Effects of Foliar Spraying of Dicarboxylicdimethylammonium Chloride on Cadmium and Arsenic Accumulation in Rice Grains
Abstract
:1. Introduction
2. Materials and Methods
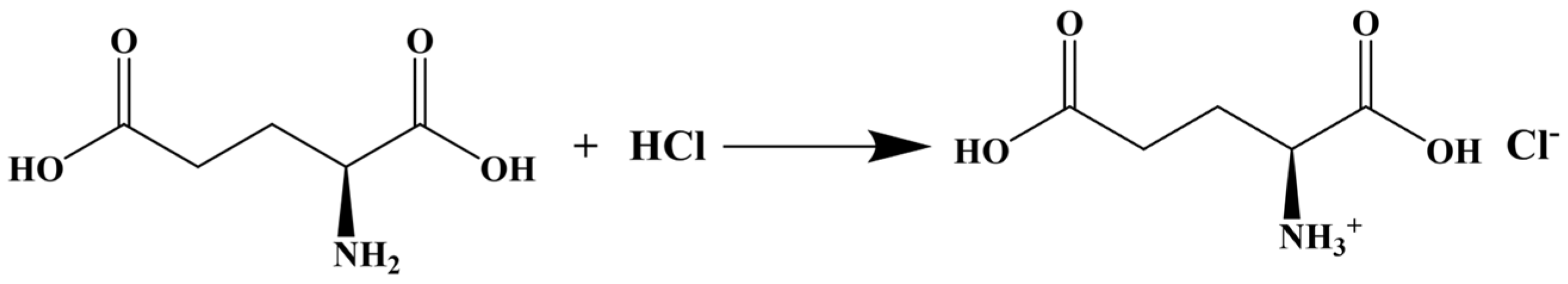
2.1. Material Preparation
2.2. Experimental Design
2.3. Determination of Cd and As Content
2.4. Determination of Essential Element Content
2.5. Determination of Total Amino Acid Content
2.6. Statistical Analysis
3. Results
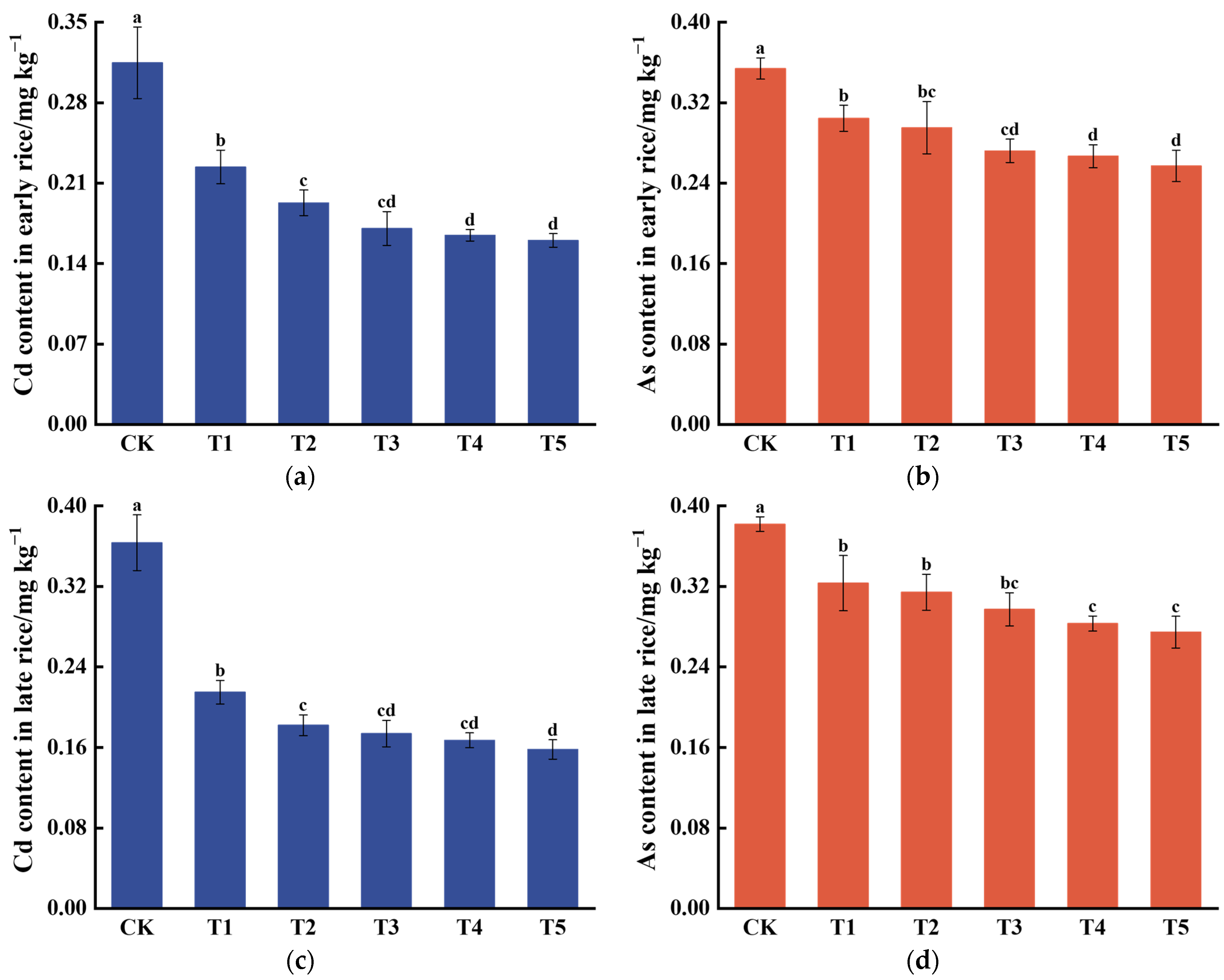
3.1. Effects of DDAC on the Acumulation of Cd and As in Rice Grains
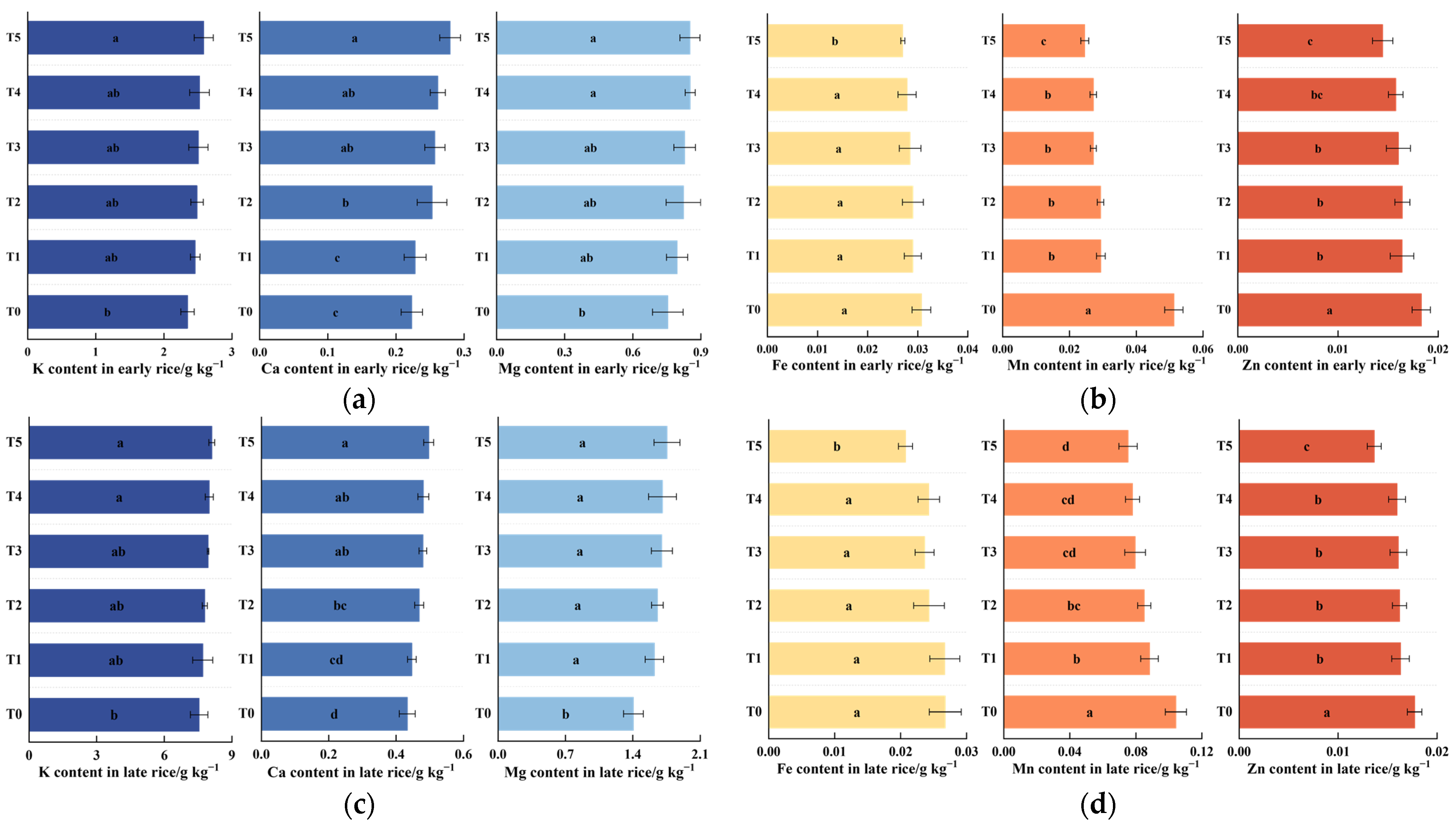
3.2. Effects of DDAC on the Essential Element Content of Rice Grains
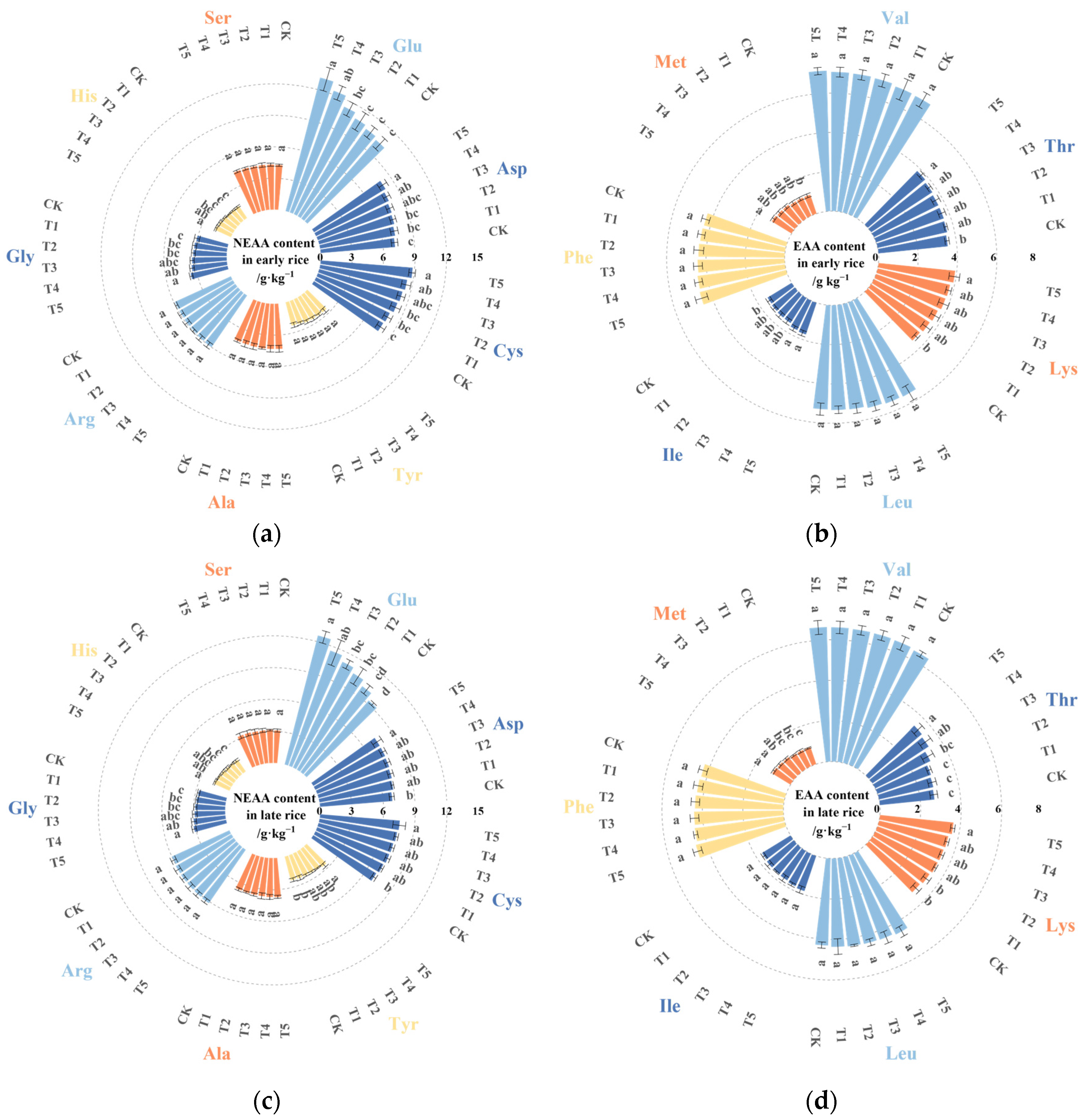
3.3. Effects of DDAC on the Total Amino Acid Content in Rice Grains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sui, F.Q.; Chang, J.D.; Tang, Z.; Liu, W.J.; Huang, X.Y.; Zhao, F.J. Nramp5 expression and functionality likely explain higher cadmium uptake in rice than in wheat and maize. Plant Soil 2018, 433, 377–389. [Google Scholar] [CrossRef]
- Su, Y.H.; McGrath, S.P.; Zhao, F.J. Rice is more efficient in arsenite uptake and translocation than wheat and barley. Plant Soil 2010, 328, 27–34. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Z.Y.; Li, F.B. Using ensemble models to identify and apportion heavy metal pollution sources in agricultural soils on a local scale. Environ. Pollut. 2015, 206, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Qiao, P.W.; Wang, S.; Li, J.B.; Shan, Y.; Wei, Y.; Zhang, Z.G.; Lei, M. Quantitative analysis of the contribution of sources, diffusion pathways, and receptor attributes for the spatial distribution of soil heavy metals and their nested structure analysis in China. Sci. Total Environ. 2023, 882, 163647. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.H.; Chen, C.; Kou, M.; Liu, Z.Y.; Wang, Z.; Cai, J.X.; Tan, W.F. Heavy metal concentrations in rice that meet safety standards can still pose a risk to human health. Commun. Earth Environ. 2023, 4, 84. [Google Scholar] [CrossRef]
- Zhao, F.J.; Wang, P. Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil 2020, 446, 1–21. [Google Scholar] [CrossRef]
- Honma, T.; Ohba, H.; Kaneko-Kadokura, A.; Makino, T.; Nakamura, K.; Katou, H. Optimal Soil Eh, pH, and Water Management for Simultaneously Minimizing Arsenic and Cadmium Concentrations in Rice Grains. Environ. Sci. Technol. 2016, 50, 4178–4185. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Wei, B.Y.; Zhou, H.; Gu, J.F.; Liao, B.H. Co-application of water management and foliar spraying silicon to reduce cadmium and arsenic uptake in rice: A two-year field experiment. Sci. Total Environ. 2022, 818, 151801. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.F.; Zhou, H.; Tang, H.L.; Yang, W.T.; Zeng, M.; Liu, Z.M.; Peng, P.Q.; Liao, B.H. Cadmium and arsenic accumulation during the rice growth period under in situ remediation. Ecotoxicol. Environ. Saf. 2019, 171, 451–459. [Google Scholar] [CrossRef]
- Xue, W.J.; Zhang, C.B.; Wang, P.P.; Wang, C.R.; Huang, Y.C.; Zhang, X.; Liu, Z.Q. Rice vegetative organs alleviate cadmium toxicity by altering the chemical forms of cadmium and increasing the ratio of calcium to manganese. Ecotoxicol. Environ. Saf. 2019, 184, 109640. [Google Scholar] [CrossRef]
- Moulick, D.; Ghosh, D.; Mandal, J.; Bhowmick, S.; Mondal, D.; Choudhury, S.; Santra, S.C.; Vithanage, M.; Biswas, J.K. A cumulative assessment of plant growth stages and selenium supplementation on arsenic and micronutrients accumulation in rice grains. J. Clean. Prod. 2023, 386, 135764. [Google Scholar] [CrossRef]
- Cui, H.; Tang, S.T.; Huang, S.Q.; Lei, L.D.; Jiang, Z.M.; Li, L.; Wei, S.Q. Simultaneous mitigation of arsenic and cadmium accumulation in rice grains by foliar inhibitor with ZIF-8@Ge-132. Sci. Total Environ. 2023, 860, 160307. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.J.; Yang, J.L.; Liu, Y.; Su, G.Q.; Zhao, M.; Wang, S.Z.; Tang, Y.T.; Qiu, R.L. Mitigation effects of foliar supply of different sulfur forms on uptake, translocation and grain accumulation of Cd and As by paddy rice on basis of liming. Sci. Total Environ. 2023, 905, 167338. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, N.; Li, Y.W.; Cai, Q.Y.; Li, H.Y.; Mo, C.H.; Wong, M.H. Cadmium in rice: Transport mechanisms, influencing factors, and minimizing measures. Environ. Pollut. 2017, 224, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.W.; Huang, P.C.; Yan, X.; Chukwuma, A.; Yang, S.; Yang, Z.H.; Li, H.; Yang, W.C. Inhibitory effect of exogenous mineral elements (Si, P, Zn, Ca, Mn, Se, Fe, S) on rice Cd accumulation and soil Cd bioavailability in Cd-contaminated farmlands: A meta-analysis. Chemosphere 2023, 343, 140282. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha. Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Mohapatra, T. Interaction between macro- and micro- nutrients in plants. Front. Plant Sci. 2021, 12, 665583. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Zhou, Y.J.; Jiang, Y. Amino acids in rice grains and their regulation by polyamines and phytohormones. Plants 2022, 11, 1581. [Google Scholar] [CrossRef] [PubMed]
- Galili, G.; Amir, R.; Fernie, A.R. The regulation of essential amino acid synthesis and accumulation in plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef]
- Chen, J.; Le, X.C.; Zhu, L.Z. Metabolomics and transcriptomics reveal defense mechanism of rice (Oryza sativa) grains under stress of 2,2′,4,4′-tetrabromodiphenyl ether. Environ. Int. 2019, 133, 105154. [Google Scholar] [CrossRef]
- Jiang, M.; Jiang, J.; Li, S.; Li, M.; Tan, Y.Y.; Song, S.Y.; Shu, Q.Y.; Huang, J.Z. Glutamate alleviates cadmium toxicity in rice via suppressing cadmium uptake and translocation. J. Hazard. Mater. 2020, 384, 121319. [Google Scholar] [CrossRef] [PubMed]
- Asgher, M.; Sehar, Z.; Rehaman, A.; Rashid, S.; Ahmed, S.; Per, T.S.; Alyemeni, M.N.; Khan, N.A. Exogenously-applied L-glutamic acid protects photosynthetic functions and enhances arsenic tolerance through increased nitrogen assimilation and antioxidant capacity in rice (Oryza sativa L.). Environ. Pollut. 2022, 301, 119008. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Fu, L.; Zhang, C.B.; Deng, J.W.; Xue, W.J.; Deng, Y. Effects of exogenous chlorinated amino acetic acid on cadmium and mineral elements in rice seedlings. Toxics 2023, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Deng, J.W.; Liu, S.Y.; Zhang, C.B.; Xue, W.J.; Mailhot, G.; Vione, D.; Deng, Y.; Wang, C.R.; Wang, L. Efficient regulation of cadmium accumulation by carboxymethylammonium chloride in rice: Correlation analysis and expression of transporter gene OsGLR3. Sci. Total Environ. 2024, 930, 172861. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chu, Y.T.; Sun, H.Y.; Bao, Q.L.; Huang, Y.Z. Melatonin alleviates arsenite toxicity by decreasing the arsenic accumulation in cell protoplasts and increasing the antioxidant capacity in rice. Chemosphere 2023, 312, 137292. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.R.; Wang, C.R.; Huang, Y.C.; Liu, B.; Liu, Z.Q.; Huang, Y.Z.; Cheng, L.L.; Huang, Y.F.; Zhang, C.B. Foliar application of the sulfhydryl compound 2,3-dimercaptosuccinic acid inhibits cadmium, lead, and arsenic accumulation in rice grains by promoting heavy metal immobilization in flag leaves. Environ. Pollut. 2021, 285, 117355. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Wang, C.R.; Zhang, C.B.; Huang, Y.C.; Wang, P.P.; Liu, Z.Q. Rice grains alleviate cadmium toxicity by expending glutamate and increasing manganese in the cadmium contaminated farmland. Environ. Pollut. 2020, 262, 114236. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Bundschuh, J.; Seneweera, S.; Ok, Y.S. An integrated approach of rice hull biochar-alternative water management as a promising tool to decrease inorganic arsenic levels and to sustain essential element contents in rice. J. Hazard. Mater. 2021, 405, 124188. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.M.; Zhou, S.L.; Zhou, Y.J.; Jia, Z.Y.; Guo, T.W.; Wang, J.X. Cadmium pollution of soil-rice ecosystems in rice cultivation dominated regions in China: A review. Environ. Pollut. 2021, 280, 116965. [Google Scholar] [CrossRef]
- Feng, X.M.; Han, L.; Chao, D.Y.; Liu, Y.; Zhang, Y.J.; Wang, R.G.; Guo, J.K.; Feng, R.W.; Xu, Y.M.; Ding, Y.Z.; et al. Ionomic and transcriptomic analysis provides new insight into the distribution and transport of cadmium and arsenic in rice. J. Hazard. Mater. 2017, 331, 246–256. [Google Scholar] [CrossRef]
- Tan, L.T.; Qu, M.M.; Zhu, Y.X.; Peng, C.; Wang, J.R.; Gao, D.Y.; Chen, C.Y. ZINC TRANSPORTER5 and ZINC TRANSPORTER9 function synergistically in zinc/cadmium uptake. Plant Physiol. 2020, 183, 1235–1249. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Zhang, C.B.; Wang, C.R.; Huang, Y.C.; Liu, Z.Q. Gadolinium inhibits cadmium transport by blocking non-selective cation channels in rice seedlings. Ecotoxicol. Environ. Saf. 2019, 179, 160–166. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, D.; Ai, H.; Mei, H.Y.; Liu, X.; Sun, S.B.; Xu, G.H.; Liu, Y.G.; Chen, Y.S.; Ma, L.N.Q. Knocking out OsPT4 gene decreases arsenate uptake by rice plants and inorganic arsenic accumulation in rice grains. Environ. Sci. Technol. 2017, 51, 12131–12138. [Google Scholar] [CrossRef]
- Shi, G.; Ma, H.; Chen, Y.; Liu, H.; Song, G.; Cai, Q.; Lou, L.; Rengel, Z. Low arsenate influx rate and high phosphorus concentration in wheat (Triticum aestivum L.): A mechanism for arsenate tolerance in wheat plants. Chemosphere 2019, 214, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Basu, S.; Rishu, A.K.; Kumar, G. Revisiting the mechanisms of arsenic uptake, transport and detoxification in plants. Environ. Exp. Bot. 2022, 194, 104730. [Google Scholar] [CrossRef]
- Zeng, F.R.; Nazir, M.M.; Ahmed, T.; Noman, M.; Ali, S.; Rizwan, M.; Alam, M.S.; Lwalaba, J.L.W.; Zhang, G.P. Calcium and L-glutamate present the opposite role in managing arsenic in barley. Environ. Pollut. 2023, 321, 121141. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Zhao, D.S.; Liu, Q.Q. Connections between amino acid metabolisms in plants: Lysine as an example. Front. Plant Sci. 2020, 11, 928. [Google Scholar] [CrossRef]
- Xue, W.J.; Zhang, C.B.; Huang, Y.C.; Wang, C.R.; Zhang, X.; Liu, Z.Q. Rice organs concentrate cadmium by chelation of amino acids containing dicarboxyl groups and enhance risks to human and environmental health in Cd-contaminated areas. J. Hazard. Mater. 2022, 426, 128130. [Google Scholar] [CrossRef]
- Cao, Z.Z.; Qin, M.L.; Lin, X.Y.; Zhu, Z.W.; Chen, M.X. Sulfur supply reduces cadmium uptake and translocation in rice grains (Oryza sativa L.) by enhancing iron plaque formation, cadmium chelation and vacuolar sequestration. Environ. Pollut. 2018, 238, 76–84. [Google Scholar] [CrossRef]
- Zhang, J.; Hamza, A.; Xie, Z.M.; Hussain, S.; Brestic, M.; Tahir, M.A.; Ulhassan, Z.; Yu, M.; Allakhverdiev, S.I.; Shabala, S. Arsenic transport and interaction with plant metabolism: Clues for improving agricultural productivity and food safety. Environ. Pollut. 2021, 290, 117987. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.J.; Zhang, X.; Zhang, C.B.; Wang, C.R.; Huang, Y.C.; Liu, Z.Q. Mitigating the toxicity of reactive oxygen species induced by cadmium via restoring citrate valve and improving the stability of enzyme structure in rice. Chemosphere 2023, 327, 138511. [Google Scholar] [CrossRef]
- Shi, S.L.; Wang, T.; Chen, Z.; Tang, Z.; Wu, Z.C.; Salt, D.E.; Chao, D.Y.; Zhao, F.J. OsHAC1;1 and OsHAC1;2 function as arsenate reductases and regulate arsenic accumulation. Plant Physiol. 2016, 172, 1708–1719. [Google Scholar] [CrossRef]
- Xu, J.M.; Shi, S.L.; Wang, L.; Tang, Z.; Lv, T.T.; Zhu, X.L.; Ding, X.M.; Wang, Y.F.; Zhao, F.J.; Wu, Z.C. OsHAC4 is critical for arsenate tolerance and regulates arsenic accumulation in rice. New Phytol. 2017, 215, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Y.; Park, J.; Mendoza-Cózatl, D.G.; Suter-Grotemeyer, M.; Shim, D.; Hörtensteiner, S.; Geisler, M.; Weder, B.; Rea, P.A.; Rentsch, D.; et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 21187–21192. [Google Scholar] [CrossRef]
- Shao, Q.L.; Gao, Q.F.; Lhamo, D.; Zhang, H.S.; Luan, S. Two glutamate- and pH-regulated Ca2+ channels are required for systemic wound signaling in Arabidopsis. Sci. Signal. 2020, 13, eaba1453. [Google Scholar] [CrossRef]
- Kong, D.; Hu, H.C.; Okuma, E.; Lee, Y.; Lee, H.S.; Munemasa, S.; Cho, D.; Ju, C.; Pedoeim, L.; Rodriguez, B.; et al. L-Met Activates Arabidopsis GLR Ca2+ Channels Upstream of ROS Production and Regulates Stomatal Movement. Cell Rep. 2016, 17, 2553–2561. [Google Scholar] [CrossRef]
- Vatsa, P.; Chiltz, A.; Bourque, S.; Wendehenne, D.; Garcia-Brugger, A.; Pugin, A. Involvement of putative glutamate receptors in plant defence signaling and NO production. Biochimie 2011, 93, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Stephens, N.R.; Spalding, E.P. Calcium entry mediated by GLR3. 3, an arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol. 2006, 142, 963–971. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, W.J.; Zhang, C.B.; Wang, C.R.; Huang, Y.C.; Wang, Y.T.; Peng, L.C.; Liu, Z.Q. Cadmium pollution leads to selectivity loss of glutamate receptor channels for permeation of Ca2+/Mn2+/Fe2+/Zn2+ over Cd2+ in rice plant. J. Hazard. Mater. 2023, 452, 131342. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, L.; Deng, J.; Lao, D.R.; Zhang, C.; Xue, W.; Deng, Y.; Luo, X. Effects of Foliar Spraying of Dicarboxylicdimethylammonium Chloride on Cadmium and Arsenic Accumulation in Rice Grains. Toxics 2024, 12, 418. https://doi.org/10.3390/toxics12060418
Fu L, Deng J, Lao DR, Zhang C, Xue W, Deng Y, Luo X. Effects of Foliar Spraying of Dicarboxylicdimethylammonium Chloride on Cadmium and Arsenic Accumulation in Rice Grains. Toxics. 2024; 12(6):418. https://doi.org/10.3390/toxics12060418
Chicago/Turabian StyleFu, Lin, Jiawei Deng, Dayliana Ruiz Lao, Changbo Zhang, Weijie Xue, Yun Deng, and Xin Luo. 2024. "Effects of Foliar Spraying of Dicarboxylicdimethylammonium Chloride on Cadmium and Arsenic Accumulation in Rice Grains" Toxics 12, no. 6: 418. https://doi.org/10.3390/toxics12060418





