Particulate Matter (PM) and Parent, Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbon (PAH) Emissions of Emulsified Heavy Fuel Oil in Marine Low-Speed Main Engine
Abstract
:1. Introduction
2. Experimental Setup and Methods
2.1. Experimental Setup
2.2. Sampling Methods and Exhaust Measurements
2.3. Chemical Analysis Methods
2.4. Calculation of Emission Factors
3. Results and Discussion
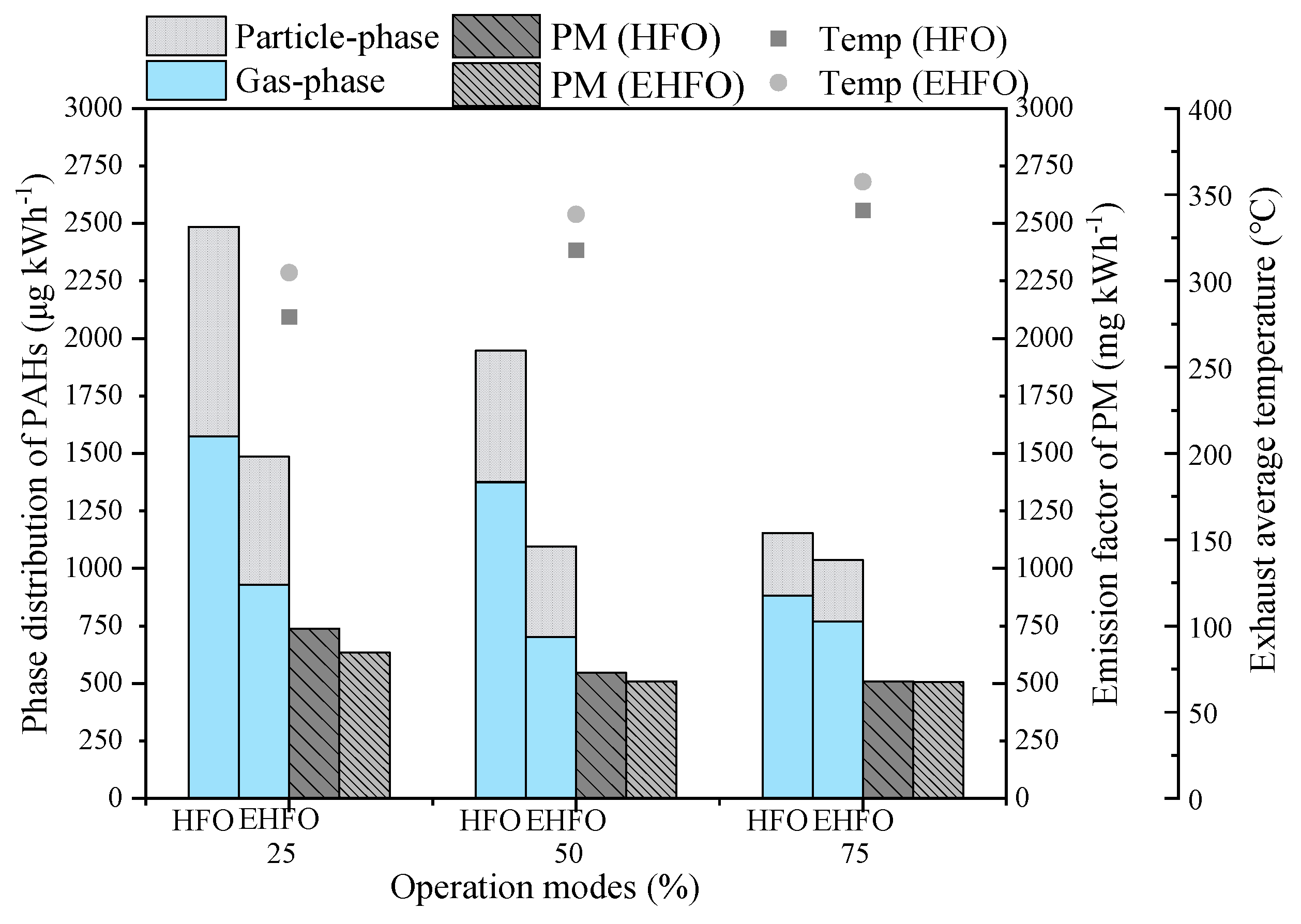
3.1. Effects on Emission Characteristics of PM and p,n,o-PAHs
3.1.1. Effects on Emission Characteristics of PM and PAHs
3.1.2. Effects on Emission Characteristics of n,o-PAHs
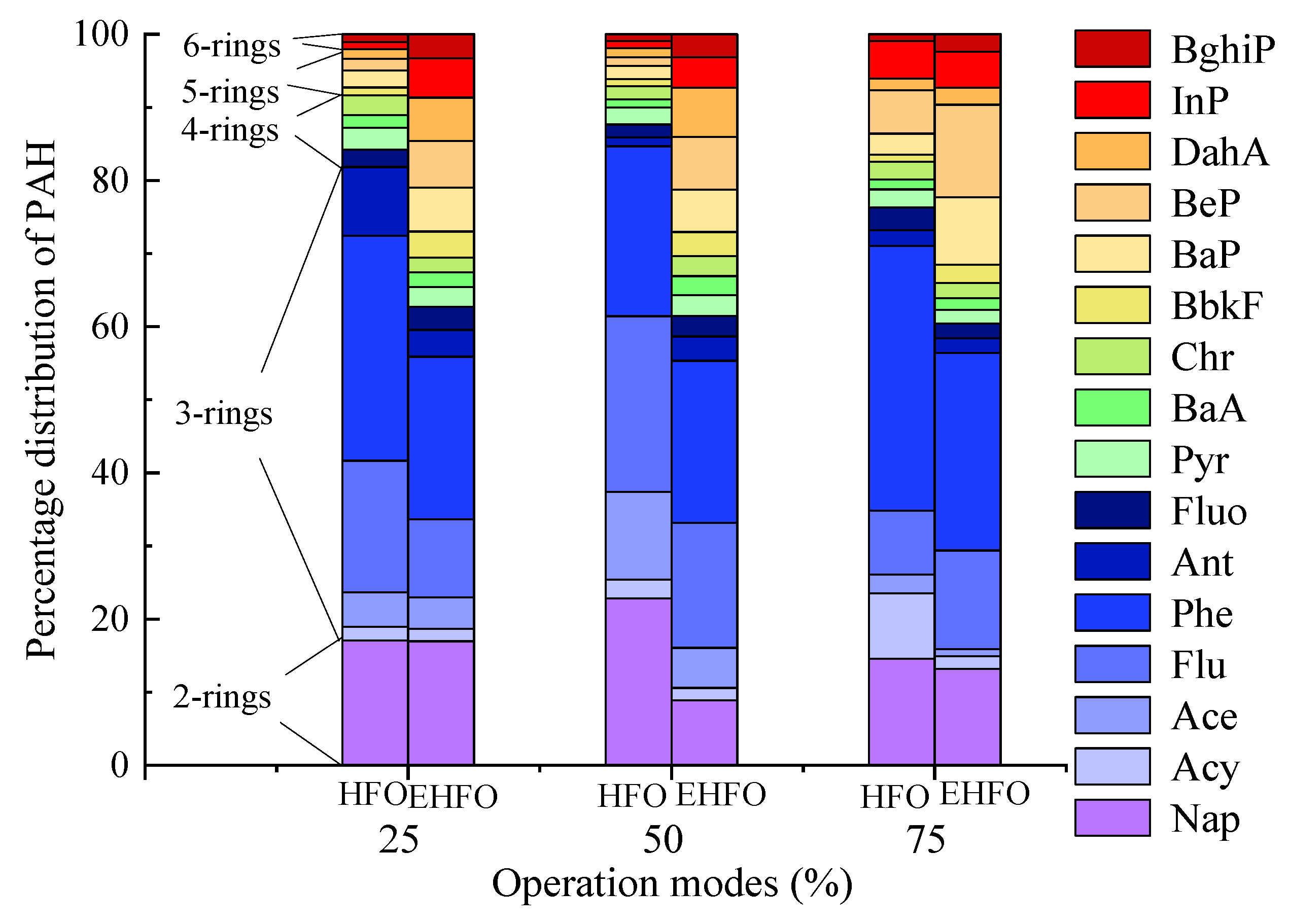
3.2. Effects on PAH Composition and Phase Distribution
3.3. Effects on Size Distributions of PM and p,n,o-PAHs
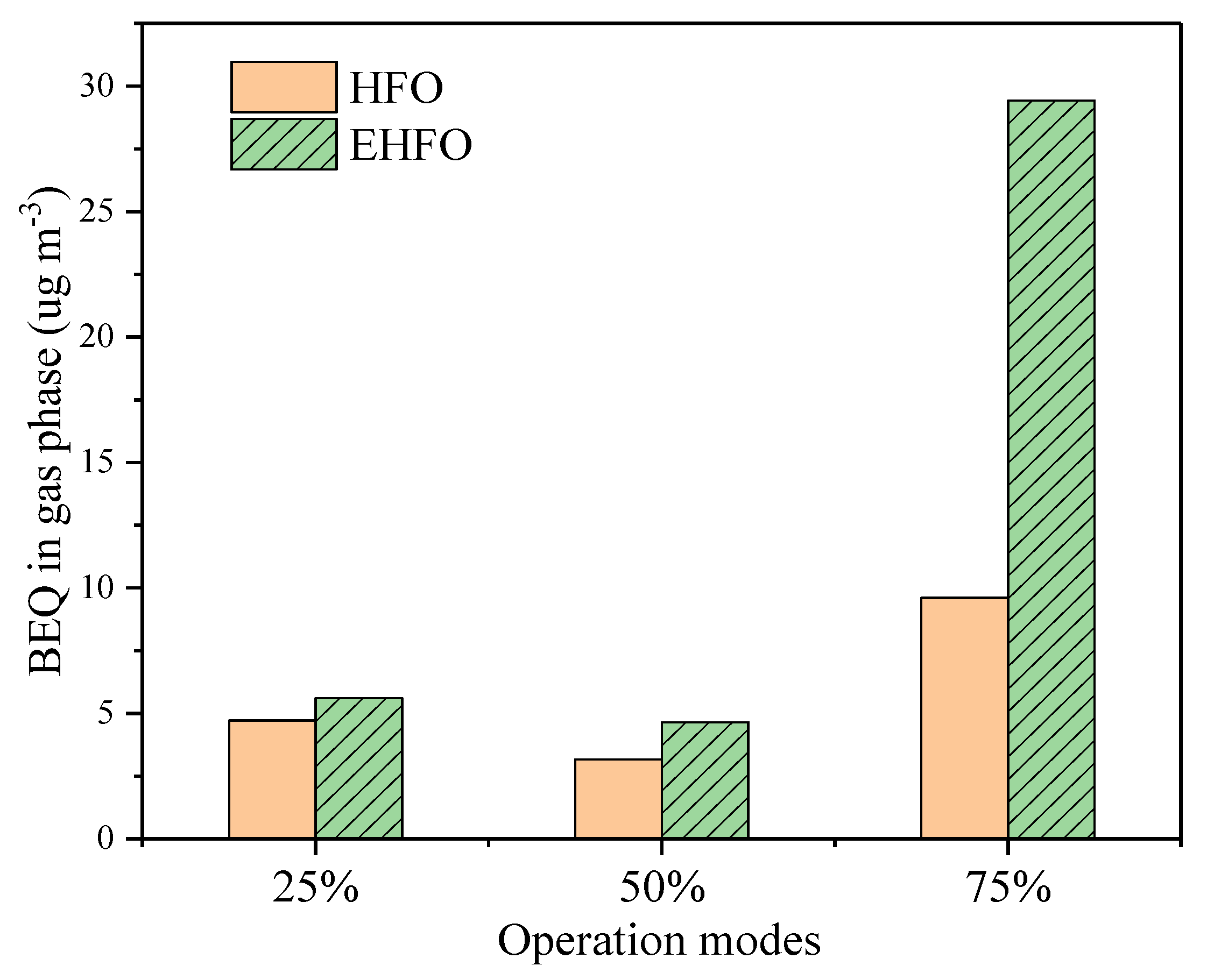
3.4. Carcinogenic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- A Pathway to Decarbonise the Shipping Sector by 2050. Available online: https://www.irena.org/publications/2021/Oct/A-Pathway-to-Decarbonise-the-Shipping-Sector-by-2050 (accessed on 27 April 2024).
- Ferraro, A.; Marino, E.; Trancone, G.; Race, M.; Mali, M.; Pontoni, L.; Fabbricino, M.; Spasiano, D.; Fratino, U. Assessment of Environmental Parameters Effect on Potentially Toxic Elements Mobility in Foreshore Sediments to Support Marine-Coastal Contamination Prediction. Mar. Pollut. Bull. 2023, 194, 115338. [Google Scholar] [CrossRef]
- IMO 2020—Cutting Sulphur Oxide Emissions. Available online: https://www.imo.org/en/MediaCentre/HotTopics/Pages/Sulphur-2020.aspx (accessed on 27 April 2024).
- Razy-Yanuv, E.; Barak, Y.; Noam, O.; Madar, D. Marine Air Pollution in Israel: Extent, Proposed Mitigation Targets, Benefits and Feasibility. Atmosphere 2022, 13, 241. [Google Scholar] [CrossRef]
- Ogugua, P.C.; Wang, E.; Jinyang, Z.; Wang, Q.; Su, H. Advancements in Low-Temperature NH3-SCR of NOx Using Ba-Based Catalysts: A Critical Review of Preparation, Mechanisms, and Challenges. Environ. Sci. Pollut. Res. 2023, 30, 84972–84998. [Google Scholar] [CrossRef]
- Muhammad Farhan, S.; Pan, W.; Zhijian, C.; JianJun, Y. Innovative Catalysts for the Selective Catalytic Reduction of NOx with H2: A Systematic Review. Fuel 2024, 355, 129364. [Google Scholar] [CrossRef]
- Wardana, M.K.A.; Lim, O. Review of Improving the NOx Conversion Efficiency in Various Diesel Engines Fitted with SCR System Technology. Catalysts 2023, 13, 67. [Google Scholar] [CrossRef]
- Ashfaque Ahmed, S.; Elahi, M.; Soudagar, M.; Rahamathullah, I.; Sadhik Basha, J.; Yunus Khan, T.M.; Javed, S.; Elfasakhany, A.; Kalam, M. Investigation of Ternary Blends of Animal Fat Biodiesel-Diethyl Ether-Diesel Fuel on CMFIS-CI Engine Characteristics. Fuel 2023, 332, 126200. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, G.-F.; Lin, Y.-C. Influence of Emulsified Biodiesel on the Emission and Health Risk of Polycyclic Aromatic Hydrocarbons in the Vapor and Particulate Phases during Engine Combustion. Environ. Sci. Pollut. Res. 2019, 26, 13510–13521. [Google Scholar] [CrossRef]
- Chen, X.; Xi, X.; Xiao, G.; Zhang, L.; Wang, Z.; Long, W. Effect of Ambient Temperature and Water Content on Emulsified Heavy Fuel Oil Droplets Evaporation: Evaporation Enhancement by Droplet Puffing and Micro-Explosion. Fuel 2023, 334, 126614. [Google Scholar] [CrossRef]
- Kim, M.; Oh, J.; Lee, C. Study on Combustion and Emission Characteristics of Marine Diesel Oil and Water-In-Oil Emulsified Marine Diesel Oil. Energies 2018, 11, 1830. [Google Scholar] [CrossRef]
- Bragadeshwaran, A.; Kasianantham, N.; Balusamy, S.; Muniappan, S.; Reddy, D.M.S.; Subhash, R.V.; Pravin, N.A.; Subbarao, R. Mitigation of NOx and Smoke Emissions in a Diesel Engine Using Novel Emulsified Lemon Peel Oil Biofuel. Environ. Sci. Pollut. Res. 2018, 25, 25098–25114. [Google Scholar] [CrossRef]
- Chu Van, T.; Ramirez, J.; Rainey, T.; Ristovski, Z.; Brown, R.J. Global Impacts of Recent IMO Regulations on Marine Fuel Oil Refining Processes and Ship Emissions. Transp. Res. Part D Transp. Environ. 2019, 70, 123–134. [Google Scholar] [CrossRef]
- Oh, J.; Im, M.; Oh, S.; Lee, C. Comparison of NOx and Smoke Characteristics of Water-in-Oil Emulsion and Marine Diesel Oil in 400-kW Marine Generator Engine. Energies 2019, 12, 228. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Tian, J.; Dong, R.; Zou, Z.; Gao, S.; Tan, D. Performance, Combustion and Emission Characteristics Investigations on a Diesel Engine Fueled with Diesel/Ethanol/n-Butanol Blends. Energy 2022, 249, 123733. [Google Scholar] [CrossRef]
- Man, X.J.; Cheung, C.S.; Ning, Z.; Wei, L.; Huang, Z.H. Influence of Engine Load and Speed on Regulated and Unregulated Emissions of a Diesel Engine Fueled with Diesel Fuel Blended with Waste Cooking Oil Biodiesel. Fuel 2016, 180, 41–49. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wang, T.; Sun, L.; Yang, Z.; Lin, Y.; Chen, Y.; Mao, H. Characterization of PM2.5-Bound Polycyclic Aromatic Hydrocarbons and Their Derivatives (Nitro-and Oxy-PAHs) Emissions from Two Ship Engines under Different Operating Conditions. Chemosphere 2019, 225, 43–52. [Google Scholar] [CrossRef]
- Lin, X.; Lin, L.; Liao, Z.; Wu, P.; Yang, C. Occurrence and Distribution of Polycyclic Aromatic Hydrocarbons (PAHs) in Marine Organisms from Shenzhen Coastal Waters and Human Health Risk Assessment. Mar. Pollut. Bull. 2023, 195, 115498. [Google Scholar] [CrossRef]
- Su, P.; Geng, P.; Wei, L.; Hou, C.; Yin, F.; Tomy, G.T.; Li, Y.; Feng, D. PM and PAHs Emissions of Ship Auxiliary Engine Fuelled with Waste Cooking Oil Biodiesel and Marine Gas Oil. IET Intell. Transp. Syst. 2019, 13, 218–227. [Google Scholar] [CrossRef]
- Xue, J. Combustion Characteristics, Engine Performances and Emissions of Waste Edible Oil Biodiesel in Diesel Engine. Renew. Sustain. Energy Rev. 2013, 23, 350–365. [Google Scholar] [CrossRef]
- Petzold, A.; Lauer, P.; Fritsche, U.; Hasselbach, J.; Lichtenstern, M.; Schlager, H.; Fleischer, F. Operation of Marine Diesel Engines on Biogenic Fuels: Modification of Emissions and Resulting Climate Effects. Environ. Sci. Technol. 2011, 45, 10394–10400. [Google Scholar] [CrossRef]
- Nabi, M.N.; Hustad, J.E. Investigation of Engine Performance and Emissions of a Diesel Engine with a Blend of Marine Gas Oil and Synthetic Diesel Fuel. Environ. Technol. 2012, 33, 9–15. [Google Scholar] [CrossRef]
- Gysel, N.R.; Russell, R.L.; Welch, W.A.; Cocker, D.R.; Ghosh, S. Impact of Sugarcane Renewable Fuel on In-Use Gaseous and Particulate Matter Emissions from a Marine Vessel. Energy Fuels 2014, 28, 4177–4182. [Google Scholar] [CrossRef]
- Xu, L.; Yu, J.; Wan, G.; Sun, L. Emission Characteristics and Source Identification of Polycyclic Aromatic Hydrocarbons (PAHs) from Used Mineral Oil Combustion. Fuel 2021, 304, 121357. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, H.; Chen, Y.; Han, Y.; Cai, J.; Peng, Y.; Feng, Y. Emission Characteristics and Influencing Mechanisms of PAHs and EC from the Combustion of Three Components (Cellulose, Hemicellulose, Lignin) of Biomasses. Sci. Total Environ. 2023, 859, 160359. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Moore, F.; Keshavarzi, B.; Moeinpour, A. Aliphatic and Polycyclic Aromatic Hydrocarbons Risk Assessment in Coastal Water and Sediments of Khark Island, SW Iran. Mar. Pollut. Bull. 2016, 108, 33–45. [Google Scholar] [CrossRef]
- Li, J.-N.; Zhang, Y.; Wang, J.-X.; Xiao, H.; Nikolaev, A.; Li, Y.-F.; Zhang, Z.-F.; Tang, Z.-H. Occurrence, Sources, and Health Risks of Polycyclic Aromatic Hydrocarbons in Road Environments from Harbin, a Megacity of China. Toxics 2023, 11, 695. [Google Scholar] [CrossRef]
- Wang, D.; Ma, J.; Li, H.; Zhang, X. Concentration and Potential Ecological Risk of PAHs in Different Layers of Soil in the Petroleum-Contaminated Areas of the Loess Plateau, China. Int. J. Environ. Res. Public Health 2018, 15, 1785. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, J.; Guo, H.; Xue, S.; Wang, X.; Wang, Z.; Chen, T.; Yang, L.; Zeng, X.; Su, P. Technical Requirements for 2023 IMO GHG Strategy. Sustainability 2024, 16, 2766. [Google Scholar] [CrossRef]
- Trancone, G.; Spasiano, D.; Race, M.; Luongo, V.; Petrella, A.; Pirozzi, F.; Fratino, U.; Piccinni, A.F. A Combined System for Asbestos-Cement Waste Degradation by Dark Fermentation and Resulting Supernatant Valorization in Anaerobic Digestion. Chemosphere 2022, 300, 134500. [Google Scholar] [CrossRef]
- Su, P.; Zhang, W.; Hao, Y.; Tomy, G.T.; Yin, F.; Chen, L.; Ding, Y.; Li, Y.; Feng, D. Polycyclic Aromatic Hydrocarbon Contaminations along Shipping Lanes and Implications of Seafarer Exposure: Based on PAHs in Ship Surface Films and a Film-Air-Water Fugacity Model. Sci. Total Environ. 2020, 731, 138943. [Google Scholar] [CrossRef]
- ISO 8178-2:2021; Reciprocating Internal Combustion Engines—Exhaust Emission Measurement—Part 2: Measurement of Gaseous and Particulate Exhaust Emissions under Field Conditions. Available online: https://www.iso.org/standard/75546.html (accessed on 27 May 2024).
- Lin, C.-Y.; Lin, S.-A. Effects of Emulsification Variables on Fuel Properties of Two- and Three-Phase Biodiesel Emulsions. Fuel 2007, 86, 210–217. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lee, W.-J.; Chen, C.-C.; Chen, C.-B. Saving Energy and Reducing Emissions of Both Polycyclic Aromatic Hydrocarbons and Particulate Matter by Adding Bio-Solution to Emulsified Diesel. Environ. Sci. Technol. 2006, 40, 5553–5559. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, B.; Li, Z.; Bennett, A.; Sioud, S.; Sarathy, S.M.; Roberts, W.L. Evolution of Oxygenated Polycyclic Aromatic Hydrocarbon Chemistry at Flame Temperatures. Combust. Flame 2019, 209, 441–451. [Google Scholar] [CrossRef]
- Tree, D.R.; Svensson, K.I. Soot Processes in Compression Ignition Engines. Prog. Energy Combust. Sci. 2007, 33, 272–309. [Google Scholar] [CrossRef]
- Cunha-Lopes, I.; Lehtoranta, K.; Almeida, S.M.; Evtyugina, M.; Vicente, A.; Vicente, E.; Kuutti, H.; Amato, F.; Alves, C.A. Chemical Speciation of PM Emissions from Heavy-Duty Vehicles. Atmos. Environ. 2023, 306, 119823. [Google Scholar] [CrossRef]
- Mwangi, J.K.; Lee, W.-J.; Whang, L.-M.; Wu, T.S.; Chen, W.-H.; Chang, J.-S.; Chen, C.-Y.; Chen, C.-L. Microalgae Oil: Algae Cultivation and Harvest, Algae Residue Torrefaction and Diesel Engine Emissions Tests. Aerosol Air Qual. Res. 2015, 15, 81–98. [Google Scholar] [CrossRef]
- Ghimire, A.; Hasan, F.; Guan, X.; Potter, P.; Guo, C.; Lomnicki, S. Oxidation 1-Methyl Naphthalene Based on the Synergy of Environmentally Persistent Free Radicals (EPFRs) and PAHs in Particulate Matter (PM) Surface. Chemosphere 2023, 341, 140002. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Lee, W.-J. Using Oily Wastewater Emulsified Fuel in Boiler: Energy Saving and Reduction of Air Pollutant Emissions. Environ. Sci. Technol. 2008, 42, 270–275. [Google Scholar] [CrossRef]
- Feng, L.; Du, B.; Tian, J.; Long, W.; Tang, B. Combustion Performance and Emission Characteristics of a Diesel Engine Using a Water-Emulsified Heavy Fuel Oil and Light Diesel Blend. Energies 2015, 8, 13628–13640. [Google Scholar] [CrossRef]
- Khan, M.Y.; Giordano, M.; Gutierrez, J.; Welch, W.A.; Asa-Awuku, A.; Miller, J.W.; Cocker, D.R.I. Benefits of Two Mitigation Strategies for Container Vessels: Cleaner Engines and Cleaner Fuels. Environ. Sci. Technol. 2012, 46, 5049–5056. [Google Scholar] [CrossRef]
- Poster, D.L.; Lopez de alda, M.J.; Schantz, M.M.; Sander, L.C.; Vangel, M.G.; Wise, S.A. Certification of a Diesel Particulate Related Standard Reference Material (SRM 1975) for PAHs. Polycycl. Aromat. Compd. 1999, 14, 23–31. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y.; Guan, C.; Cheung, C.S.; Huang, Z. Effect of Biodiesel on PAH, OPAH, and NPAH Emissions from a Direct Injection Diesel Engine. Environ. Sci. Pollut. Res. 2018, 25, 34131–34138. [Google Scholar] [CrossRef]
- Karavalakis, G.; Deves, G.; Fontaras, G.; Stournas, S.; Samaras, Z.; Bakeas, E. The Impact of Soy-Based Biodiesel on PAH, Nitro-PAH and Oxy-PAH Emissions from a Passenger Car Operated over Regulated and Nonregulated Driving Cycles. Fuel 2010, 89, 3876–3883. [Google Scholar] [CrossRef]
- Edwards, D.E.; Zubarev, D.Y.; Lester, W.A.; Frenklach, M. Pathways to Soot Oxidation: Reaction of OH with Phenanthrene Radicals. J. Phys. Chem. A 2014, 118, 8606–8613. [Google Scholar] [CrossRef]
- Frenklach, M.; Liu, Z.; Singh, R.I.; Galimova, G.R.; Azyazov, V.N.; Mebel, A.M. Detailed, Sterically-Resolved Modeling of Soot Oxidation: Role of O Atoms, Interplay with Particle Nanostructure, and Emergence of Inner Particle Burning. Combust. Flame 2018, 188, 284–306. [Google Scholar] [CrossRef]
- Cooper, D.A. Exhaust Emissions from Ships at Berth. Atmos. Environ. 2003, 37, 3817–3830. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.; Cui, M.; Feng, Y.; Yang, X.; Chen, J.; Zhang, Y.; Gao, H.; Tian, C.; Matthias, V.; et al. Emission Factors and Environmental Implication of Organic Pollutants in PM Emitted from Various Vessels in China. Atmos. Environ. 2019, 200, 302–311. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.; Su, P.; Cui, M.; Han, Y.; Matthias, V.; Wang, G. Variations and Characteristics of Carbonaceous Substances Emitted from a Heavy Fuel Oil Ship Engine under Different Operating Loads. Environ. Pollut. 2021, 284, 117388. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, L.; Zhou, Q.; Zhang, X.; Xing, W.; Wei, Y.; Hu, M.; Zhao, L.; Toriba, A.; Hayakawa, K.; et al. Size Distribution of Particulate Polycyclic Aromatic Hydrocarbons in Fresh Combustion Smoke and Ambient Air: A Review. J. Environ. Sci. 2020, 88, 370–384. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, J.; Zhu, Y. Chemical Composition and Size Distribution of Particulate Matters from Marine Diesel Engines with Different Fuel Oils. Fuel 2019, 235, 972–983. [Google Scholar] [CrossRef]
- Leermakers, C.A.J.; Musculus, M.P.B. In-Cylinder Soot Precursor Growth in a Low-Temperature Combustion Diesel Engine: Laser-Induced Fluorescence of Polycyclic Aromatic Hydrocarbons. Proc. Combust. Inst. 2015, 35, 3079–3086. [Google Scholar] [CrossRef]
- Lu, T.; Huang, Z.; Cheung, C.S.; Ma, J. Size Distribution of EC, OC and Particle-Phase PAHs Emissions from a Diesel Engine Fueled with Three Fuels. Sci. Total Environ. 2012, 438, 33–41. [Google Scholar] [CrossRef]
- Yu, H.; Duan, S.; Sun, P. Comparative Analysis between Natural Gas/Diesel (Dual Fuel) and Pure Diesel on the Marine Diesel Engine. J. Eng. Res. 2015, 3, 37. [Google Scholar] [CrossRef]
- Su, P.; Yue, H.; Zhang, W.; Tomy, G.T.; Yin, F.; Sun, D.; Ding, Y.; Li, Y.; Feng, D. Application of a Fugacity Model to Estimate Emissions and Environmental Fate of Ship Stack PAHs in Shanghai, China. Chemosphere 2021, 281, 130710. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Mu, J.; Wang, Z.; Cong, Y.; Yao, Z.; Lin, Z. Aquatic Predicted No-Effect Concentrations of 16 Polycyclic Aromatic Hydrocarbons and Their Ecological Risks in Surface Seawater of Liaodong Bay, China. Environ. Toxicol. Chem. 2016, 35, 1587–1593. [Google Scholar] [CrossRef]
- Rönkkö, T.; Virtanen, A.; Kannosto, J.; Keskinen, J.; Lappi, M.; Pirjola, L. Nucleation Mode Particles with a Nonvolatile Core in the Exhaust of a Heavy Duty Diesel Vehicle. Environ. Sci. Technol. 2007, 41, 6384–6389. [Google Scholar] [CrossRef]
- Anderson, M.; Salo, K.; Hallquist, Å.M.; Fridell, E. Characterization of Particles from a Marine Engine Operating at Low Loads. Atmos. Environ. 2015, 101, 65–71. [Google Scholar] [CrossRef]
- Ntziachristos, L.; Saukko, E.; Lehtoranta, K.; Rönkkö, T.; Timonen, H.; Simonen, P.; Karjalainen, P.; Keskinen, J. Particle Emissions Characterization from a Medium-Speed Marine Diesel Engine with Two Fuels at Different Sampling Conditions. Fuel 2016, 186, 456–465. [Google Scholar] [CrossRef]
- Zhao, P.; Li, T.; Li, A.; Ma, Y.; Fang, M.; Li, X. Comprehensive Study on Particle Size Distribution, MAH and PAH Profiles during Alkylbenzene Pyrolysis. Fuel 2023, 352, 129099. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Guo, D.; Dang, J. Characteristics and Risk Assessment of PAH Pollution in Soil of a Retired Coking Wastewater Treatment Plant in Taiyuan, Northern China. Toxics 2023, 11, 415. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Jaafarzadeh Haghighi Fard, N.; Ramezani, Z.; Ahmadi, M.; Jorfi, S. Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Seawater and Sediments, Human and Ecological Risks, Northern Coastline of Persian Gulf. Bull. Environ. Contam. Toxicol. 2023, 110, 39. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, K.; Zhang, Y.; Fu, D.; Jiang, K.; Luo, J.; Li, Y.; Shen, G.; Liu, W.; Tao, S.; et al. Polycyclic Aromatic Compounds (PACs) in Industrial Soils from Northwestern of China: Occurrence, Distribution, Exposure Risk, and Implications on Risk-Based Controls. Environ. Geochem. Health 2024, 46, 135. [Google Scholar] [CrossRef]
- Johansson, K.O.; Head-Gordon, M.P.; Schrader, P.E.; Wilson, K.R.; Michelsen, H.A. Resonance-Stabilized Hydrocarbon-Radical Chain Reactions May Explain Soot Inception and Growth. Science 2018, 361, 997–1000. [Google Scholar] [CrossRef]
- Nisbet, I.C.T.; LaGoy, P.K. Toxic Equivalency Factors (TEFs) for Polycyclic Aromatic Hydrocarbons (PAHs). Regul. Toxicol. Pharmacol. 1992, 16, 290–300. [Google Scholar] [CrossRef]
- Agrawal, H.; Malloy, Q.G.; Welch, W.A.; Miller, J.W.; Cocker, D.R. In-use gaseous and particulate matter emissions from a modern ocean going container vessel. Atmos. Environ. 2008, 42, 5504–5510. [Google Scholar] [CrossRef]
- Collins, J.F.; Brown, J.P.; Alexeeff, G.V.; Salmon, A.G. Potency Equivalency Factors for Some Polycyclic Aromatic Hydrocarbons and Polycyclic Aromatic Hydrocarbon Derivatives. Regul. Toxicol. Pharmacol. 1998, 28, 45–54. [Google Scholar] [CrossRef]
- Khan, M.Y.; Ranganathan, S.; Agrawal, H.; Welch, W.A.; Laroo, C.; Miller, J.W.; Cocker, D.R. Measuring in-use ship emissions with international and U.S. federal methods. J. Air Waste Manag. Assoc. 2012, 63, 284–291. [Google Scholar] [CrossRef]
- Sax, T.; Alexis, A. A Critical Review of Ocean-Going Vessel Particulate Matter Emission Factors; California Air Resource Board: Sacramento, CA, USA, 2007. [Google Scholar]
- Sippula, O.; Stengel, B.; Sklorz, M.; Streibel, T.; Rabe, R.; Orasche, J.; Lintelmann, J.; Michalke, B.; Abbaszade, G.; Radischat, C.; et al. Particle Emissions from a Marine Engine: Chemical Composition and Aromatic Emission Profiles under Various Operating Conditions. Environ. Sci. Technol. 2014, 48, 11721–11729. [Google Scholar] [CrossRef]
- van der Gon, H.D.; Hulskotte, J. Methodologies for Estimating Shipping Emissions in The Netherlands: A Documentation of Currently Used Emission Factors and Related Activity Data; Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2010. [Google Scholar]
- Zhang, F.; Chen, Y.; Tian, C.; Lou, D.; Li, J.; Zhang, G.; Matthias, V. Emission factors for gaseous and particulate pollutants from offshore diesel engine vessels in China. Atmos. Meas. Technol. 2016, 16, 6319–6334. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Chang, J.; Peng, S.; Hong, N.; Hu, J.; Lv, J.; Wang, T.; Mao, H. Emission characteristics and temporal variation of PAHs and their derivatives from an ocean-going cargo vessel. Chemosphere 2020, 249, 126194. [Google Scholar] [CrossRef]

| Engine Mode | 6S35ME-B9 |
|---|---|
| Type | 6-cylinder, two strokes |
| Bore/stroke | 350/1550 mm |
| Revolutions per minute | 142 rpm |
| Max continuous output | 3570 kW |
| Parameter | HFO | EHFO |
|---|---|---|
| Density at 15 °C, kg/m3 | 976 | 982 |
| Viscosity (CST) at 50 °C | 161 | 159 |
| Water content, % (v/v) | 0.32 | 4.15 |
| Flash Point (Close), °C | 81.0 | 63.0 |
| Carbon content, wt.% | 86.17 | 82.85 |
| Hydrogen content, wt.% | 13.01 | 12.94 |
| Oxygen content, wt.% | 0.68 | 4.07 |
| Lower heating value, kJ/g | 42.56 | 39.00 |
| Operation Modes | 25% | 50% | 75% |
|---|---|---|---|
| HFO | 211.9 | 237.4 | 180.4 |
| EHFO | 227.0 | 219.7 | 202.6 |
| N-PAHs | RT | T | N-PAHs | RT | T |
|---|---|---|---|---|---|
| 1-Nitronaphthalene | 8.995 | 173,174 | 3-Nitrophenanthrene | 16 | 223,224 |
| 2-Nitronaphthalene | 9.374 | 173,174 | 2-Nitroanthracene | 16.804 | 223,224 |
| 2-Nitrobiphenyl | 11.054 | 199,200 | 3-Nitrofluoranthene | 22.293 | 247,248 |
| 3-Nitrobiphenyl | 12.446 | 199,200 | 1-Nitropyrene | 23.253 | 247,248 |
| 5-Nitroacenaphthene | 12.859 | 199,200 | 7Nitrobenzo(a)anthracene | 25.926 | 273,274 |
| 2-Nitrofluorene | 13.909 | 211,212 | 6-Nitrochrysene | 26.836 | 273,274 |
| 9-Nitroanthracene | 14.314 | 223,224 | 6-Nitrobenzo[a]pyrene | 29.624 | 297,298 |
| 9-Nitrophenanthrene | 15.292 | 223,224 |
| O-PAHs | RT | T |
|---|---|---|
| Naphthalene-1-alsehyde | 8.058 | 156,157 |
| 9-Fluorenone | 10.259 | 180,181 |
| 9-Formylphenanthrene | 14.06 | 206,207 |
| 9,10-Anthraquinone | 12.454 | 208,209 |
| 1,4-Anthraquinone | 13.126 | 208,209 |
| Benzanthrone | 21.097 | 230,231 |
| Benz(a)anthracene-7,12-dione | 23.024 | 258,259 |
| Reactions | k | T (K) |
|---|---|---|
| 2.7 × 10−16T1.42exp(−732/T) | 400–1495 | |
| 3.7 × 10−11exp(−2280/T) | 298–1400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, P.; Zhang, H.; Peng, L.; Zhu, L.; Li, T.; Tang, X.; Zhu, Y. Particulate Matter (PM) and Parent, Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbon (PAH) Emissions of Emulsified Heavy Fuel Oil in Marine Low-Speed Main Engine. Toxics 2024, 12, 404. https://doi.org/10.3390/toxics12060404
Su P, Zhang H, Peng L, Zhu L, Li T, Tang X, Zhu Y. Particulate Matter (PM) and Parent, Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbon (PAH) Emissions of Emulsified Heavy Fuel Oil in Marine Low-Speed Main Engine. Toxics. 2024; 12(6):404. https://doi.org/10.3390/toxics12060404
Chicago/Turabian StyleSu, Penghao, Hanzhe Zhang, Liming Peng, Lihong Zhu, Tie Li, Xiaojia Tang, and Yimin Zhu. 2024. "Particulate Matter (PM) and Parent, Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbon (PAH) Emissions of Emulsified Heavy Fuel Oil in Marine Low-Speed Main Engine" Toxics 12, no. 6: 404. https://doi.org/10.3390/toxics12060404
APA StyleSu, P., Zhang, H., Peng, L., Zhu, L., Li, T., Tang, X., & Zhu, Y. (2024). Particulate Matter (PM) and Parent, Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbon (PAH) Emissions of Emulsified Heavy Fuel Oil in Marine Low-Speed Main Engine. Toxics, 12(6), 404. https://doi.org/10.3390/toxics12060404





