Biotransformation of Chlorpyrifos Shewanella oneidensis MR-1 in the Presence of Goethite: Experimental Optimization and Degradation Products
Abstract
1. Introduction
2. Materials and Method
2.1. Chemicals and Reagents
2.2. Synthesis of Goethite
2.3. S. oneidensis MR-1 and Its Culture Condition
2.3.1. Strain and Culture Methods
2.3.2. Acclimated Processes of S. oneidensis MR-1
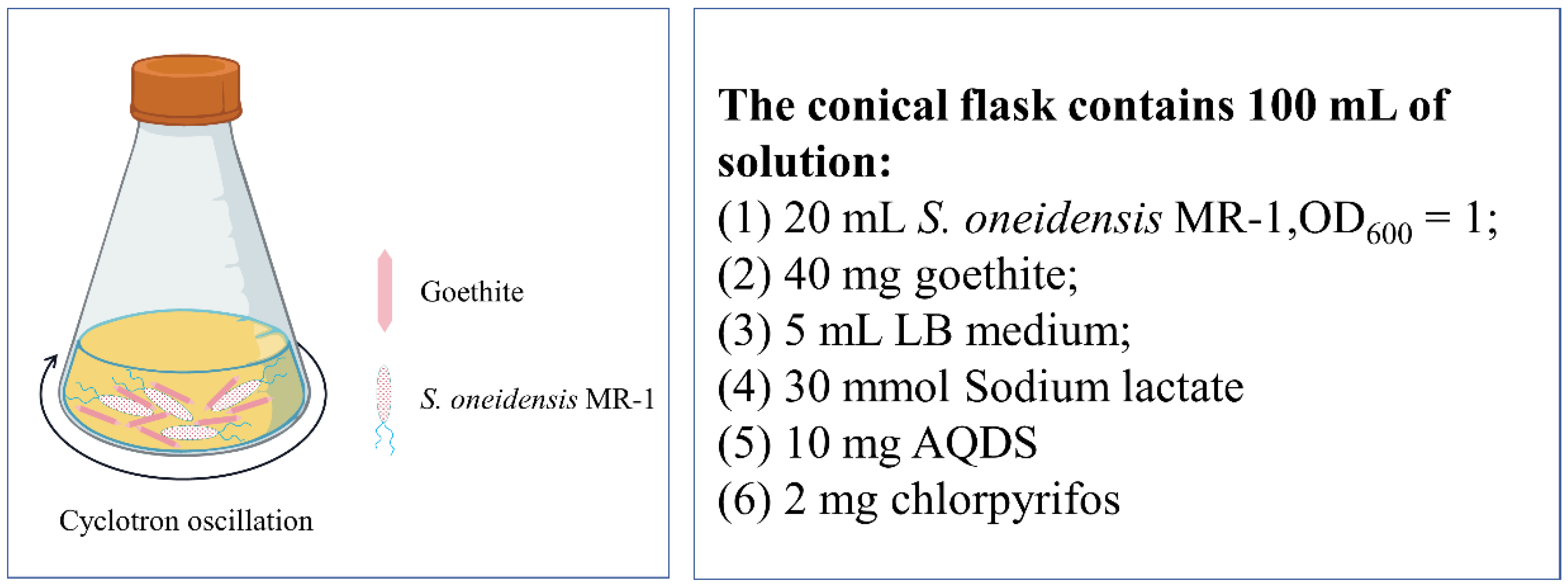
2.4. Design and Optimization of Chlorpyrifos Degradation Experiment
2.5. Analytical Method
2.5.1. Sample Characterization
2.5.2. Analysis of Chlorpyrifos
3. Results and Discussion
3.1. Experimental Optimization Results and Model Analysis
3.2. Characterization of Goethite
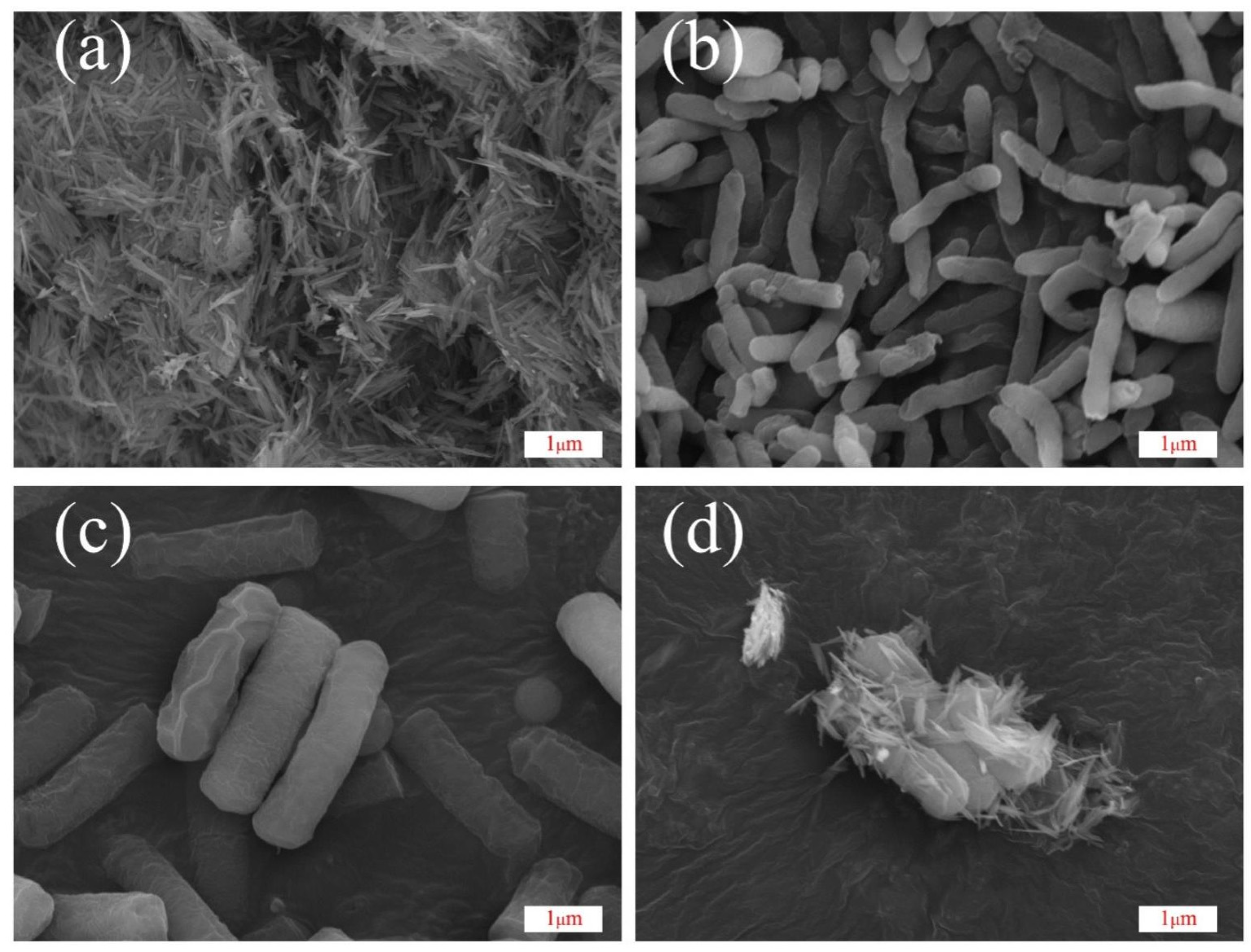
3.2.1. SEM Analysis
3.2.2. FT-IR Analysis
3.2.3. XRD Analysis
3.2.4. XPS Analysis
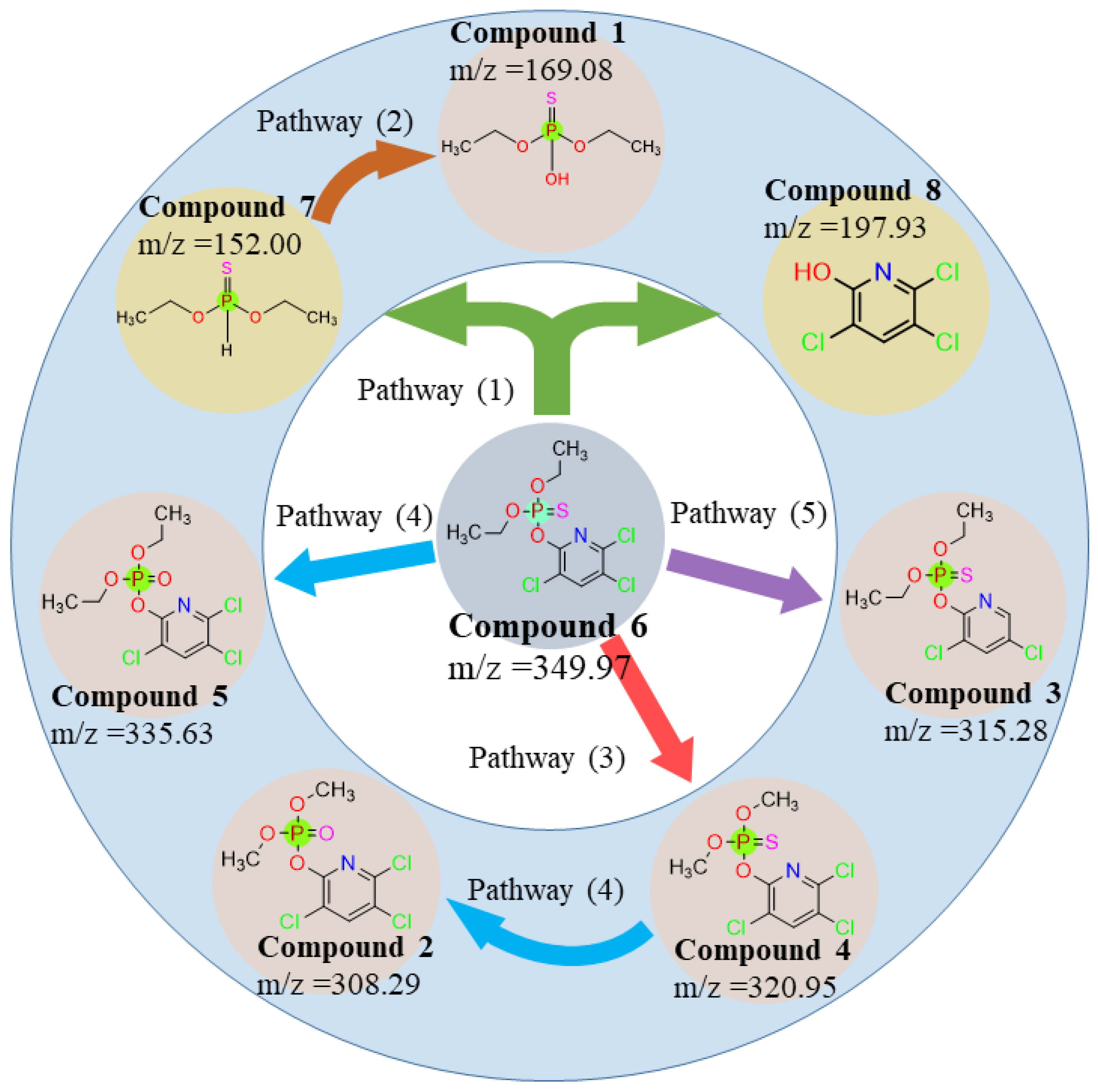
3.3. Interference of Degradation Products and Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dar, M.A.; Kaushik, G.; Villarreal-Chiu, J.F. Pollution Status and Bioremediation of Chlorpyrifos in Environmental Matrices by the Application of Bacterial Communities: A Review. J. Environ. Manag. 2019, 239, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Cui, H.; Duan, W. Ecotoxicity of Chlorpyrifos to Aquatic Organisms: A Review. Ecotoxicol. Environ. Saf. 2020, 200, 110731. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, J.; Xue, N.; Abdulkreem AL-Huqail, A.; Majdi, H.S.; Darvishmoghaddam, E.; Assilzadeh, H.; Khadimallah, M.A.; Ali, H.E. Risk Assessment of Organophosphorus Pesticide Residues in Drinking Water Resources: Statistical and Monte-Carlo Approach. Chemosphere 2022, 307, 135632. [Google Scholar] [CrossRef] [PubMed]
- Stalin, A.; Suganthi, P.; Mathivani, S.; Paray, B.A.; Al-Sadoon, M.K.; Gokula, V.; Musthafa, M.S. Impact of Chlorpyrifos on Behavior and Histopathological Indices in Different Tissues of Freshwater Fish Channa Punctatus (Bloch). Environ. Sci. Pollut. Res. 2019, 26, 17623–17631. [Google Scholar] [CrossRef]
- Nandi, N.K.; Vyas, A.; Akhtar, M.J.; Kumar, B. The Growing Concern of Chlorpyrifos Exposures on Human and Environmental Health. Pestic. Biochem. Physiol. 2022, 185, 105138. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Oladoye, P.O.; Olanrewaju, C.A.; Akinsola, G.O. Organophosphorus Pesticides: Impacts, Detection and Removal Strategies. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100655. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Vo, D.-V.N.; Rajamohan, N.; Saravanan, R. Microbial Degradation of Recalcitrant Pesticides: A Review. Environ. Chem. Lett. 2021, 19, 3209–3228. [Google Scholar] [CrossRef]
- Jiang, Z.; Shi, M.; Shi, L. Degradation of Organic Contaminants and Steel Corrosion by the Dissimilatory Metal-Reducing Microorganisms Shewanella and Geobacter spp. Int. Biodeterior. Biodegrad. 2020, 147, 104842. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, C.; Zhao, G.; Chen, Y. Versatile Mechanisms and Enhanced Strategies of Pollutants Removal Mediated by Shewanella oneidensis: A Review. J. Hazard. Mater. 2022, 440, 129703. [Google Scholar] [CrossRef]
- Govarthanan, M.; Ameen, F.; Kamala-Kannan, S.; Selvankumar, T.; Almansob, A.; Alwakeel, S.S.; Kim, W. Rapid Biodegradation of Chlorpyrifos by Plant Growth-Promoting Psychrophilic Shewanella sp. BT05: An Eco-Friendly Approach to Clean up Pesticide-Contaminated Environment. Chemosphere 2020, 247, 125948. [Google Scholar] [CrossRef]
- Xiao, X.; Han, X.; Wang, L.-G.; Long, F.; Ma, X.-L.; Xu, C.-C.; Ma, X.-B.; Wang, C.-X.; Liu, Z.-Y. Anaerobically Photoreductive Degradation by CdS Nanocrystal: Biofabrication Process and Bioelectron-Driven Reaction Coupled with Shewanella oneidensis MR-1. Biochem. Eng. J. 2020, 154, 107466. [Google Scholar] [CrossRef]
- Xiao, X.; Ma, X.-L.; Liu, Z.-Y.; Li, W.-W.; Yuan, H.; Ma, X.-B.; Li, L.-X.; Yu, H.-Q. Degradation of Rhodamine B in a Novel Bio-Photoelectric Reductive System Composed of Shewanella oneidensis MR-1 and Ag3PO4. Environ. Int. 2019, 126, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Buchman, J.T.; Hudson-Smith, N.V.; Landy, K.M.; Haynes, C.L. Understanding Nanoparticle Toxicity Mechanisms to Inform Redesign Strategies to Reduce Environmental Impact. Acc. Chem. Res. 2019, 52, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xue, T.; Xiang, F.; Zhang, S.; Hanamoto, S.; Sun, P.; Zhao, L. Toxicity and Combined Effects of Antibiotics and Nano ZnO on a Phosphorus-Removing Shewanella Strain in Wastewater Treatment. J. Hazard. Mater. 2021, 416, 125532. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Chen, Y.; Yan, Z.; Zhang, J.; Ji, X.; Wang, H.; Dahlgren, R.A.; Chen, F.; Shang, X.; Chen, Z. Recent Advances in the Roles of Minerals for Enhanced Microbial Extracellular Electron Transfer. Renew. Sustain. Energy Rev. 2020, 134, 110404. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, H.; Yang, Y.; Wu, B.; Li, X.; Wang, K.; Wang, P.; Zhang, C. Goethite and Riboflavin Synergistically Enhance Cr(VI) Reduction by Shewanella oneidensis MR-1. Biodegradation 2023, 34, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Wang, C.; Dong, Z.; Che, Q.; Wang, Z.; Zhu, Y. The Effects of Aniline-Promoted Electron Shuttle-Mediated Goethite Reduction by Shewanella oneidensis MR-1 and the Degradation of Aniline. Water 2023, 15, 3686. [Google Scholar] [CrossRef]
- Barik, M.; Das, C.P.; Kumar Verma, A.; Sahoo, S.; Sahoo, N.K. Metabolic Profiling of Phenol Biodegradation by an Indigenous Rhodococcus pyridinivorans Strain PDB9T N-1 Isolated from Paper Pulp Wastewater. Int. Biodeterior. Biodegrad. 2021, 158, 105168. [Google Scholar] [CrossRef]
- Poddar, K.; Sarkar, D.; Chakraborty, D.; Patil, P.B.; Maity, S.; Sarkar, A. Paracetamol Biodegradation by Pseudomonas Strain PrS10 Isolated from Pharmaceutical Effluents. Int. Biodeterior. Biodegrad. 2022, 175, 105490. [Google Scholar] [CrossRef]
- Srivastava, V.; Srivastava, T.; Kumar, M.S. Fate of the Persistent Organic Pollutant (POP) Hexachlorocyclohexane (HCH) and Remediation Challenges. Int. Biodeterior. Biodegrad. 2019, 140, 43–56. [Google Scholar] [CrossRef]
- Gao, L.; Gu, J.-D. A New Unified Conceptual Framework Involving Maintenance Energy, Metabolism and Toxicity for Research on Degradation of Organic Pollutants. Int. Biodeterior. Biodegrad. 2021, 162, 105253. [Google Scholar] [CrossRef]
- Bose, S.; Kumar, P.S.; Vo, D.-V.N. A Review on the Microbial Degradation of Chlorpyrifos and Its Metabolite TCP. Chemosphere 2021, 283, 131447. [Google Scholar] [CrossRef] [PubMed]
- Echeverri-Jaramillo, G.; Jaramillo-Colorado, B.; Sabater-Marco, C.; Castillo-López, M.Á. Acute Toxicity of Chlorpyrifos and Its Metabolite 3,5,6-Trichloro-2-Pyridinol Alone and in Combination Using a Battery of Bioassays. Environ. Sci. Pollut. Res. 2020, 27, 32770–32778. [Google Scholar] [CrossRef] [PubMed]
- Lockridge, O.; Verdier, L.; Schopfer, L.M. Half-Life of Chlorpyrifos Oxon and Other Organophosphorus Esters in Aqueous Solution. Chem. Biol. Interact. 2019, 311, 108788. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Banerjee, S.; Mani, R.; Chattopadhyaya, M.C. Synthesis, Characterization and Application of Goethite Mineral as an Adsorbent. J. Environ. Chem. Eng. 2013, 1, 281–289. [Google Scholar] [CrossRef]
- Mao, F.; Liu, X.; Wu, K.; Zhou, C.; Si, Y. Biodegradation of Sulfonamides by Shewanella oneidensis MR-1 and Shewanella sp. Strain MR-4. Biodegradation 2018, 29, 129–140. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiao, C.; Zhao, F. Long-Term Adaptive Evolution of Shewanella oneidensis MR-1 for Establishment of High Concentration Cr (VI) Tolerance. Front. Environ. Sci. Eng 2020, 14, 3. [Google Scholar]
- Dalvand, K.; Ghiasvand, A. Simultaneous Analysis of PAHs and BTEX in Soil by a Needle Trap Device Coupled with GC-FID and Using Response Surface Methodology Involving Box-Behnken Design. Anal. Chim. Acta 2019, 1083, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Mosca Angelucci, D.; Piscitelli, D.; Tomei, M.C. Pentachlorophenol Biodegradation in Two-Phase Bioreactors Operated with Absorptive Polymers: Box-Behnken Experimental Design and Optimization by Response Surface Methodology. Process. Saf. Environ. Prot. 2019, 131, 105–115. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Eletta, O.A.A. Response Surface Modelling of the Biosorption of Zn(II) and Pb(II) onto Micropogonias Undulatus Scales: Box–Behnken Experimental Approach. Appl. Water Sci. 2020, 10, 197. [Google Scholar] [CrossRef]
- Mohamed, A.; Yu, L.; Fang, Y.; Ashry, N.; Riahi, Y.; Uddin, I.; Dai, K.; Huang, Q. Iron Mineral-Humic Acid Complex Enhanced Cr(VI) Reduction by Shewanella oneidensis MR-1. Chemosphere 2020, 247, 125902. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ren, X.; Hu, L.; Guo, W.; Zhan, J. Facet-Controlled Activation of Persulfate by Goethite for Tetracycline Degradation in Aqueous Solution. Chem. Eng. J. 2021, 412, 128628. [Google Scholar] [CrossRef]
- Zhu, M.; Hu, X.; Tu, C.; Zhang, H.; Song, F.; Luo, Y.; Christie, P. Sorption Mechanisms of Diphenylarsinic Acid on Ferrihydrite, Goethite and Hematite Using Sequential Extraction, FTIR Measurement and XAFS Spectroscopy. Sci. Total Environ. 2019, 669, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, E.J.; Huang, J.-H.; Chorover, J.; Kretzschmar, R. ATR-FTIR Spectroscopy Study of the Influence of pH and Contact Time on the Adhesion of Shewanella putrefaciens Bacterial Cells to the Surface of Hematite. Environ. Sci. Technol. 2012, 46, 12848–12855. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wu, F.; Song, K.; Lin, Y.; Bai, Y.; Zhu, Y.; Giesy, J.P. Competitive Interaction between Soil-Derived Humic Acid and Phosphate on Goethite. Appl. Geochem. 2013, 36, 125–131. [Google Scholar] [CrossRef]
- Zhao, G.; Li, E.; Li, J.; Xu, M.; Huang, Q.; Rong, X. Effects of Interfaces of Goethite and Humic Acid-Goethite Complex on Microbial Degradation of Methyl Parathion. Front. Microbiol. 2018, 9, 1748. [Google Scholar] [CrossRef] [PubMed]
- Thakar, S.P.; Dabhi, R.C.; Rathod, S.L.; Patel, U.P.; Rana, A.; Shrivastav, P.S.; George, L.-B.; Highland, H. In Situ Chlorpyrifos (CPF) Degradation by Acrobeloides maximus: Insights from Chromatographic Analysis. J. Chromatogr. A 2024, 1714, 464555. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Koopal, L.K.; Wang, M.; Xiong, J.; Hou, J.; Li, Y.; Tan, W. Phosphate Speciation on Al-Substituted Goethite: ATR-FTIR/2D-COS and CD-MUSIC Modeling. Environ. Sci. Nano 2019, 6, 3625–3637. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Gypser, S.; Leinweber, P.; Freese, D.; Kühn, O. Infrared Spectroscopic Characterization of Phosphate Binding at the Goethite–Water Interface. Phys. Chem. Chem. Phys. 2019, 21, 4421–4434. [Google Scholar] [CrossRef]
- Guo, H.; Barnard, A.S. Naturally Occurring Iron Oxide Nanoparticles: Morphology, Surface Chemistry and Environmental Stability. J. Mater. Chem. A 2013, 1, 27–42. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, A.; Shan, C.; Gao, G.; Pan, B. Enhanced Fe(III)-Mediated Fenton Oxidation of Atrazine in the Presence of Functionalized Multi-Walled Carbon Nanotubes. Water Res. 2018, 137, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-S.; Chen, J.-J.; Cheng, R.-F.; Min, Y.; Yu, H.-Q. Iron Cycle Tuned by Outer-Membrane Cytochromes of Dissimilatory Metal-Reducing Bacteria: Interfacial Dynamics and Mechanisms In Vitro. Environ. Sci. Technol. 2021, 55, 11424–11433. [Google Scholar] [CrossRef]
- Yu, H.; Liu, G.; Dong, B.; Jin, R.; Zhou, J. Synergistic Catalytic Fenton-like Degradation of Sulfanilamide by Biosynthesized Goethite-Reduced Graphene Oxide Composite. J. Hazard. Mater. 2021, 415, 125704. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, G.; Dong, B.; Jin, R.; Zhou, J. Humic Acids Promote Hydroxyl Radical Production during Transformation of Biogenic and Abiogenic Goethite under Redox Fluctuation. Chem. Eng. J. 2021, 424, 130359. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, J.; Zhao, N.; Yang, X.; Chen, C.; Shang, J. Goethite Modified Biochar as a Multifunctional Amendment for Cationic Cd(II), Anionic As(III), Roxarsone, and Phosphorus in Soil and Water. J. Clean. Prod. 2020, 247, 119579. [Google Scholar] [CrossRef]
- Ding, J.; Shen, L.; Yan, R.; Lu, S.; Zhang, Y.; Zhang, X.; Zhang, H. Heterogeneously Activation of H2O2 and Persulfate with Goethite for Bisphenol A Degradation: A Mechanistic Study. Chemosphere 2020, 261, 127715. [Google Scholar] [CrossRef] [PubMed]
- Elshikh, M.S.; Alarjani, K.M.; Huessien, D.S.; Elnahas, H.A.M.; Esther, A.R. Enhanced Biodegradation of Chlorpyrifos by Bacillus Cereus CP6 and Klebsiella Pneumoniae CP19 from Municipal Waste Water. Environ. Res. 2022, 205, 112438. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, A.R.; Harshiny, M.; Gummadi, S.N. Chlorpyrifos in Environment and Food: A Critical Review of Detection Methods and Degradation Pathways. Environ. Sci. Process. Impacts 2021, 23, 1255–1277. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, W.; Pang, S.; Chen, J.; Bhatt, P.; Mishra, S.; Chen, S. Insights into the Microbial Degradation and Catalytic Mechanisms of Chlorpyrifos. Environ. Res. 2021, 194, 110660. [Google Scholar] [CrossRef]
- Sheikhi, S.; Dehghanzadeh, R.; Maryamabadi, A.; Aslani, H. Chlorpyrifos Removal from Aqueous Solution through Sequential Use of Coagulation and Advanced Oxidation Processes: By-Products, Degradation Pathways, and Toxicity Assessment. Environ. Technol. Innov. 2021, 23, 101564. [Google Scholar] [CrossRef]
- Bouteh, E.; Ahmadi, N.; Abbasi, M.; Torabian, A.; Van Loosdrecht, M.C.M.; Ducoste, J. Biodegradation of Organophosphorus Pesticides in Moving Bed Biofilm Reactors: Analysis of Microbial Community and Biodegradation Pathways. J. Hazard. Mater. 2021, 408, 124950. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, M.; Verma, S.; Chatterjee, S. Profenofos, an Acetylcholinesterase-Inhibiting Organophosphorus Pesticide: A Short Review of Its Usage, Toxicity, and Biodegradation. J. Environ. Qual. 2016, 45, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Hadibarata, T.; Kristanti, R.A.; Bilal, M.; Yilmaz, M.; Sathishkumar, P. Biodegradation Mechanism of Chlorpyrifos by Halophilic Bacterium Hortaea sp. B15. Chemosphere 2023, 312, 137260. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Ahmad, M.; Kanwal, A.; Butt, Z.A.; Khan, Q.F.; Raza, S.A.; Qayyum, H.; Wahid, A. Biodegradation of Chlorpyrifos Using Isolates from Contaminated Agricultural Soil, Its Kinetic Studies. Sci. Rep. 2021, 11, 10320. [Google Scholar] [CrossRef]
- Verma, S.; Singh, D.; Chatterjee, S. Biodegradation of Organophosphorus Pesticide Chlorpyrifos by Sphingobacterium sp. C1B, a Psychrotolerant Bacterium Isolated from Apple Orchard in Himachal Pradesh of India. Extremophiles 2020, 24, 897–908. [Google Scholar] [CrossRef]




| Variable | Units | Experimental Levels | ||
|---|---|---|---|---|
| Lowest (−1) | Middle (0) | Highest (+1) | ||
| = Initial pH | / | 5 | 7 | 9 |
| = Concentration | mg·L−1 | 15 | 20 | 25 |
| = Temperature | °C | 25 | 30 | 35 |
| Run | pH | Concentration (mg·L−1) | Temperature (°C) | Chlorpyrifos Removal Rate (%) |
|---|---|---|---|---|
| 1 | 9 | 20 | 35 | 41.52 |
| 2 | 9 | 15 | 30 | 35.95 |
| 3 | 7 | 25 | 35 | 56.94 |
| 4 | 7 | 20 | 30 | 74.36 |
| 5 | 7 | 15 | 25 | 62.59 |
| 6 | 7 | 20 | 30 | 74.41 |
| 7 | 5 | 20 | 25 | 52.68 |
| 8 | 7 | 20 | 30 | 70.48 |
| 9 | 7 | 15 | 35 | 65.81 |
| 10 | 7 | 20 | 30 | 70.59 |
| 11 | 7 | 20 | 30 | 72.65 |
| 12 | 7 | 25 | 25 | 51.65 |
| 13 | 9 | 20 | 25 | 33.24 |
| 14 | 5 | 15 | 30 | 40.28 |
| 15 | 5 | 20 | 35 | 45.69 |
| 16 | 5 | 25 | 30 | 37.68 |
| 17 | 9 | 25 | 30 | 30.56 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 3883.14 | 9 | 431.46 | 52.69 | <0.0001 |
| = pH | 153.65 | 1 | 153.65 | 18.76 | 0.0034 |
| = Concentration | 96.61 | 1 | 96.61 | 11.8 | 0.0109 |
| = Temperature | 12 | 1 | 12 | 1.47 | 0.2653 |
| 1.95 | 1 | 1.95 | 0.2376 | 0.6408 | |
| 58.29 | 1 | 58.29 | 7.12 | 0.0321 | |
| 1.07 | 1 | 1.07 | 0.1308 | 0.7283 | |
| 2884.26 | 1 | 2884.26 | 352.22 | <0.0001 | |
| 438.73 | 1 | 438.73 | 53.58 | 0.0002 | |
| 38.98 | 1 | 38.98 | 4.76 | 0.0655 | |
| Residual | 57.32 | 7 | 8.19 | ||
| Lock of Fit | 42.46 | 3 | 14.15 | 3.81 | 0.1145 |
| Pure error | 14.86 | 4 | 3.71 | ||
| C.V. % | 5.30 | ||||
| R2 | 0.9855 | ||||
| Adjusted R2 | 0.9667 |
| No. | m/z | Molecular Formula | Ionization Mode | Molecular Weight (g/mol) | Name of Degradation Products |
|---|---|---|---|---|---|
| 1 | 169.08 | C4H11O3PS | [M − H]+ | 170.17 | O,O-Diethyl phosphorothionate |
| 2 | 308.29 | C7H7Cl3NO4P | [M+2H]+ | 306.47 | Chlorpyrifos methyl oxon |
| 3 | 315.28 | C9H11Cl2NO3PS | [M − Cl]+ | 315.28 | Dechlorination of Chlorpyrifos |
| 4 | 320.95 | C7H7Cl3NO3PS | [M − H]+ | 322.53 | Chlorpyrifos-methyl |
| 5 | 335.63 | C9H11Cl3NO4P | [M+H]+ | 334.52 | Chlorpyrifos oxon |
| 6 | 349.97 | C9H11Cl3NO3PS | [M]+ | 350.58 | Chlorpyrifos |
| 7 | 152.00 | C4H11O2PS | [M − H]− | 154.17 | O,O-diethyl thiophosphonate |
| 8 | 196.92 | C5H2Cl3NO | [M − H]− | 198.43 | 2-Hydroxy-3,5,6-trichloropyridine |
| Strains | Biodegradation Potential | References |
|---|---|---|
| Hortaea sp. B15 | For chlorpyrifos at 400 mg·L−1, 91.1% degradation was achieved in 20 h. The degradation products were 3,5,6-trichloropyridin-2-ol and 2-pyridinol. | [53] |
| Bacillus sp. CP6 and Klebsiella pneumoniae sp. CP19 | For chlorpyrifos at 250 mg·L−1, 93.4 ± 2.8% degradation was achieved in 16 days. Degradation products were not reported. | [47] |
| Bacillus sp. Ct3 | For chlorpyrifos at 125 mg·L−1, 88% degradation was achieved in 8 days. The degradation product was 3,5,6-trichloro-2-pyridinol (TCP). | [54] |
| Sphingobacterium sp. C1B | For chlorpyrifos at 50 mg·L−1, 84% degradation was achieved in 14 days. The degradation products were 3,5,6-trichloro-2-pyridinol to benzene and 1,3-bis (1,1-dimethylethyl). | [55] |
| Shewanella sp. BT05 | For chlorpyrifos at 10 mg·L−1, the degradation rate was 94.3% at 24 h. The degradation product was 3,5,6-trichloro-2-pyridinol (TCP). | [10] |
| Shewanella oneidensis MR-1 | For chlorpyrifos at 19.18 mg·L−1, 75.71% degradation was achieved in 10 days. The degradation products were O,O-Diethyl phosphorothionate, Chlorpyrifos methyl oxon, Dechlorination of Chlorpyrifos, Chlorpyrifos-methyl, Chlorpyrifos oxon, O,O-diethyl thiophosphonate, and 2-Hydroxy-3,5,6-trichloropyridine. | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Li, Y.; Zhu, Z.; Wang, Y.; Peng, Y.; Zhang, J.; Nong, P.; Pan, S.; Fan, Y.; Zhu, Y. Biotransformation of Chlorpyrifos Shewanella oneidensis MR-1 in the Presence of Goethite: Experimental Optimization and Degradation Products. Toxics 2024, 12, 402. https://doi.org/10.3390/toxics12060402
Tang S, Li Y, Zhu Z, Wang Y, Peng Y, Zhang J, Nong P, Pan S, Fan Y, Zhu Y. Biotransformation of Chlorpyrifos Shewanella oneidensis MR-1 in the Presence of Goethite: Experimental Optimization and Degradation Products. Toxics. 2024; 12(6):402. https://doi.org/10.3390/toxics12060402
Chicago/Turabian StyleTang, Shen, Yanhong Li, Zongqiang Zhu, Yaru Wang, Yuqing Peng, Jing Zhang, Peijie Nong, Shufen Pan, Yinming Fan, and Yinian Zhu. 2024. "Biotransformation of Chlorpyrifos Shewanella oneidensis MR-1 in the Presence of Goethite: Experimental Optimization and Degradation Products" Toxics 12, no. 6: 402. https://doi.org/10.3390/toxics12060402
APA StyleTang, S., Li, Y., Zhu, Z., Wang, Y., Peng, Y., Zhang, J., Nong, P., Pan, S., Fan, Y., & Zhu, Y. (2024). Biotransformation of Chlorpyrifos Shewanella oneidensis MR-1 in the Presence of Goethite: Experimental Optimization and Degradation Products. Toxics, 12(6), 402. https://doi.org/10.3390/toxics12060402






