Leukocyte Telomere Length Mediates the Associations between Blood Lead and Cadmium with Hypertension among Adults in the United States: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposure Measurement
2.3. LTL Measurement
2.4. Outcome Ascertainment
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Blood Metal Concentrations and Hypertension Risk
3.3. Blood Metal Concentrations and LTL
3.4. LTL and Hypertension Risk
3.5. Mediation Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Collaborators GBDCoD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yuan, Y.; Xiao, Y.; Long, P.; Li, W.; Yu, Y.; Liu, Y.; Liu, K.; Wang, H.; Zhou, L.; et al. Associations of plasma metal concentrations with the risks of all-cause and cardiovascular disease mortality in Chinese adults. Environ. Int. 2021, 157, 106808. [Google Scholar] [CrossRef] [PubMed]
- Solenkova, N.V.; Newman, J.D.; Berger, J.S.; Thurston, G.; Hochman, J.S.; Lamas, G.A. Metal pollutants and cardiovascular disease: Mechanisms and consequences of exposure. Am. Heart J. 2014, 168, 812–822. [Google Scholar] [CrossRef]
- Wang, B.; Chen, C.; Zhang, W.; Chen, Y.; Xia, F.; Wang, N.; Lu, Y. Exposure to lead and cadmium is associated with fasting plasma glucose and type 2 diabetes in Chinese adults. Diabetes Metab. Res. Rev. 2022, 38, e3578. [Google Scholar] [CrossRef] [PubMed]
- Gambelunghe, A.; Sallsten, G.; Borne, Y.; Forsgard, N.; Hedblad, B.; Nilsson, P.; Fagerberg, B.; Engstrom, G.; Barregard, L. Low-level exposure to lead, blood pressure, and hypertension in a population-based cohort. Environ. Res. 2016, 149, 157–163. [Google Scholar] [CrossRef]
- Orr, S.; Bridges, C. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef]
- Wang, K.; Mao, Y.; Liu, Z.; Li, Y.; Li, Z.; Sun, Y.; Ding, Y.; Liu, X.; Hong, J.; Xu, D.; et al. Association of Blood Heavy Metal Exposure with Atherosclerotic Cardiovascular Disease (ASCVD) Among White Adults: Evidence from NHANES 1999–2018. Biol. Trace Elem. Res. 2022, 201, 4321–4333. [Google Scholar] [CrossRef]
- Lee, B.K.; Ahn, J.; Kim, N.S.; Lee, C.B.; Park, J.; Kim, Y. Association of Blood Pressure with Exposure to Lead and Cadmium: Analysis of Data from the 2008–2013 Korean National Health and Nutrition Examination Survey. Biol. Trace Elem. Res. 2016, 174, 40–51. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, N.S.; Lee, B.K.; Park, J.; Kim, Y. Association of Blood Pressure with Blood Lead and Cadmium Levels in Korean Adolescents: Analysis of Data from the 2010-2016 Korean National Health and Nutrition Examination Survey. J. Korean Med. Sci. 2018, 33, e278. [Google Scholar] [CrossRef] [PubMed]
- Lanphear, B.P.; Rauch, S.; Auinger, P.; Allen, R.W.; Hornung, R.W. Low-level lead exposure and mortality in US adults: A population-based cohort study. Lancet Public Health 2018, 3, e177–e184. [Google Scholar] [CrossRef] [PubMed]
- de Lange, T. How Telomeres Solve the End-Protection Problem. Science 2009, 326, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W. Telomeres and aging. Curr. Opin. Cell Biol. 2018, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, Y.; Wang, J. Telomeres and essential hypertension. Clin. Biochem. 2015, 48, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, Y.; Ma, L. Telomere length and associated factors in older adults with hypertension. J. Int. Med. Res. 2019, 47, 5465–5474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, Y.; Wang, Y.; Liu, P.; Zhang, M.; Zhang, C.; Hu, F.B.; Hui, R. Short telomere length in blood leucocytes contributes to the presence of atherothrombotic stroke and haemorrhagic stroke and risk of post-stroke death. Clin. Sci. 2013, 125, 27–36. [Google Scholar] [CrossRef]
- Wilson, W.R.W.; Herbert, K.E.; Mistry, Y.; Stevens, S.E.; Patel, H.R.; Hastings, R.A.; Thompson, M.M.; Williams, B. Blood leucocyte telomere DNA content predicts vascular telomere DNA content in humans with and without vascular disease. Eur. Heart J. 2008, 29, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. Int. 2016, 23, 8244–8259. [Google Scholar] [CrossRef]
- Zota, A.R.; Needham, B.L.; Blackburn, E.H.; Lin, J.; Park, S.K.; Rehkopf, D.H.; Epel, E.S. Associations of Cadmium and Lead Exposure With Leukocyte Telomere Length: Findings From National Health and Nutrition Examination Survey, 1999–2002. Am. J. Epidemiol. 2015, 181, 127–136. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC): National Health and Nutrition Examination Survey 1999–2000 Data Documentation, Codebook, and Frequencies: Cadmium, Lead, Mercury, Cotinine & Nutritional Biochemistries (LAB06). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/LAB06.htm (accessed on 22 May 2024).
- Centers for Disease Control and Prevention (CDC): National Health and Nutrition Examination Survey 2000–2001 Data Documentation, Codebook, and Frequencies: Cadmium, Lead, Mercury, Cotinine & Nutritional Biochemistries (LAB06). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2001-2002/L06_B.htm (accessed on 22 May 2024).
- Guallar, E.; Silbergeld, E.K.; Navas-Acien, A.; Malhotra, S.; Astor, B.C.; Sharrett, A.R.; Schwartz, B.S. Confounding of the Relation between Homocysteine and Peripheral Arterial Disease by Lead, Cadmium, and Renal Function. Am. J. Epidemiol. 2006, 163, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention: National Health and Nutrition Examination Survey 1999–2000 Data Documentation, Codebook, and Frequencies: Telomere Mean and Standard Deviation (Surplus) (TELO_A). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/TELO_A.htm (accessed on 22 May 2024).
- Centers for Disease Control and Prevention: National Health and Nutrition Examination Survey 2001–2002 Data Documentation, Codebook, and Frequencies: Telomere Mean and Standard Deviation (Surplus) (TELO_B). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2001-2002/TELO_B.htm (accessed on 22 May 2024).
- Centers for Disease Control and Prevention: National Health and Nutrition Examination Survey 1999–2000 Data Documentation, Codebook, and Frequencies: Blood Pressure (BPX). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/BPX.htm (accessed on 22 May 2024).
- Centers for Disease Control and Prevention: National Health and Nutrition Examination Survey 2000–2001 Data Documentation, Codebook, and Frequencies: Blood Pressure (BPX_B). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2001-2002/BPX_B.htm (accessed on 22 May 2024).
- Reboussin, D.M.; Allen, N.B.; Griswold, M.E.; Guallar, E.; Hong, Y.; Lackland, D.T.; Miller, E.R.; Polonsky, T.; Thompson-Paul, A.M.; Vupputuri, S. Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018, 138, e595–e616. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, T.; Wan, Z.; Lu, Q.; Zhang, X.; Qiu, Z.; Li, L.; Zhu, K.; Liu, L.; Pan, A.; et al. Associations of Serum Folate and Vitamin B12 Levels With Cardiovascular Disease Mortality Among Patients With Type 2 Diabetes. JAMA Netw. Open 2022, 5, e2146124. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, A.; Yeung, S.; Woo, J.; Lo, K. Joint Associations of Food Groups with All-Cause and Cause-Specific Mortality in the Mr. OS and Ms. OS Study: A Prospective Cohort. Nutrients 2022, 14, 3915. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chan, K.; Choi, C.; Yang, A.; Lo, K. Identifying Effects of Urinary Metals on Type 2 Diabetes in U.S. Adults: Cross-Sectional Analysis of National Health and Nutrition Examination Survey 2011–2016. Nutrients 2022, 14, 1552. [Google Scholar] [CrossRef]
- Kilpatrick, E.S.; Bloomgarden, Z.T.; Zimmet, P.Z. International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care 2009, 32, e159. [Google Scholar] [CrossRef]
- Zhang, N.-H.; Luo, R.; Cheng, Y.-C.; Ge, S.-W.; Xu, G. Leisure-Time Physical Activity and Mortality in CKD: A 1999–2012 NHANES Analysis. Am. J. Nephrol. 2020, 51, 919–929. [Google Scholar] [CrossRef]
- Wu, S.; Li, L.; Ji, G.; Xing, X.; Li, J.; Ma, A.; Wei, Y.; Zhao, D.; Huang, H.; Ma, W.; et al. Association of multi-metals with the risk of hypertension and the interaction with obesity: A cross-sectional study in China. Front. Public Health 2023, 11, 1090935. [Google Scholar] [CrossRef]
- Huang, Z. Association Between Blood Lead Level With High Blood Pressure in US (NHANES 1999–2018). Front. Public Health 2022, 10, 836357. [Google Scholar] [CrossRef]
- Angeli, J.K.; Cruz Pereira, C.A.; de Oliveira Faria, T.; Stefanon, I.; Padilha, A.S.; Vassallo, D.V. Cadmium exposure induces vascular injury due to endothelial oxidative stress: The role of local angiotensin II and COX-2. Free Radic. Biol. Med. 2013, 65, 838–848. [Google Scholar] [CrossRef]
- Vaziri, N.D. Mechanisms of lead-induced hypertension and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H454–H465. [Google Scholar] [CrossRef] [PubMed]
- Morty, R.E.; Simões, M.R.; Ribeiro Júnior, R.F.; Vescovi, M.V.A.; de Jesus, H.C.; Padilha, A.S.; Stefanon, I.; Vassallo, D.V.; Salaices, M.; Fioresi, M. Acute Lead Exposure Increases Arterial Pressure: Role of the Renin-Angiotensin System. PLoS ONE 2011, 6, e18730. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, Y.; Ni, N.; Bao, B.; Zhang, C.; Lu, L. High lead exposure is associated with telomere length shortening in Chinese battery manufacturing plant workers. Occup. Environ. Med. 2012, 69, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Cowell, W.; Colicino, E.; Tanner, E.; Amarasiriwardena, C.; Andra, S.S.; Bollati, V.; Kannan, S.; Ganguri, H.; Gennings, C.; Wright, R.O.; et al. Prenatal toxic metal mixture exposure and newborn telomere length: Modification by maternal antioxidant intake. Environ. Res. 2020, 190, 110009. [Google Scholar] [CrossRef] [PubMed]
- Houben, J.M.J.; Moonen, H.J.J.; van Schooten, F.J.; Hageman, G.J. Telomere length assessment: Biomarker of chronic oxidative stress? Free Radic. Biol. Med. 2008, 44, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Lichterfeld, M.; O’Donovan, A.; Pantell, M.S.; Puterman, E.; Dhabhar, F.S.; Blackburn, E.H.; Yaffe, K.; Cawthon, R.M.; Opresko, P.L.; Hsueh, W.-C.; et al. Cumulative Inflammatory Load Is Associated with Short Leukocyte Telomere Length in the Health, Aging and Body Composition Study. PLoS ONE 2011, 6, e19687. [Google Scholar] [CrossRef]
- Cheng, G.; Wang, L.; Dai, M.; Wei, F.; Xu, D. Shorter Leukocyte Telomere Length coupled with lower expression of Telomerase Genes in patients with Essential Hypertension. Int. J. Med. Sci. 2020, 17, 2180–2186. [Google Scholar] [CrossRef] [PubMed]
- Tamim, H.; Isma’eel, H.; Nakhoul, N.; Nasrallah, M.; Nasreddine, L.; Sleiman, F.; Zgheib, N.K. Short Telomere Length is Associated with Aging, Central Obesity, Poor Sleep and Hypertension in Lebanese Individuals. Aging Dis. 2018, 9, 77. [Google Scholar] [CrossRef]
- Yu, S.-N.; Chen, S.-Q.; Fan, G.-Q.; Pan, W.-Z.; Jia, J.; Wang, Q.; Ma, L.; Li, B.; Qiang, M.; Qiu, Y.-L.; et al. Relative Telomere Length in Peripheral Blood Cells and Hypertension Risk among Mine Workers: A Case-Control Study in Chinese Coal Miners. BioMed Res. Int. 2020, 2020, 5681096. [Google Scholar] [CrossRef]
- Yeh, J.-K.; Wang, C.-Y. Telomeres and Telomerase in Cardiovascular Diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef]
- Yegorov, Y.E.; Poznyak, A.V.; Nikiforov, N.G.; Starodubova, A.V.; Orekhov, A.N. Role of Telomeres Shortening in Atherogenesis: An Overview. Cells 2021, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Agostini, C.; Sartore, S.; Avogaro, A. Endothelial progenitor cells in the natural history of atherosclerosis. Atherosclerosis 2007, 194, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.-K.; Lin, M.-H.; Wang, C.-Y. Telomeres as Therapeutic Targets in Heart Disease. JACC Basic Transl. Sci. 2019, 4, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, Q.; Zhou, F.; Li, G.; Liu, J.; Lv, J.; Li, L.; Chang, D. Telomere length and the risk of cardiovascular diseases: A Mendelian randomization study. Front. Cardiovasc. Med. 2022, 9, 1012615. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.G.; Ives, S.J.; Walker, A.E.; Cawthon, R.M.; Andtbacka, R.H.I.; Noyes, D.; Lesniewski, L.A.; Richardson, R.S.; Donato, A.J. Role of arterial telomere dysfunction in hypertension: Relative contributions of telomere shortening and telomere uncapping. J. Hypertens. 2014, 32, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Koriath, M.; Müller, C.; Pfeiffer, N.; Nickels, S.; Beutel, M.; Schmidtmann, I.; Rapp, S.; Münzel, T.; Westermann, D.; Karakas, M.; et al. Relative Telomere Length and Cardiovascular Risk Factors. Biomolecules 2019, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Liu, F.; Chen, H.; Tan, T.; Yao, P.; Tang, Y. Biological aging mediates the associations between urinary metals and osteoarthritis among U.S. adults. BMC Med. 2022, 20, 207. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, J.; Kang, H.; Han, Z. Mediating effect of telomere length on relationship between lead and cadmium coexposure and blood glucose. J. Environ. Occup. Med. 2022, 39, 841–848. [Google Scholar] [CrossRef]
- Li, Y.; Guo, J.; Gao, Y.; Kang, H. Mediating role of peripheral blood telomere length in relationship between environmental lead exposure and glomerular filtration rate. J. Occup. Environ. Med. 2023, 38, 1327–1332. [Google Scholar] [CrossRef]


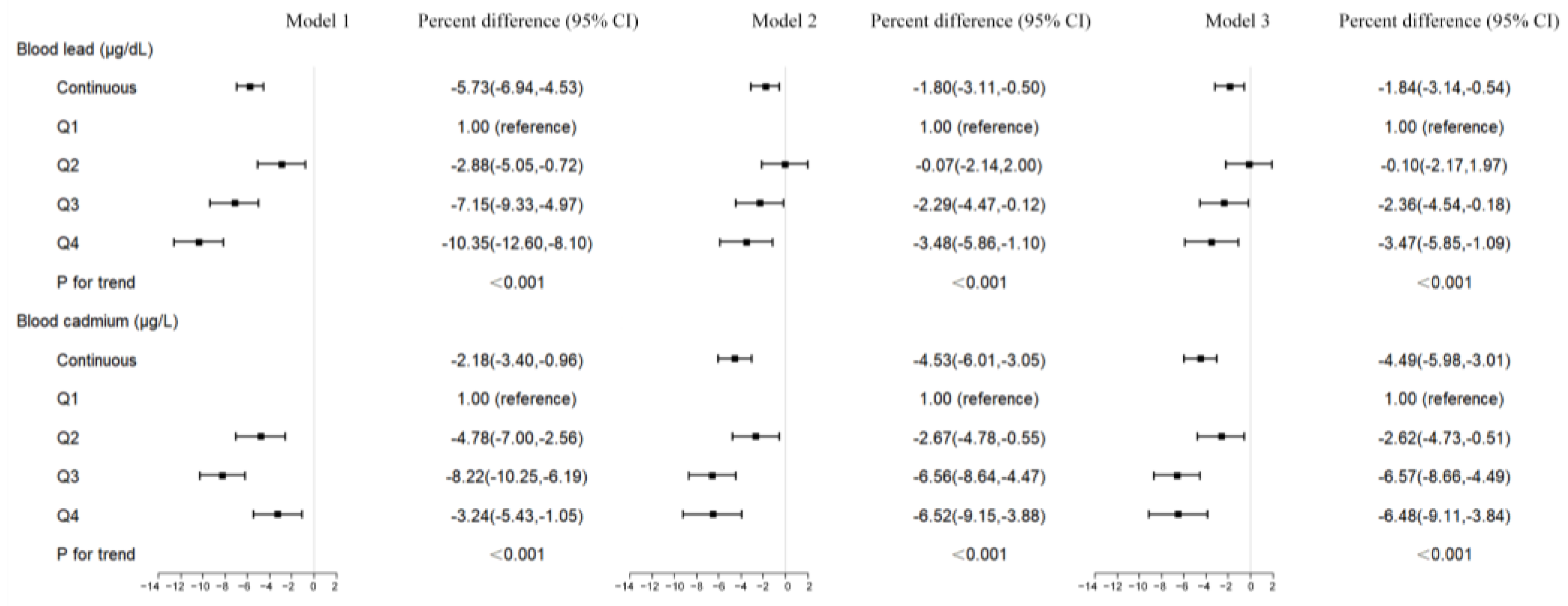
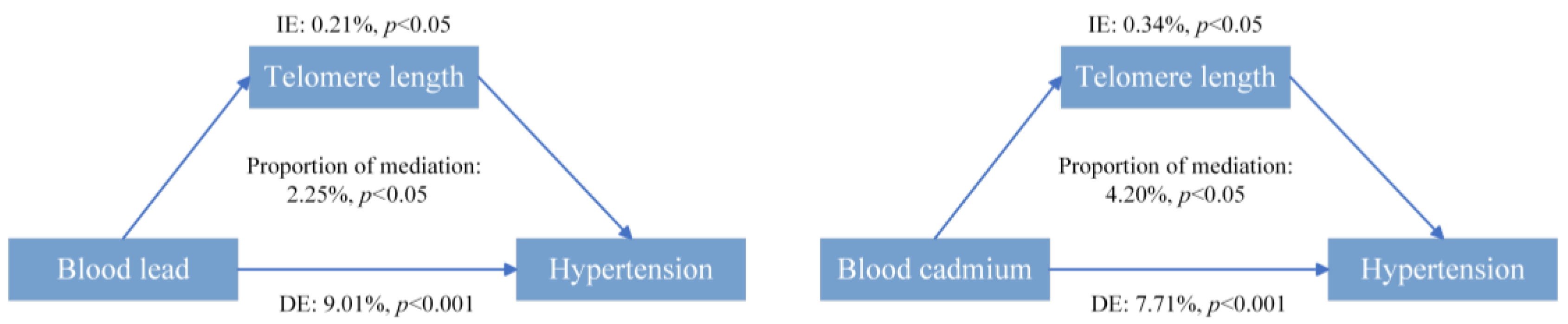
| Blood Metals | Continuous | Q1 | Q2 | Q3 | Q4 | p for Trend * |
|---|---|---|---|---|---|---|
| Blood lead (μg/dL) | ≤1.2 | 1.2–1.8 | 1.8–2.8 | >2.8 | ||
| Model 1 | 1.71 (1.54, 1.90) | 1.00 (reference) | 1.56 (1.30, 1.88) | 1.93 (1.60, 2.33) | 2.66 (2.19, 3.24) | <0.001 |
| Model 2 | 1.43 (1.26, 1.63) | 1.00 (reference) | 1.38 (1.13, 1.70) | 1.48 (1.20, 1.83) | 1.96 (1.55, 2.48) | <0.001 |
| Model 3 | 1.46 (1.28, 1.66) | 1.00 (reference) | 1.40 (1.14, 1.72) | 1.52 (1.23, 1.89) | 2.02 (1.59, 2.56) | <0.001 |
| Blood cadmium (μg/L) | ≤0.3 | 0.3–0.4 | 0.4–0.7 | >0.7 | ||
| Model 1 | 1.10 (0.99, 1.22) | 1.00 (reference) | 1.19 (0.98, 1.44) | 1.65 (1.39, 1.97) | 1.13 (0.94, 1.36) | 0.004 |
| Model 2 | 1.34 (1.16, 1.55) | 1.00 (reference) | 1.11 (0.90, 1.37) | 1.60 (1.30, 1.97) | 1.53 (1.17, 1.99) | <0.001 |
| Model 3 | 1.35 (1.16, 1.57) | 1.00 (reference) | 1.10 (0.89, 1.36) | 1.62 (1.32, 2.00) | 1.54 (1.18, 2.01) | <0.001 |
| Leukocyte Telomere Length | OR | 95% CI | p Value |
|---|---|---|---|
| Model 1 | 0.26 | 0.19, 0.34 | <0.001 |
| Model 2 | 0.62 | 0.45, 0.85 | 0.003 |
| Model 3 | 0.65 | 0.47, 0.89 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, C.; Yang, Y.; Pan, J.; Liu, H.; Wang, X.; Zhou, S.; Shi, X.; Zhang, Y.; Wang, D.; Hu, X. Leukocyte Telomere Length Mediates the Associations between Blood Lead and Cadmium with Hypertension among Adults in the United States: A Cross-Sectional Study. Toxics 2024, 12, 409. https://doi.org/10.3390/toxics12060409
Ouyang C, Yang Y, Pan J, Liu H, Wang X, Zhou S, Shi X, Zhang Y, Wang D, Hu X. Leukocyte Telomere Length Mediates the Associations between Blood Lead and Cadmium with Hypertension among Adults in the United States: A Cross-Sectional Study. Toxics. 2024; 12(6):409. https://doi.org/10.3390/toxics12060409
Chicago/Turabian StyleOuyang, Changping, Yinan Yang, Jinhua Pan, Heming Liu, Xuemei Wang, Shengze Zhou, Xiaoru Shi, Yanxia Zhang, Dan Wang, and Xiaobin Hu. 2024. "Leukocyte Telomere Length Mediates the Associations between Blood Lead and Cadmium with Hypertension among Adults in the United States: A Cross-Sectional Study" Toxics 12, no. 6: 409. https://doi.org/10.3390/toxics12060409
APA StyleOuyang, C., Yang, Y., Pan, J., Liu, H., Wang, X., Zhou, S., Shi, X., Zhang, Y., Wang, D., & Hu, X. (2024). Leukocyte Telomere Length Mediates the Associations between Blood Lead and Cadmium with Hypertension among Adults in the United States: A Cross-Sectional Study. Toxics, 12(6), 409. https://doi.org/10.3390/toxics12060409





