Heavy metal contamination and exposure risk assessment via drinking groundwater in Vehari, Pakistan. Environ. Sci. Pollut. Res. 2020, 27, 39852–39864. [Google Scholar] [CrossRef]
- Pan, Y.; Ding, L.; Xie, S.; Zeng, M.; Zhang, J.; Peng, H. Spatiotemporal simulation, early warning, and policy recommendations of the soil heavy metal environmental capacity of the agricultural land in a typical industrial city in China: Case of Zhongshan City. J. Clean. Prod. 2021, 285, 124849. [Google Scholar] [CrossRef]
- Zhao, F.-j.; Ma, Y.; Zhu, Y.-g.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.-N.; Qiu, B.; Wu, F.-B.; Zhang, G.-P. The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ. Pollut. 2011, 15, 84–91. [Google Scholar] [CrossRef]
- Change, I.C. Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; United Nations Economic Commission for Africa: Addis Ababa, Ethiopia, 2014; Volume 1454, p. 147. [Google Scholar]
- Mouginot, C.; Kawamura, R.; Matulich, K.L.; Berlemont, R.; Allison, S.D.; Amend, A.S.; Martiny, A.C. Elemental stoichiometry of Fungi and Bacteria strains from grassland leaf litter. Soil Biol. Biochem. 2014, 76, 278–285. [Google Scholar] [CrossRef]
- Fu, Q.L.; Weng, N.Y.; Fujii, M.; Zhou, D.M. Temporal variability in Cu speciation, phytotoxicity, and soil microbial activity of Cu-polluted soils as affected by elevated temperature. Chemosphere 2018, 194, 285–296. [Google Scholar] [CrossRef]
- Duval, B.; Tessier, E.; Kortazar, L.; Fernandez, L.A.; de Diego, A.; Amouroux, D. Dynamics, distribution, and transformations of mercury species from pyrenean high-altitude lakes. Environ. Res. 2023, 216, 114611. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.Q.; Cang, L.; Liu, H.; Zhou, D.M. Effects of warming on uptake and translocation of cadmium (Cd) and copper (Cu) in a contaminated soil-rice system under Free Air Temperature Increase (FATI). Chemosphere 2016, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Cang, L.; Ata-Ul-Karim, S.T.; Yang, J.; Zhou, D. Effects of various warming patterns on Cd transfer in soil-rice systems under Free Air Temperature Increase (FATI) conditions. Ecotoxicol. Environ. Saf. 2019, 168, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, L.; Chen, J.; Zhou, H.; Wan, Y.; Qu, Q.; Wang, M.; Xue, S. Changes in soil microbial activity and their linkages with soil carbon under global warming. Catena 2023, 232, 107419. [Google Scholar] [CrossRef]
- González-Alcaraz, M.N.; Loureiro, S.; van Gestel, C.A.M. Toxicokinetics of Zn and Cd in the earthworm Eisenia andrei exposed to metal-contaminated soils under different combinations of air temperature and soil moisture content. Chemosphere 2018, 197, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Cang, L.; Liu, H.; Zhou, D. Effects of different warming patterns on the translocations of cadmium and copper in a soil-rice seedling system. Environ. Sci. Pollut. Res. Int. 2015, 22, 15835–15843. [Google Scholar] [CrossRef] [PubMed]
- Medfu Tarekegn, M.; Zewdu Salilih, F.; Ishetu, A.I. Microbes used as a tool for bioremediation of heavy metal from the environment. Cogent Food Agric. 2020, 6, 1783174. [Google Scholar] [CrossRef]
- Bouabidi, Z.B.; El-Naas, M.H.; Zhang, Z. Immobilization of microbial cells for the biotreatment of wastewater: A review. Environ. Chem. Lett. 2019, 17, 241–257. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 2022, 307, 135957. [Google Scholar] [CrossRef] [PubMed]
- Roobaert, A.; Laruelle, G.G.; Resplandy, L.; Landschützer, P.; Gruber, N.; Liao, E.; Chou, L.; Régnier, P. The Spatiotemporal Dynamics of the Sources and Sinks of CO2 in the Global Coastal Ocean. Glob. Biogeochem. Cycles 2019, 33, 1693–1714. [Google Scholar] [CrossRef]
- Sulman, B.N.; Phillips, R.P.; Oishi, A.C.; Shevliakova, E.; Pacala, S.W. Microbe-driven turnover offsets mineral-mediated storage of soil carbon under elevated CO2. Nat. Clim. Chang. 2014, 4, 1099–1102. [Google Scholar] [CrossRef]
- Logan, C.A. A Review of Ocean Acidification and America’s Response. Bioscience 2010, 60, 819–828. [Google Scholar] [CrossRef]
- Ardelan, M.V.; Steinnes, E.; Lierhagen, S.; Linde, S.O. Effects of experimental CO2 leakage on solubility and transport of seven trace metals in seawater and sediment. Sci. Total Environ. 2009, 40, 6255–6266. [Google Scholar] [CrossRef]
- Martín-Torre, M.C.; Cifrian, E.; Ruiz, G.; Galán, B.; Viguri, J.R. Estuarine sediment resuspension and acidification: Release behaviour of contaminants under different oxidation levels and acid sources. J. Environ. Manag. 2017, 199, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Worm, B.; Lotze, H.K. Marine biodiversity and climate change. In Climate Change; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Kroeker, K.J.; Sanford, E. Ecological Leverage Points: Species Interactions Amplify the Physiological Effects of Global Environmental Change in the Ocean. Annu. Rev. Mar. Sci. 2021, 14, 75–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Zhao, P.; Chen, L.; Yan, C.; Yan, Y.; Chi, Q. Metal release from contaminated coastal sediments under changing pH conditions: Implications for metal mobilization in acidified oceans. Mar. Pollut. Bull. 2015, 101, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Batley, G. Sediment Quality Assessment: A Practical Guide; CSIRO Publishing: Clayton, Australia, 2016. [Google Scholar]
- Liu, J.; Chen, X.; Shu, H.-Y.; Lin, X.-R.; Zhou, Q.-X.; Bramryd, T.; Shu, W.-S.; Huang, L.-N. Microbial community structure and function in sediments from e-waste contaminated rivers at Guiyu area of China. Environ. Pollut. 2018, 235, 171–179. [Google Scholar] [CrossRef]
- Vega, F.A.; Weng, L. Speciation of heavy metals in River Rhine. Water Res. 2013, 47, 363–372. [Google Scholar] [CrossRef]
- Su, R.; Wu, X.; Hu, J.; Li, H.; Xiao, H.; Zhao, J.; Hu, R. Warming promotes the decomposition of oligotrophic bacterial-driven organic matter in paddy soil. Soil Biol. Biochem. 2023, 186, 109156. [Google Scholar] [CrossRef]
- Trenberth, K.E. Changes in precipitation with climate change. Clim. Res. 2011, 47, 123–138. [Google Scholar] [CrossRef]
- Srivastava, V.; Sarkar, A.; Singh, S.; Singh, P.; de Araujo, A.S.F.; Singh, R.P. Agroecological Responses of Heavy Metal Pollution with Special Emphasis on Soil Health and Plant Performances. Front. Environ. Sci. 2017, 5, 64. [Google Scholar] [CrossRef]
- María-Cervantes, A.; Conesa, H.M.; González-Alcaraz, M.N.; Álvarez-Rogel, J. Rhizosphere and flooding regime as key factors for the mobilisation of arsenic and potentially harmful metals in basic, mining-polluted salt marsh soils. Appl. Geochem. 2010, 25, 1722–1733. [Google Scholar] [CrossRef]
- Tan, X.; He, J.; Nie, Y.; Ni, X.; Ye, Q.; Ma, L.; Megharaj, M.; He, W.; Shen, W. Climate and edaphic factors drive soil enzyme activity dynamics and tolerance to Cd toxicity after rewetting of dry soil. Sci. Total Environ. 2023, 855, 158926. [Google Scholar] [CrossRef] [PubMed]
- Pesce, S.; Lambert, A.-S.; Morin, S.; Foulquier, A.; Coquery, M.; Dabrin, A. Experimental warming differentially influences the vulnerability of phototrophic and heterotrophic periphytic communities to copper toxicity. Front. Microbiol. 2018, 9, 1424. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals-Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Wu, J.; Lu, J.; Zhang, C.; Zhang, Y.X.; Lin, Y.C.; Xu, J. Pollution, sources, and risks of heavy metals in coastal waters of China. Hum. Ecol. Risk Assess. 2020, 26, 2011–2026. [Google Scholar] [CrossRef]
- Yin, K.; Wang, Q.; Lv, M.; Chen, L. Microorganism remediation strategies towards heavy metals. Chem. Eng. J. 2019, 360, 1553–1563. [Google Scholar] [CrossRef]
- Wen, J.; Zou, D.-h. Interactive effects of increasing atmospheric CO2 and copper exposure on the growth and photosynthesis in the young sporophytes of Sargassum fusiforme (Phaeophyta). Chemosphere 2020, 269, 129397. [Google Scholar] [CrossRef]
- Tripathi, S.; Poluri, K.M. Heavy metal detoxification mechanisms by microalgae: Insights from transcriptomics analysis. Environ. Pollut. 2021, 285, 117443. [Google Scholar] [CrossRef]
- Múgica, M.; Izagirre, U.; Marigómez, I.J.A.T. Lysosomal responses to heat-shock of seasonal temperature extremes in Cd-exposed mussels. Aquat. Toxicol. 2015, 164, 99–107. [Google Scholar] [CrossRef]
- Pančić, M.; Kiørboe, T. Phytoplankton defence mechanisms: Traits and trade-offs. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1269–1303. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Pan, Y.; Zhu, X. Salinization and heavy metal cadmium impair growth but have contrasting effects on defensive colony formation of Scenedesmus obliquus. Sci. Total Environ. 2023, 862, 160693. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.X.; Wang, Y.Y.; Hou, X.Y.; Kong, Q.D.; Sun, Y.F.; Wang, J.; Huang, Y.; Yang, Z. High temperature promotes the inhibition effect of Zn2+ on inducible defense of Scenedesmus obliquus. Chemosphere 2019, 216, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.K.; Sim, K.S.; Poong, S.W.; Wei, D.; Phang, S.M.; Lim, P.E. Interactive effects of warming and copper toxicity on a tropical freshwater green microalgaChloromonas augustae (Chlorophyceae). J. Appl. Phycol. 2021, 33, 67–77. [Google Scholar] [CrossRef]
- Biscéré, T.; Lorrain, A.; Rodolfo-Metalpa, R.; Gilbert, A.; Wright, A.; Devissi, C.; Peignon, C.; Farman, R.; Duvieilbourg, E.; Payri, C.; et al. Nickel and ocean warming affect scleractinian coral growth. Mar. Pollut. Bull. 2017, 120, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhang, J.; Zeng, X.; Huang, J.; Lin, J.; Lu, Y.; Liang, S.; Ye, M.; Xiao, M.; Zhao, J.; et al. Adaptation of a marine diatom to ocean acidification increases its sensitivity to toxic metal exposure. Mar. Pollut. Bull. 2022, 183, 114056. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.H.; Guo, Y.Y.; Liang, Z.; Huang, Q.T.; Lu, H.; Pan, J.M.; Li, P.Y.; Jin, P.; Xia, J.R. Adaptation of a marine diatom to ocean acidification and warming reveals constraints and trade-offs. Sci. Total Environ. 2021, 771, 145167. [Google Scholar] [CrossRef] [PubMed]
- Fietzke, J.; Ragazzola, F.; Halfar, J.; Dietze, H.; Foster, L.C.; Hansteen, T.H.; Eisenhauer, A.; Steneck, R.S. Century-scale trends and seasonality in pH and temperature for shallow zones of the Bering Sea. Proc. Natl. Acad. Sci. USA 2015, 112, 2960–2965. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Stow, C.A.; Gronewold, A.D.; Mason, L.A.; McCormick, M.J.; Qian, S.S.; Ruberg, S.A.; Beadle, K.; Constant, S.A.; Hawley, N. Seasonal overturn and stratification changes drive deep-water warming in one of Earth’s largest lakes. Nat. Commun. 2021, 12, 1688. [Google Scholar] [CrossRef]
- Fadhlaoui, M.; Laderriere, V.; Lavoie, I.; Fortin, C. Influence of temperature and nickel on algal biofilm fatty acid composition. Environ. Toxicol. 2020, 39, 1566–1577. [Google Scholar] [CrossRef]
- Pascual, G.; Sano, D.; Sakamaki, T.; Akiba, M.; Nishimura, O. The water temperature changes the effect of pH on copper toxicity to the green microalgae Raphidocelis subcapitata. Chemosphere 2022, 291, 133110. [Google Scholar] [CrossRef] [PubMed]
- De Schamphelaere, K.A.; Janssen, C.R. Bioavailability models for predicting copper toxicity to freshwater green microalgae as a function of water chemistry. Environ. Sci. Technol. 2006, 40, 4514–4522. [Google Scholar] [CrossRef] [PubMed]
- Nardi, A.; Mincarelli, L.F.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Regoli, F. Indirect effects of climate changes on cadmium bioavailability and biological effects in the Mediterranean mussel Mytilus galloprovincialis. Chemosphere 2017, 169, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Katsikatsou, M.; Anestis, A.; Portner, H.O.; Kampouris, T.; Michaelidis, B. Field studies on the relation between the accumulation of heavy metals and metabolic and HSR in the bearded horse mussel Modiolus barbatus. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2011, 153, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Nardi, A.; Benedetti, M.; d’Errico, G.; Fattorini, D.; Regoli, F. Effects of ocean warming and acidification on accumulation and cellular responsiveness to cadmium in mussels Mytilus galloprovincialis: Importance of the seasonal status. Aquat. Toxicol. 2018, 204, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kwak, I.-S. Environmental co-exposure of high temperature and Cu induce hormonal disturbance of cortisol signaling and altered responses of cellular defense genes in zebrafish. Sci. Total Environ. 2022, 842, 156555. [Google Scholar] [CrossRef] [PubMed]
- Sappal, R.; Fast, M.; Stevens, D.; Kibenge, F.; Siah, A.; Kamunde, C. Effects of copper, hypoxia and acute temperature shifts on mitochondrial oxidation in rainbow trout (Oncorhynchus mykiss) acclimated to warm temperature. Aquat. Toxicol. 2015, 169, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Zebral, Y.D.; Roza, M.; da Silva Fonseca, J.; Costa, P.G.; de Oliveira, C.S.; Zocke, T.G.; Dal Pizzol, J.L.; Robaldo, R.B.; Bianchini, A. Waterborne copper is more toxic to the killifish Poecilia vivipara in elevated temperatures: Linking oxidative stress in the liver with reduced organismal thermal performance. Aquat. Toxicol. 2019, 209, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Han, E.J.; Ahn, G.; Kwak, I.S. Effects of combined stressors to cadmium and high temperature on antioxidant defense, apoptotic cell death, and DNA methylation in zebrafish (Danio rerio) embryos. Sci. Total Environ. 2020, 716, 137130. [Google Scholar] [CrossRef]
- Park, K.; Han, E.J.; Ahn, G.; Kwak, I.S. Effects of thermal stress-induced lead (Pb) toxicity on apoptotic cell death, inflammatory response, oxidative defense, and DNA methylation in zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2020, 224, 105479. [Google Scholar] [CrossRef]
- Jin, M.; Ji, X.; Zhang, B.; Sheng, W.; Wang, R.; Liu, K. Synergistic effects of Pb and repeated heat pulse on developmental neurotoxicity in zebrafish. Ecotoxicol. Environ. Saf. 2019, 172, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, A.P.; Li, W.F.; Shi, R.; Jin, H.T.; Wei, J.F. Sensitive biomarkers identification for differentiating Cd and Pb induced toxicity on zebrafish embryos. Environ. Toxicol. Pharmacol. 2017, 56, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Frakes, J.I.; Malison, R.L.; Sydor, M.J.; Arthur Woods, H. Exposure to copper increases hypoxia sensitivity and decreases upper thermal tolerance of giant salmonfly nymphs (Pteronarcys californica). J. Insect Physiol. 2022, 143, 104455. [Google Scholar] [CrossRef] [PubMed]
- Sartori, D.; Scatena, G.; Vrinceanu, C.A.; Gaion, A. Increased sensitivity of sea urchin larvae to metal toxicity as a consequence of the past two decades of Climate Change and Ocean Acidification in the Mediterranean Sea. Mar. Pollut. Bull. 2023, 194, 115274. [Google Scholar] [CrossRef] [PubMed]
- Desforges, J.P.; Outridge, P.; Hobson, K.A.; Heide-Jørgensen, M.P.; Dietz, R. Anthropogenic and Climatic Drivers of Long-Term Changes of Mercury and Feeding Ecology in Arctic Beluga (Delphinapterus leucas) Populations. Environ. Sci. Technol. 2022, 56, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Loseto, L.L.; Stern, G.A.; Macdonald, R.W. Distant drivers or local signals: Where do mercury trends in western Arctic belugas originate? Sci. Total Environ. 2015, 509–510, 226–236. [Google Scholar] [CrossRef]
- Loseto, L.L.; Stern, G.A.; Ferguson, S.H. Size and biomagnification: How Habitat selection explains beluga mercury levels. Environ. Sci. Technol. 2008, 42, 3982–3988. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, A.A.; Abdel-Wahhab, M.A. Occurrence of trace metals in foodstuffs and their health impact. Trends Food Sci. Technol. 2018, 75, 36–45. [Google Scholar] [CrossRef]
- Li, X.; Peng, P.; Long, J.; Dong, X.; Jiang, K.; Hou, H. Plant-induced insoluble Cd mobilization and Cd redistribution among different rice cultivars. J. Clean. Prod. 2020, 256, 120494. [Google Scholar] [CrossRef]
- Mitra, S.; Pramanik, K.; Sarkar, A.; Ghosh, P.K.; Soren, T.; Maiti, T.K. Bioaccumulation of cadmium by Enterobacter sp. and enhancement of rice seedling growth under cadmium stress. Ecotoxicol. Environ. Saf. 2018, 156, 183–196. [Google Scholar] [CrossRef]
- Ziska, L.H.; Bunce, J.; Shimono, H.; Gealy, D.R.; Baker, J.T.; Newton, P.C.D.; Reynolds, M.P.; Jagadish, K.S.V.; Zhu, C.; Howden, M.; et al. Food security and climate change: On the potential to adapt global crop production by active selection to rising atmospheric carbon dioxide. Proc. R. Soc. B Biol. Sci. 2012, 279, 4097–4105. [Google Scholar] [CrossRef] [PubMed]
- Högy, P.; Kottmann, L.; Schmid, I.; Fangmeier, A.J.J.o.A. Heat, wheat and CO2: The relevance of timing and the mode of temperature stress on biomass and yield. J. Agron. Crop Sci. 2019, 205, 608–615. [Google Scholar] [CrossRef]
- Peijnenburg, W.J.G.M.; Zablotskaja, M.; Vijver, M.G. Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotoxicol. Environ. Saf. 2007, 67, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Holmstrup, M.; Bindesbøl, A.-M.; Oostingh, G.J.; Duschl, A.; Scheil, V.; Köhler, H.-R.; Loureiro, S.; Soares, A.M.V.M.; Ferreira, A.L.G.; Kienle, C.; et al. Interactions between effects of environmental chemicals and natural stressors: A review. Sci. Total Environ. 2010, 408, 3746–3762. [Google Scholar] [CrossRef] [PubMed]
- González-Alcaraz, M.N.; van Gestel, C.A.M. Climate change effects on enchytraeid performance in metal-polluted soils explained from changes in metal bioavailability and bioaccumulation. Environ. Res. 2015, 142, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.B.; Pomati, F.; Eggen, R.I.L. The toxicity of chemical pollutants in dynamic natural systems: The challenge of integrating environmental factors and biological complexity. Sci. Total Environ. 2013, 449, 253–259. [Google Scholar] [CrossRef]
- Silva, A.R.R.; Malheiro, C.; Loureiro, S.; González-Alcaraz, M.N. Toxicity of historically metal(loid)-contaminated soils to Folsomia candida under the influence of climate change alterations. Environ. Pollut. 2022, 305, 119256. [Google Scholar] [CrossRef]
- Ge, J.; Slotsbo, S.; Sørensen, J.G.; Holmstrup, M. Copper-contaminated soil compromises thermal performance in the springtail Folsomia candida (Collembola). Sci. Total Environ. 2023, 897, 165334. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Högy, P.; Zikeli, S.; Pignata, M.L.; Rodriguez, J.H. Assessment of elevated CO2 concentrations and heat stress episodes in soybean cultivars growing in heavy metal polluted soils: Crop nutritional quality and food safety. Environ. Pollut. 2022, 303, 119123. [Google Scholar] [CrossRef]
- Nechita, C.; Iordache, A.M.; Lemr, K.; Levanič, T.; Pluhacek, T. Evidence of declining trees resilience under long term heavy metal stress combined with climate change heating. J. Clean. Prod. 2021, 317, 128428. [Google Scholar] [CrossRef]
- Rejomon, G.; Kumar, P.D.; Nair, M.; Muraleedharan, K.J.E.t. Trace metal dynamics in zooplankton from the Bay of Bengal during summer monsoon. Environ. Toxicol. 2010, 25, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Zelano, I.; Malandrino, M.; Giacomino, A.; Buoso, S.; Conca, E.; Sivry, Y.; Benedetti, M.; Abollino, O. Element variability in lacustrine systems of Terra Nova Bay (Antarctica) and concentration evolution in surface waters. Chemosphere 2017, 180, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Huang, M.; You, W.; Cai, L.; Hong, Y.; Xiao, Q.; Zheng, X.; Lin, R. Spatial analysis and risk assessment of heavy metal pollution in rice in Fujian Province, China. Front. Environ. Sci. 2022, 10, 2422. [Google Scholar] [CrossRef]
- Singh, G.; Patel, N.; Jindal, T.; Ranjan, M.R. Heavy metal contamination in soils and crops irrigated by Kali River in Uttar Pradesh, India. Bull. Environ. Contam. Toxicol. Mech. Methods 2021, 107, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Ramlan;Basir-Cyio, M.; Napitupulu, M.; Inoue, T.; Anshary, A.; Mahfudz;Isrun;Rusydi, M.; Sulbadanam, G. Pollution and contamination level of Cu, Cd, and Hg heavy metals in soil and food crop. Int. J. Environ. Sci. Technol. 2022, 19, 1153–1164. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Z.; Liu, Y.; Shan, B.; Yu, W.; Li, H.; Sun, D. Heavy metal pollution and stable isotope ratios (δ13C and δ15N) in marine organisms from the Northern Beibu Gulf, South China Sea. Mar. Pollut. Bull. 2021, 166, 112230. [Google Scholar] [CrossRef]
- Liu, H.; Liu, G.; Yuan, Z.; Ge, M.; Wang, S.; Liu, Y.; Da, C. Occurrence, potential health risk of heavy metals in aquatic organisms from Laizhou Bay, China. Mar. Pollut. Bull. 2019, 140, 388–394. [Google Scholar] [CrossRef]

| Location | HM (ppm) | References | ||||||
|---|---|---|---|---|---|---|---|---|
| Cr | Co | Cu | Pb | Cd | Ni | Zn | ||
| Kongsfjorden, Arctic (1925–2018) | 29.27–234.18 | 14.091–77.24 | 16.05–401.75 | 6.83–98.98 | 0.14–1.05 | 31.1–178.82 | 46.48–190.62 | [35,36,37] |
| Tibetan Plateau (1836–2014) | 85.73–1244 | 60.07–118.6 | 20.36–37.85 | 0.13–0.44 | 52.65–335.6 | 80.58–168.7 | [38] | |
| Renuka Lake (1839–2003) | 5–62 | 7–51 | 11–40 | 21–27 | 11–33 | 43–127 | [39] | |
| Poyang lake (1950–2005) | 48.30–54.53 | 0.23–0.59 | [40] | |||||
| Romanian Black Sea (1996–2012) | 1.29–15.01 | 19.36–96 | 0.15–27.70 | 0.45–14.5 | 20–106.34 | [41] | ||
| Location | Sample | HM | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Pb | Cd | Zn | Cr | Ni | Hg | |||
| South Durban beaches | Sediments (ppm) | 50.53 | 14.00 | 0.70 | 64.56 | 302 | 33.32 | 0.98 | [78] |
| South Durban beaches | Sediments (ppm) | 33.63 | 16.06 | 0.41 | 44.57 | 298 | 101.98 | [79] | |
| Suez Gulf, Egypt | Coastal sediments (ppm) | 13.73 | 49.25 | 5.8 | 48.59 | 12.89 | 53.59 | [80] | |
| South Australian coastline | Coastal sediments (ppm) | 50.22 | 662 | 22.12 | 1609 | 26.62 | 662 | [81] | |
| Spain | Coastal sediments (ppm) | 31.8 | 22.2 | 0.30 | 70.2 | 18.5 | 13.8 | [82] | |
| Bahia Solano Beaches, Colombia | Coastal sediments (ppm) | 53.93 | 409.67 | 125.80 | 269.17 | [83] | |||
| Acapulco beach, Mexico | Coastal sediments (ppm) | 0.76–26.97 | 0.13–20.46 | 0.10–12.51 | 3.54–96.23 | 0.64–105.50 | 0.33–16.35 | [84] | |
| Marmara Sea, Turkey | Coastal sediments (ppm) | 20 | 25.4 | 0.50 | 43 | 47.8 | [85] | ||
| Nepal | Surface soil (ppm) | 132–1010 | 59.8–294 | 27.2–93 | 606–4260 | 135–393 | 606–4260 | [86] | |
| European mountain beech forests | Soils (ppm) | 11.3–39.8 | 1.38–91.8 | 0.99–6.03 | 32.5–252 | 4.22–83.4 | 4.79–56.3 | 32.5–252 | [87] |
| Kuril-Kamchatka Trench | Surface sediments (ppm) | 86.2 | 16.9 | 86.3 | 38.2 | 76 (ppb) | [88] | ||
| Bering Sea | Surface sediments (ppm) | 36.4 | 8.7 | 100 | 34.6 | 55 (ppb) | |||
| Sea of Okhotsk | Surface sediments (ppm) | 47.6 | 18.6 | 97.80 | 41.6 | 88 (ppb) | |||
| Shenzhen | Soils (ppm) | 57.46 ± 0.73 | 72.96 ± 0.87 | 2.24 ± 0.33 | 234.82 ± 15.61 | 110.43 ± 0.94 | 37.98 ± 0.16 | 0.46 ± 0.06 | [89] |
| Nigeria | Water samples (ppm) | 0.03–0.30 | 0.02–0.08 | 0.02–0.08 * | 0.55–1.33 * | 0.03–0.30 * | 0.01–0.04 * | [90] | |
| Mt. Gongga (3600–3700 m) | Soils (ppm) | 133.5 | 17.9 | 0.2 | 47.8 | 133.5 | 17.9 | 56.6 (ppb) | [91] |
| Natal | RDS, DS, RS (ppm) | 26.9–108.4 | 49.7–119.4 | 3.2–4.8 | 31.8–149.8 | 19.5–33.7 | [92] | ||
| Beijing | Soils (ppm) | 0.12 | 26.8 | 29.8 | 26.8 | [93] | |||
| China | Sediment of lake (ppm) | 33.93 ± 5.21–64.91 ± 3.16 | 20.49 ± 1.68–30.83 ± 0.55 | 0.25 ± 0.05–0.39 ± 0.08 | 120.17 ± 10.25–128.86 ± 6.69 | 87.83 ± 4.81–100.33 ± 10.04 | 44.16 ± 2.97–62.87 ± 3.83 | 23.25 ± 3.16–76.00 ± 4.31 (ppb) | [94] |
| Yangxin County | Soils (ppm) | 144.9 * | 69.4 | 2.9 * | 188.3 | 55.5 | 137.0 * | [95] | |
| Morocco | Soils (ppm) | 36.65 ± 2.63 | 53.25 ± 3.23 | 14.2 ± 0.81 | 85.21 ± 2.42 | 27.33 ± 1.58 | [96,97] | ||
| India | Groundwater (ppb) | 56.30 | 29.75 * | 12.94 | 800 | 102.94 * | 40.60 | [71] | |
| India | Seawater (ppb) | 2022.7 * | 30.42 * | 5.70 | 252.9 | 1118.5 * | 450 * | ||
| Odisha coastal plains | groundwater (ppm) | 4.09 ± 1.73 | 0.05 ± 0.14 | 1.46 ± 2.44 | 0.28 ± 0.83 | 1.46 ± 2.44 | [98] | ||
| Pakistan | groundwater (ppm) | 0.09–2.63 | 0.0–1.05 | 0.0–0.94 | 0.09–2.63 | [99] | |||
| Guangzhou | Soil (ppm) | 56.81 | 50.67 | 131.33 | 77.20 | 38.31 | 0.19 | [100] | |
| Area | Organism | HM (ppm) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Hg | Cd | Cr | Pb | Ni | Co | Zn | |||
| Bay of Bengal | Zooplankton | 84.9 ± 6.7 | 46.2 ± 5.6 | 19.2 ± 2.6 | 62.8 ± 6.5 | 46.2 ± 4.6 | [178] | |||
| Antarctica | Algae | 0.30–36.70 | 1.06–3.34 | 1.72–29.85 | 0.26–3.15 | 1.32–19.13 | [179] | |||
| southeast coast of China | rice | 1.170–26.616 * | 0.002–0.063 * | 0.000–0.684 * | 0.001–0.897 | 0.008–0.302 * | 0.013–0.884 * | [180] | ||
| Kali River | crops | 3.8–42.7 | 0.06− 0.69 * | 0.23–90.7 | 7.05–65.05 | 19–29.4 | 12.9–86.2 | [181] | ||
| Grand Forest Park | leaf | 0.10–0.54 | 0.12–0.14 | 2.35–3.60 | [182] | |||||
| The Beibu Gulf | Charybdis miles | 29.94 ± 3.02 | 569.9 ± 541.0 (ppb) | 230.7 ± 181.3 (ppb) | 52.1 ± 4.9 (ppb) | 118.6 ± 72.6 (ppb) | 123.86 ± 39.85 | [183] | ||
| Laizhou Bay | Aquatic organisms | 19.60 | 0.40 | 0.39 | 0.25 | 0.51 | 60.11 | [184] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, W.; Zhang, Y.; Chen, X.; Sha, A.; Xiong, Z.; Luo, Y.; Peng, L.; Zou, L.; Zhao, C.; Li, Q. The Easily Overlooked Effect of Global Warming: Diffusion of Heavy Metals. Toxics 2024, 12, 400. https://doi.org/10.3390/toxics12060400
Xiao W, Zhang Y, Chen X, Sha A, Xiong Z, Luo Y, Peng L, Zou L, Zhao C, Li Q. The Easily Overlooked Effect of Global Warming: Diffusion of Heavy Metals. Toxics. 2024; 12(6):400. https://doi.org/10.3390/toxics12060400
Chicago/Turabian StyleXiao, Wenqi, Yunfeng Zhang, Xiaodie Chen, Ajia Sha, Zhuang Xiong, Yingyong Luo, Lianxin Peng, Liang Zou, Changsong Zhao, and Qiang Li. 2024. "The Easily Overlooked Effect of Global Warming: Diffusion of Heavy Metals" Toxics 12, no. 6: 400. https://doi.org/10.3390/toxics12060400






