Shift in Bacterial Community Structure in the Biodegradation of Benzene and Toluene under Sulfate-Reducing Condition
Abstract
1. Introduction
2. Materials and Methods
2.1. Groundwater Sampling and Analysis
2.2. Artificial Sample Setup
2.3. Analytical Method
2.4. Analysis of Bacterial Community Sturcture
3. Results
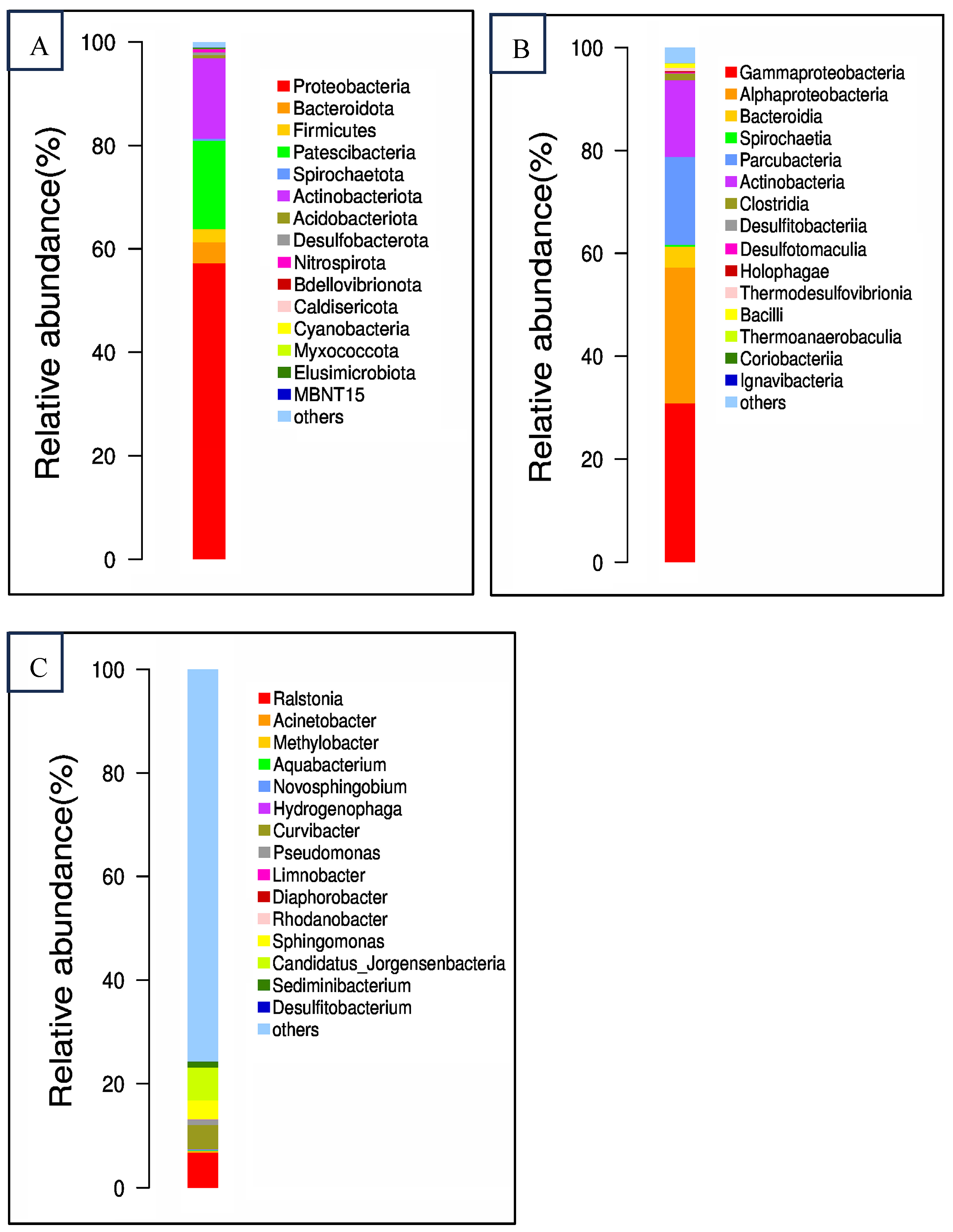
3.1. Physicochemical Properties and Bacterial Communitry Structure of the Collected Groundwater
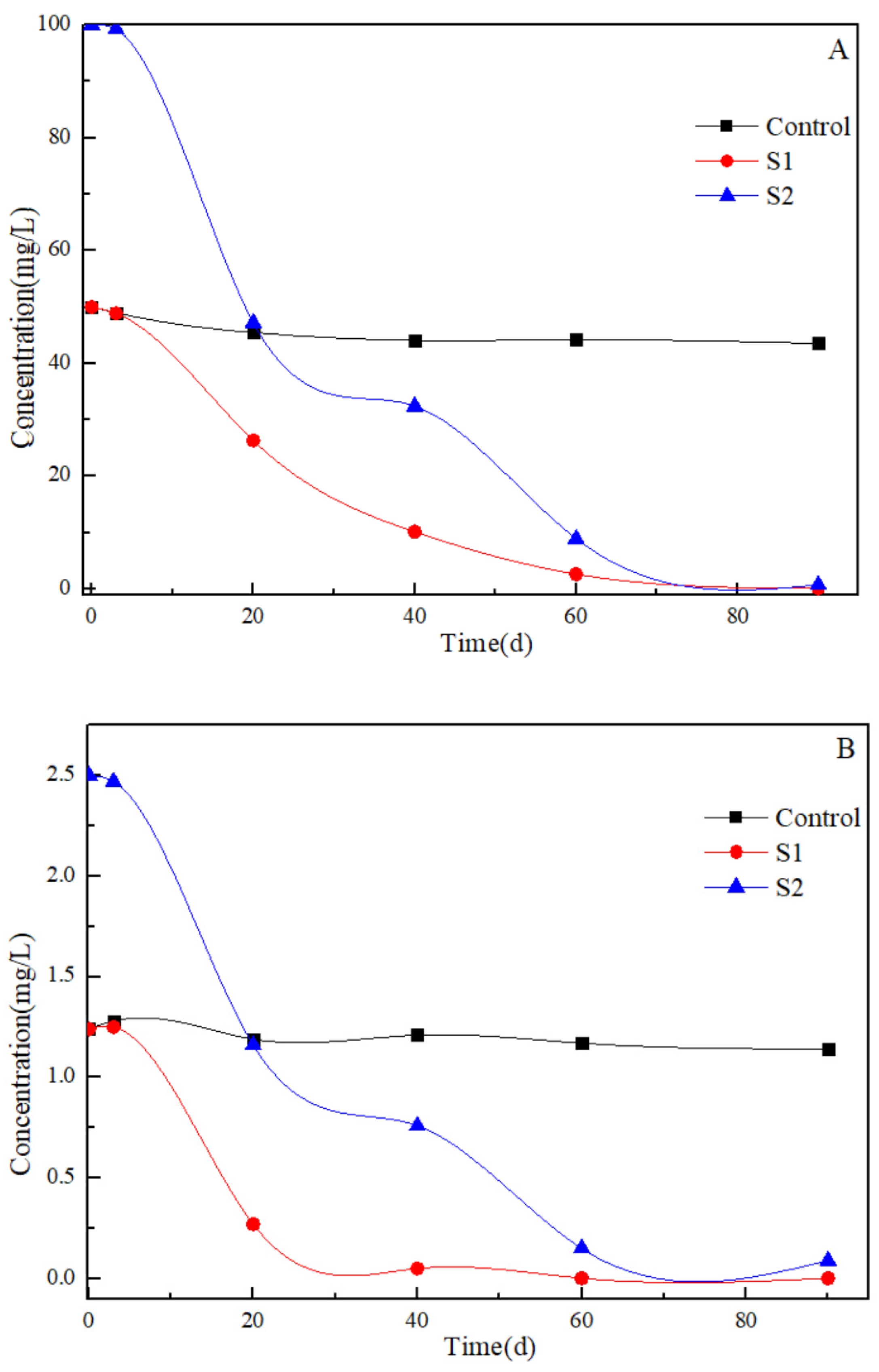
3.2. Biodegradation of Benzene and Toluene in the Artificial Samples
3.3. Changes of Geochemcial Parameters in the Artificial Samples
3.4. Changes of Bacterial Community Structures in the Artificial Samples
- Enhancing activities: By adjusting environmental parameters such as pH, ORP, and sulfate availability for the growth and metabolic activity of these bacteria, it is possible to enhance their degradation capabilities.
- Targeted introduction: Knowing these bacteria are most effective at degrading benzene can guide the targeted introduction of these species into contaminated environments. This approach can be more effective than a general inoculation with an undefined microbial mixture.
- Monitoring and adjustment: Regular monitoring of the microbial community structure can help assess the progress of bioremediation and allow for timely adjustments to the strategy.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, N.; Chandran, P. Microbial degradation of petroleum hydrocarbon contaminants: An overview. Biotechnol. Res. Int. 2011, 2011, 941810–941822. [Google Scholar] [CrossRef]
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, Y.; Zhao, J.; Li, S.; Sarah, S.; Deng, L. BTEX biodegradation is linked to bacterial community assembly patterns. J. Hazard. Mater. 2021, 419, 5. [Google Scholar] [CrossRef]
- Chen, X.-X.; Zhang, S.; Yi, L.-J.; Liu, Z.-W.; Ye, X.-Y.; Yu, B.; Shi, S.; Lu, X.-X. Evaluation of Biodegradation of BTEX in the Subsurface of a Petrochemical Site near the Yangtze River, China. Int. J. Environ. Res. Public Health 2022, 19, 16449. [Google Scholar] [CrossRef]
- Christian, W.; Sabine, S.; Tillmann, L. Detection of anaerobic toluene and hydrocarbon degraders in contaminated aquifers using benzylsuccinate synthase (bssA) genes as a functional marker. Environ. Microbiol. 2007, 9, 1035–1046. [Google Scholar]
- Todd, H.-W.; John, T.-W.; Donald, H.-K. Technical Protocol for Implementing Intrinsic Remediation with Long-Term Monitoring for Natural Attenuation of Fuel Contamination Dissolved in Groundwater; Air Force Center for Environmental Excellence: Brooks, TX, USA, 1999; pp. B5-4–B5-31. [Google Scholar]
- Naraboyina, D.; Rastogi, A.-K. Remediation techniques for BTEX contamination of groundwater—A review. Int. J. Eng. Res. Technol. 2015, 3, 1–6. [Google Scholar]
- Yu, M.; Michael, S.-W.; Shaily, M.; Nicholas, W.-J.; Kimberly, H.; Guo, S.-J.; Camilah, D.-P.; Thien, P.; Phillip, B.-G.; David, T.-A.; et al. Microbial responses to combined oxidation and catalysis treatment of 1,4-dioxane and co-contaminants in groundwater and soil. Front. Environ. Sci. Eng. 2018, 12, 2. [Google Scholar]
- Thierrin, J.; Davis, G.-B.; Barber, C.; Patterson, B.-M.; Pribac, F.; Power, T.-R.; Lambert, M. Natural degradation rates of BTEX compounds and naphthalene in a sulfate reducing groundwater environment. Hydrol. Sci. J. 1993, 38, 309–322. [Google Scholar] [CrossRef]
- Edwards, E.-A.; Wills, L.-E.; Reinhard, M.; Grbic-Galic, D. Anaerobic degradation of toluene and xylene by aquifer microorganisms under sulfate-reducing conditions. Appl. Environ. Microb. 1992, 58, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.-R.; Coates, J.-D.; Woodward, J.-C.; Phillips, E.-J. Benzene oxidation coupled to sulfate reduction. Appl. Environ. Microb. 1995, 61, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Firmino, P.-I.; Farias, R.-S.; Buarque, P.-M.-C.; Costa, M.-C.; Rodríguez, E.; Lopes, A.-C. Engineering and microbiological aspects of BTEX removal in bioreactors under sulfate-reducing conditions. Chem. Eng. J. 2015, 260, 503–512. [Google Scholar] [CrossRef]
- Huang, W.-H.; Kao, C.-M. Bioremediation of petroleum-hydrocarbon contaminated groundwater under sulfate-reducing conditions: Effectiveness and mechanism study. J. Environ. Eng. 2015, 142, 04015089. [Google Scholar] [CrossRef]
- Huang, W.-H.; Dong, C.-D.; Chen, C.-W.; Surampalli, R.-Y.; Kao, C.-M. Application of sulfate reduction mechanisms for the simultaneous bioremediation of toluene and copper contaminated groundwater. Int. Biodeter. Biodegr. 2017, 124, 215–222. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, B.-Y.; Zhou, C.; Chen, H.; Chen, G.; Tang, Y.-N. Determination of growth kinetics of microorganisms linked with 1,4-dioxane degradation in a consortium based on two improved methods. Front. Environ. Sci. Eng. 2022, 16, 62. [Google Scholar] [CrossRef]
- Müller, J.B.; Ramos, D.T.; Larose, C.; Fernandes, M.; Lazzarin, H.S.; Vogel, T.M.; Corseuil, H.X. Combined iron and sulfate reduction biostimulation as a novel approach to enhance BTEX and PAH source-zone biodegradation in biodiesel blend-contaminated groundwater. J. Hazard. Mater. 2017, 326, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Norma, P.; Alex, O.-S.; Esteban, B.; Pamela, S.; Isabel, D.; Homero, U. Performance of two differently designed permeable reactive barriers with sulfate and zinc solutions. Sci. Total Environ. 2018, 642, 894–903. [Google Scholar]
- Volatile Organic Compounds in Water by Purge and Trap Capillary Column Gas Chromatography with Photoionization and Electrolytic Conductivity Detectors in Series. Revision 2.1. 1995. Available online: https://nepis.epa.gov/Exe/ZyNET.exe/P1018ORA.TXT?ZyActionD=ZyDocument&Client=EPA&Index=1986+Thru+1990&Docs=&Query=&Time=&EndTime=&SearchMethod=1&TocRestrict=n&Toc=&TocEntry=&QField=&QFieldYear=&QFieldMonth=&QFieldDay=&IntQFieldOp=0&ExtQFieldOp=0&XmlQuery=&File=D%3A%5Czyfiles%5CIndex%20Data%5C86thru90%5CTxt%5C00000039%5CP1018ORA.txt&User=ANONYMOUS&Password=anonymous&SortMethod=h%7C-&MaximumDocuments=1&FuzzyDegree=0&ImageQuality=r75g8/r75g8/x150y150g16/i425&Display=hpfr&DefSeekPage=x&SearchBack=ZyActionL&Back=ZyActionS&BackDesc=Results%20page&MaximumPages=1&ZyEntry=1&SeekPage=x&ZyPURL (accessed on 10 April 2024).
- Nossa, C.W.; Oberdorf, W.E.; Yang, L.; Aas, J.A.; Paster, B.J.; DeSantis, T.Z.; Brodie, E.L.; Malamud, D.; Poles, M.A.; Pei, Z. Design of 16S rRNA gene primers for 454 pyrosequencing of the human foregut microbiome. World J. Gastroenterol. 2010, 16, 4135–4144. [Google Scholar] [CrossRef]
- Kao, C.-M.; Chen, C.-S.; Tsa, F.-Y.; Yang, K.-H.; Chen, C.; Liang, S.; Yang, C.; Chen, S.-C. Application of real-time PCR, DGGE fingerprinting, and culture based method to evaluate the effectiveness of intrinsic bioremediation on the control of petroleum-hydrocarbon plume. J. Hazard. Mater. 2010, 178, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-L.; Zhang, Y.-L.; Ding, Y.; Song, H.-W.; Liu, T. Analysis of microbial community resistance mechanisms in groundwater contaminated with SAs and high NH4+-Fe-Mn. Sci. Total Environ. 2022, 817, 153036. [Google Scholar] [CrossRef]
- Abbas, H.; Ahmad, A. A novel nitrile-degrading enzyme (nitrile hydratase) from Ralstonia sp. ZA96 isolated from oil-contaminated soils. Biocatal. Agric. Biotechnol. 2019, 21, 101285. [Google Scholar]
- Allison, S.-D.; Lu, Y.; Claudia, W.; Goulden, M.-L.; Martiny, A.-C.; Martiny, T. Microbial abundance and composition influence litter decomposition response to environmental change. Ecology 2013, 94, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Dou, J.-F.; Liu, X.; Hu, Z.-F.; Deng, D. Anaerobic BTEX biodegradation linked to nitrate and sulfate reduction. J. Hazard. Mater. 2008, 151, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zuo, R.; Wang, J.-S.; Yang, J.; Li, Q.; Chen, M.-H. The spatial variations of correlation between microbial diversity and groundwater quality derived from a riverbank filtration site, northeast China. Sci. Total Environ. 2020, 706, 135855. [Google Scholar] [CrossRef]
- van der Meer, J.R. Environmental pollution promotes selection of microbial degradation pathways. Front. Ecol. Environ. 2006, 4, 35–42. [Google Scholar] [CrossRef]





| Artificial Sample | Components |
|---|---|
| Control | contaminated groundwater containing benzene (50 mg/L) and toluene (1.24 mg/L) + 250 mg/L HgCl2+ sulfate (470 mg/L) + yeast extract (50 mg/L) |
| S1 | contaminated groundwater containing benzene (50 mg/L) and toluene (1.24 mg/L) + sulfate (470 mg/L) + yeast extract (50 mg/L) |
| S2 | contaminated groundwater containing benzene (100 mg/L) and toluene (2.5 mg/L) + sulfate (940 mg/L) + yeast extract (50 mg/L) |
| pH | ORP (mV) | DO (mg/L) | NO3− (mg/L) | SO42− (mg/L) | Fe2+ (mg/L) | Mn2+ (mg/L) | Benzene (mg/L) | Toluene (mg/L) | Ethylbenzene (mg/L) | m, p-Xylene (mg/L) | o-Xylene (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5.89 | −86 | 0.75 | 0.797 | 2.82 | 8.84 | 2.65 | 209.000 | 5.180 | 0.460 | 0.110 | 0.188 |
| Samples | Chao1 | Shannon | Simpson | Observed Species | Goods Coverage | PD-Whole Tree |
|---|---|---|---|---|---|---|
| S11 (3 d) | 581.33 | 7.18 | 0.9674 | 580.50 | 0.9999 | 50.24 |
| S12 (40 d) | 133.13 | 2.56 | 0.7016 | 132.60 | 0.9999 | 17.44 |
| S13 (90 d) | 1429.26 | 8.81 | 0.9780 | 1422.90 | 0.9994 | 101.80 |
| S21 (3 d) | 513.53 | 6.41 | 0.9430 | 512.70 | 0.9999 | 46.19 |
| S22 (40 d) | 109.72 | 2.08 | 0.6815 | 108.50 | 0.9999 | 15.82 |
| S23 (90 d) | 1072.56 | 7.18 | 0.9075 | 1068.00 | 0.9996 | 77.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Lin, X.; Wang, X.; Sun, M.; Ma, S.; Zhang, S. Shift in Bacterial Community Structure in the Biodegradation of Benzene and Toluene under Sulfate-Reducing Condition. Toxics 2024, 12, 423. https://doi.org/10.3390/toxics12060423
Liu Z, Lin X, Wang X, Sun M, Ma S, Zhang S. Shift in Bacterial Community Structure in the Biodegradation of Benzene and Toluene under Sulfate-Reducing Condition. Toxics. 2024; 12(6):423. https://doi.org/10.3390/toxics12060423
Chicago/Turabian StyleLiu, Zhengwei, Xiaoyu Lin, Xinzhe Wang, Mingbo Sun, Shici Ma, and Shucai Zhang. 2024. "Shift in Bacterial Community Structure in the Biodegradation of Benzene and Toluene under Sulfate-Reducing Condition" Toxics 12, no. 6: 423. https://doi.org/10.3390/toxics12060423
APA StyleLiu, Z., Lin, X., Wang, X., Sun, M., Ma, S., & Zhang, S. (2024). Shift in Bacterial Community Structure in the Biodegradation of Benzene and Toluene under Sulfate-Reducing Condition. Toxics, 12(6), 423. https://doi.org/10.3390/toxics12060423






