Associations of Urinary Heavy Metal Mixtures with High Remnant Cholesterol among US Adults: Evidence from the National Health and Nutrition Examination Survey (1998–2018)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Evaluation of High Remnant Cholesterol (HRC)
2.3. Metal Measurements
2.4. Potential Covariates
2.5. Statistical Analyses
2.5.1. Weighted Multivariable Logistic Regression
2.5.2. Weighted Quantile Sum (WQS) Regression
2.5.3. Quantile g-Computation (qgcomp)
2.5.4. Bayesian Kernel Machine Regression (BKMR)
2.5.5. Subgroup and Sensitivity Analysis
3. Results
3.1. Baseline Characteristics
3.2. Associations between Single Metal Concentrations and HRC: Logistic Regression
3.3. Associations between Co-Exposure to Metals and HRC: WQS, qgcomp, and BKMR Models
3.4. Subgroup and Sensitivity Analysis
4. Discussion
4.1. Key Findings
4.2. Single Metals
4.3. Combined Exposure
4.4. Subgroup Analysis
4.5. Implications for Public Health
4.6. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jørgensen, A.B.; Frikke-Schmidt, R.; West, A.S.; Grande, P.; Nordestgaard, B.G.; Tybjærg-Hansen, A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur. Heart J. 2013, 34, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Wadström, B.N.; Wulff, A.B.; Pedersen, K.M.; Jensen, G.B.; Nordestgaard, B.G. Elevated remnant cholesterol increases the risk of peripheral artery disease, myocardial infarction, and ischaemic stroke: A cohort-based study. Eur. Heart J. 2022, 43, 3258–3269. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Vine, D.; Proctor, S.; Grzelakowska, K.; Berti, S.; Kubica, J.; Raggi, P. Independent Causal Effect of Remnant Cholesterol on Atherosclerotic Cardiovascular Outcomes: A Mendelian Randomization Study. Arterioscler. Thromb. Vasc. Biol. 2023, 43, e373–e380. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Packard, C.J.; Chapman, M.J.; Borén, J.; Aguilar-Salinas, C.A.; Averna, M.; Ference, B.A.; Gaudet, D.; Hegele, R.A.; Kersten, S.; et al. Triglyceride-rich lipoproteins and their remnants: Metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021, 42, 4791–4806. [Google Scholar] [CrossRef] [PubMed]
- Quispe, R.; Martin, S.S.; Michos, E.D.; Lamba, I.; Blumenthal, R.S.; Saeed, A.; Lima, J.; Puri, R.; Nomura, S.; Tsai, M.; et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: A primary prevention study. Eur. Heart J. 2021, 42, 4324–4332. [Google Scholar] [CrossRef]
- Zou, Y.; Lan, J.; Zhong, Y.; Yang, S.; Zhang, H.; Xie, G. Association of remnant cholesterol with nonalcoholic fatty liver disease: A general population-based study. Lipids Health Dis. 2021, 20, 139. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.H.; Roh, E.; Lee, S.J.; Ihm, S.H.; Han, K.D.; Kang, J.G. Remnant Cholesterol Is an Independent Predictor of Type 2 Diabetes: A Nationwide Population-Based Cohort Study. Diabetes Care 2023, 46, 305–312. [Google Scholar] [CrossRef]
- Zou, Y.; Kuang, M.; Zhong, Y.; Jiang, C. Remnant cholesterol can identify individuals at higher risk of metabolic syndrome in the general population. Sci. Rep. 2023, 13, 5957. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chuang, C.-S.; Lin, C.-I.; Wang, C.-L.; Huang, Y.-C.; Chuang, H.-Y. The association of the blood lead level and serum lipid concentrations may be modified by the genetic combination of the metallothionein 2A polymorphisms rs10636 GC and rs28366003 AA. J. Clin. Lipidol. 2017, 11, 234–241. [Google Scholar] [CrossRef]
- Bulka, C.M.; Persky, V.W.; Daviglus, M.L.; Durazo-Arvizu, R.A.; Argos, M. Multiple metal exposures and metabolic syndrome: A cross-sectional analysis of the National Health and Nutrition Examination Survey 2011–2014. Environ. Res. 2019, 168, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yin, G.; Xu, J.; Ge, X.; Li, A.; Mei, Y.; Wu, J.; Liu, X.; Wei, L.; Xu, Q. Independent, combine and interactive effects of heavy metal exposure on dyslipidemia biomarkers: A cross-sectional study in northeastern China. Ecotoxicol. Environ. Saf. 2023, 250, 114494. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.H.; Pu, Y.Q.; Liu, M.L.; Hu, L.X.; Bao, W.W.; Zhang, Y.S.; Kakaer, A.; Zhao, Y.; Chen, Y.C.; Pu, X.Y.; et al. Joint effect of whole blood metals exposure with dyslipidemia in representative US adults in NHANES 2011–2020. Environ. Sci. Pollut. Res. Int. 2023, 30, 96604–96616. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.W.; Kim, S.H.; Park, M.J. An association of blood mercury levels and hypercholesterolemia among Korean adolescents. Sci. Total Environ. 2020, 709, 135965. [Google Scholar] [CrossRef]
- Wan, H.; Wang, D.; Liang, Y.; He, Y.; Ma, Q.; Li, T.; He, Y.; Guo, H.; Wang, J.; Li, Z.; et al. Single and combined associations of blood lead and essential metals with serum lipid profiles in community-dwelling adults. Front. Nutr. 2023, 10, 1129169. [Google Scholar] [CrossRef] [PubMed]
- Souza-Arroyo, V.; Fabián, J.J.; Bucio-Ortiz, L.; Miranda-Labra, R.U.; Gomez-Quiroz, L.E.; Gutiérrez-Ruiz, M.C. The mechanism of the cadmium-induced toxicity and cellular response in the liver. Toxicology 2022, 480, 153339. [Google Scholar] [CrossRef] [PubMed]
- Ingawale, D.K.; Mandlik, S.K.; Naik, S.R. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism(s): A critical discussion. Environ. Toxicol. Pharmacol. 2014, 37, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yin, H.; Yang, Z.; Tan, M.; Wang, F.; Chen, K.; Zuo, Z.; Shu, G.; Cui, H.; Ouyang, P.; et al. Vitamin E protects against cadmium-induced sub-chronic liver injury associated with the inhibition of oxidative stress and activation of Nrf2 pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111610. [Google Scholar] [CrossRef] [PubMed]
- Elwej, A.; Grojja, Y.; Ghorbel, I.; Boudawara, O.; Jarraya, R.; Boudawara, T.; Zeghal, N. Barium chloride induces redox status unbalance, upregulates cytokine genes expression and confers hepatotoxicity in rats-alleviation by pomegranate peel. Environ. Sci. Pollut. Res. Int. 2016, 23, 7559–7571. [Google Scholar] [CrossRef]
- Renu, K.; Chakraborty, R.; Myakala, H.; Koti, R.; Famurewa, A.C.; Madhyastha, H.; Vellingiri, B.; George, A.; Valsala Gopalakrishnan, A. Molecular mechanism of heavy metals (Lead, Chromium, Arsenic, Mercury, Nickel and Cadmium)—Induced hepatotoxicity—A review. Chemosphere 2021, 271, 129735. [Google Scholar] [CrossRef]
- Perak, A.M.; Ning, H.; Kit, B.K.; de Ferranti, S.D.; Van Horn, L.V.; Wilkins, J.T.; Lloyd-Jones, D.M. Trends in Levels of Lipids and Apolipoprotein B in US Youths Aged 6 to 19 Years, 1999–2016. JAMA 2019, 321, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Langsted, A.; Mora, S.; Kolovou, G.; Baum, H.; Bruckert, E.; Watts, G.F.; Sypniewska, G.; Wiklund, O.; Borén, J.; et al. Fasting is not routinely required for determination of a lipid profile: Clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur. Heart J. 2016, 37, 1944–1958. [Google Scholar] [PubMed]
- Chen, L.; Sun, Q.; Peng, S.; Tan, T.; Mei, G.; Chen, H.; Zhao, Y.; Yao, P.; Tang, Y. Associations of blood and urinary heavy metals with rheumatoid arthritis risk among adults in NHANES, 1999–2018. Chemosphere 2022, 289, 133147. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Kim, Y.; Boudreau, N. Association of smoking with serum and dietary levels of antioxidants in adults: NHANES III, 1988–1994. Am. J. Public Health 2001, 91, 258–264. [Google Scholar] [PubMed]
- Carrico, C.; Gennings, C.; Wheeler, D.C.; Factor-Litvak, P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J. Agric. Biol. Environ. Stat. 2015, 20, 100–120. [Google Scholar] [CrossRef]
- Keil, A.P.; Buckley, J.P.; O‘Brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect. 2020, 128, 47004. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Valeri, L.; Claus Henn, B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Pan, Y.; Zhang, A.; Zhu, C.; Liu, D.; Xu, G.; Zheng, Y.; Yan, H. Distribution of rubidium, cesium, beryllium, strontium, and barium in blood and urine in general Chinese population. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2015, 33, 894–899. [Google Scholar]
- Crinnion, W.J. The CDC Fourth National Report on Human Exposure to Environmental Chemicals: What it tells us about our toxic burden and how it assists environmental medicine physicians. Altern. Med. Rev. 2010, 15, 101. [Google Scholar]
- Ding, C.; Pan, Y.; Zhang, A.; Wu, B.; Huang, H.; Zhu, C.; Liu, D.; Zhu, B.; Xu, G.; Shao, H.; et al. Study of distribution and influencing factors of lead and cadmium in whole blood and urine among population in 8 provinces in China. Zhonghua Yu Fang Yi Xue Za Zhi 2014, 48, 91–96. [Google Scholar] [PubMed]
- Becker, K.; Schulz, C.; Kaus, S.; Seiwert, M.; Seifert, B. German Environmental Survey 1998 (GerES III): Environmental pollutants in the urine of the German population. Int. J. Hyg. Environ. Health 2003, 206, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Fang, L.; Chen, Y.; Ma, Y.; Zhao, H.; Wu, Y.; Xu, S.; Cai, G.; Pan, F. Association between exposure to mixture of heavy metals and hyperlipidemia risk among U.S. adults: A cross-sectional study. Chemosphere 2023, 344, 140334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, J.; Li, H.; Yang, Y.; Cai, C.; Gao, Q.; Xing, Y.; Shao, B.; Li, G. Multiple-element exposure and metabolic syndrome in Chinese adults: A case-control study based on the Beijing population health cohort. Environ. Int. 2020, 143, 105959. [Google Scholar] [CrossRef]
- Shan, Q. Trend analysis of the association of urinary metals and obesity in children and adolescents. Chemosphere 2022, 307 Pt 1, 135617. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Liu, Q.; He, X.; Liu, H.; Gu, A.; Jiang, Z. Association between level of urinary trace heavy metals and obesity among children aged 6–19 years: NHANES 1999–2011. Environ. Sci. Pollut. Res. Int. 2017, 24, 11573–11581. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Aimuzi, R.; Si, M.; Qu, Y.; Jiang, Y. Associations of metal mixtures with metabolic-associated fatty liver disease and non-alcoholic fatty liver disease: NHANES 2003–2018. Front. Public Health 2023, 11, 1133194. [Google Scholar] [CrossRef]
- Tang, P.; Liao, Q.; Tang, Y.; Yao, X.; Du, C.; Wang, Y.; Song, F.; Deng, S.; Wang, Y.; Qiu, X.; et al. Independent and combined associations of urinary metals exposure with markers of liver injury: Results from the NHANES 2013–2016. Chemosphere 2023, 338, 139455. [Google Scholar] [CrossRef]
- Elwej, A.; Chaabane, M.; Ghorbel, I.; Chelly, S.; Boudawara, T.; Zeghal, N. Effects of barium graded doses on redox status, membrane bound ATPases and histomorphological aspect of the liver in adult rats. Toxicol. Mech. Methods 2017, 27, 677–686. [Google Scholar] [CrossRef]
- Kim, K. Blood cadmium concentration and lipid profile in Korean adults. Environ. Res. 2012, 112, 225–229. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Skalnaya, M.G.; Aaseth, J.; Bjørklund, G.; Gatiatulina, E.R.; Popova, E.V.; Nemereshina, O.N.; Huang, P.T.; et al. Cadmium and atherosclerosis: A review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ. Res. 2018, 162, 240–260. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Hsu, S.H.; Chen, C.W.; Wang, C.; Sung, F.C.; Su, T.C. Association of Urinary Lead and Cadmium Levels, and Serum Lipids with Subclinical Arteriosclerosis: Evidence from Taiwan. Nutrients 2023, 15, 571. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, A.; Wang, X.; Zeng, X.; Xing, H. AMPK/PPAR-γ/NF-κB axis participates in ROS-mediated apoptosis and autophagy caused by cadmium in pig liver. Environ. Pollut. 2022, 294, 118659. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Su, Q.; Yue, C.; Zou, H.; Zhu, J.; Zhao, H.; Song, R.; Liu, Z. The Effect of Oxidative Stress-Induced Autophagy by Cadmium Exposure in Kidney, Liver, and Bone Damage, and Neurotoxicity. Int. J. Mol. Sci. 2022, 23, 13491. [Google Scholar] [CrossRef]
- Wang, X.; Karvonen-Gutierrez, C.A.; Herman, W.H.; Mukherjee, B.; Park, S.K. Metals and risk of incident metabolic syndrome in a prospective cohort of midlife women in the United States. Environ. Res. 2022, 210, 112976. [Google Scholar] [CrossRef] [PubMed]
- Zha, B.; Liu, Y.; Xu, H. Associations of mixed urinary metals exposure with metabolic syndrome in the US adult population. Chemosphere 2023, 344, 140330. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.; Grandcolas, L.; Grison, S.; Gourmelon, P.; Guéguen, Y.; Veyssière, G.; Souidi, M. Molecular modifications of cholesterol metabolism in the liver and the brain after chronic contamination with cesium 137. Food Chem. Toxicol. 2009, 47, 1642–1647. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, Z.; Shen, J.; Wu, Y.; Fang, L.; Xu, S.; Ma, Y.; Zhao, H.; Pan, F. Associations of exposure to blood and urinary heavy metal mixtures with psoriasis risk among U.S. adults: A cross-sectional study. Sci. Total Environ. 2023, 887, 164133. [Google Scholar] [CrossRef]

| Characteristics | Total | HRC | Non-HRC | p Value |
|---|---|---|---|---|
| Age, n (%) | <0.001 | |||
| 20–59 | 3996 (70.23) | 937 (77.62) | 3059 (80.09) | |
| ≥60 | 1694 (29.77) | 471 (22.38) | 1223 (19.91) | |
| Gender, n (%) | <0.001 | |||
| Male | 2725 (47.89) | 785 (58.30) | 1940 (43.93) | |
| Female | 2965 (52.11) | 623 (41.70) | 2342 (56.07) | |
| Race/ethnicity, n (%) | <0.001 | |||
| Mexican American | 1020 (17.93) | 344 (12.31) | 676 (8.77) | |
| Other Hispanic | 1217 (21.39) | 168 (6.62) | 1049 (13.04) | |
| Non-Hispanic White | 2290 (40.25) | 604 (67.48) | 1686 (65.17) | |
| Non-Hispanic Black | 562 (9.88) | 168 (6.56) | 394 (5.90) | |
| Other race/multiracial | 601 (10.56) | 124 (7.04) | 477 (7.12) | |
| BMI, n (%) | <0.001 | |||
| Normal or low weight | 1754 (30.83) | 222 (13.91) | 1532 (38.26) | |
| Overweight | 1837 (32.28) | 495 (35.85) | 1342 (31.06) | |
| Obese | 2034 (35.75) | 672 (49.08) | 1362 (29.91) | |
| Missing | 65 (1.14) | 19 (1.16) | 46 (0.76) | |
| Education level, n (%) | <0.001 | |||
| Below high school | 1506 (26.47) | 477 (21.87) | 1029 (15.93) | |
| High school or equivalent | 1320 (23.2) | 607 (29.05) | 962 (22.72) | |
| Above high school | 2864 (50.33) | 573 (49.08) | 2291 (61.35) | |
| PIR, n (%) | 0.420 | |||
| 0–1.3 | 1634 (28.72) | 435 (21.80) | 1199 (19.75) | |
| 1.31–3.50 | 2055 (36.12) | 503 (35.20) | 1552 (34.96) | |
| >3.50 | 1523 (26.77) | 340 (36.57) | 1183 (38.52) | |
| Missing | 478 (8.4) | 130 (6.44) | 348 (6.77) | |
| Marital status, n (%) | <0.001 | |||
| Married/cohabiting | 3428 (60.25) | 893 (65.95) | 2535 (63.25) | |
| Widowed/divorced/separated | 1216 (21.37) | 335 (20.69) | 881 (17.08) | |
| Never married | 1046 (18.38) | 180 (13.36) | 866 (19.67) | |
| Serum cotinine, n (%) | 0.060 | |||
| <14 ng/ml | 4279 (75.2) | 1022 (71.57) | 3257 (75.05) | |
| ≥14 ng/ml | 1411 (24.8) | 386 (28.43) | 1025 (24.95) | |
| Drinking status, n (%) | 0.010 | |||
| Never | 730 (12.83) | 184 (10.26) | 546 (9.68) | |
| Former | 809 (14.22) | 247 (14.74) | 562 (10.98) | |
| Now | 3555 (62.48) | 852 (67.15) | 2703 (70.20) | |
| Missing | 596 (10.47) | 125 (7.85) | 471 (9.13) | |
| HEI-2015, n (%) | 0.870 | |||
| Inadequate | 2703 (47.5) | 692 (49.74) | 2011 (48.71) | |
| Average | 2183 (38.37) | 541 (38.70) | 1642 (38.09) | |
| Optimal | 487 (8.56) | 118 (7.99) | 369 (8.46) | |
| Missing | 317 (5.57) | 57 (3.58) | 260 (4.74) | |
| Physical activity, n (%) | 0.55 | |||
| Poor | 1219 (21.42) | 289 (22.70) | 930 (22.28) | |
| Intermediate | 656 (11.53) | 149 (11.17) | 507 (12.83) | |
| Ideal | 2351 (41.32) | 543 (41.88) | 1808 (45.19) | |
| Missing | 1464 (27.01) | 427 (24.26) | 1037 (19.70) | |
| Hypertension, n (%) | <0.001 | |||
| No | 4989 (87.68) | 1138 (85.13) | 3851 (93.17) | |
| Yes | 701 (12.32) | 270 (14.87) | 431 (6.83) | |
| Diabetes, n (%) | <0.001 | |||
| No | 3544 (62.28) | 730 (54.10) | 2814 (71.58) | |
| Yes | 2146 (37.72) | 678 (45.90) | 1468 (28.42) |
| Urine Metals (μg/g Creatinine) | Continuous | Q1 | Q2 | Q3 | Q4 | p for Trend |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| Ba | ||||||
| Model 1 | 1.10 (1.03, 1.18) | 1.00 (reference) | 0.87 (0.67, 1.13) | 1.40 (1.11, 1.76) | 1.29 (1.01,1.65) | 0.003 |
| Model 2 | 1.11 (1.04, 1.20) | 1.00 (reference) | 0.94 (0.72, 1.23) | 1.49 (1.17, 1.91) | 1.33 (1.01, 1.75) | 0.005 |
| Cd | ||||||
| Model 1 | 1.17 (1.10, 1.25) | 1.00 (reference) | 1.12 (0.89, 1.40) | 1.46 (1.14, 1.87) | 1.54 (1.23, 1.93) | <0.001 |
| Model 2 | 1.16 (1.08, 1.25) | 1.00 (reference) | 1.08 (0.85, 1.37) | 1.38 (1.04, 1.82) | 1.50 (1.16, 1.94) | 0.002 |
| Co | ||||||
| Model 1 | 1.05 (0.94, 1.16) | 1.00 (reference) | 1.15 (0.96, 1.38) | 1.10 (0.85, 1.41) | 1.08 (0.84, 1.40) | 0.666 |
| Model 2 | 1.03 (0.93, 1.15) | 1.00 (reference) | 1.13 (0.92, 1.40) | 1.10 (0.82, 1.46) | 1.09 (0.82, 1.46) | 0.641 |
| Cs | ||||||
| Model 1 | 0.98 (0.86, 1.11) | 1.00 (reference) | 1.18 (0.94, 1.47) | 0.94 (0.76, 1.18) | 1.00 (0.78, 1.29) | 0.668 |
| Model 2 | 0.94 (0.82, 1.09) | 1.00 (reference) | 1.12 (0.89, 1.42) | 0.86 (0.67, 1.10) | 0.97 (0.73, 1.28) | 0.477 |
| Hg | ||||||
| Model 1 | 1.02 (0.97, 1.09) | 1.00 (reference) | 1.19 (0.94, 1.49) | 1.09 (0.87, 1.36) | 1.15 (0.87, 1.36) | 0.431 |
| Model 2 | 1.09 (1.03, 1.16) | 1.00 (reference) | 1.34 (1.04, 1.71) | 1.22 (0.95, 1.57) | 1.52 (1.15, 2.01) | 0.007 |
| Mo | ||||||
| Model 1 | 1.07 (0.98, 1.17) | 1.00 (reference) | 1.02 (0.79, 1.31) | 1.04 (0.83, 1.31) | 1.20 (0.98, 1.48) | 0.097 |
| Model 2 | 1.04 (0.94, 1.15) | 1.00 (reference) | 0.99 (0.76, 1.30) | 1.06 (0.82, 1.35) | 1.14 (0.90, 1.43) | 0.257 |
| Pb | ||||||
| Model 1 | 1.08 (1.00, 1.15) | 1.00 (reference) | 1.19 (0.96, 1.47) | 1.33 (1.08, 1.65) | 1.28 (1.03, 1.59) | 0.014 |
| Model 2 | 1.09 (1.00, 1.18) | 1.00 (reference) | 1.20 (0.95, 1.52) | 1.37 (1.09, 1.71) | 1.35 (1.06, 1.73) | 0.007 |
| Sb | ||||||
| Model 1 | 0.98 (0.91, 1.05) | 1.00 (reference) | 1.01 (0.82, 1.26) | 1.21 (0.98, 1.50) | 0.96 (0.76, 1.20) | 0.967 |
| Model 2 | 1.01 (0.93, 1.09) | 1.00 (reference) | 1.02 (0.81, 1.27) | 1.20 (0.96,1.50) | 1.02 (0.81, 1.29) | 0.618 |
| Tl | ||||||
| Model 1 | 0.99 (0.88, 1.12) | 1.00 (reference) | 1.17 (0.94, 1.47) | 0.95 (0.75, 1.19) | 1.04 (0.80, 1.36) | 0.883 |
| Model 2 | 1.05 (0.92, 1.19) | 1.00 (reference) | 1.25 (0.99, 1.58) | 0.97 (0.76, 1.25) | 1.18 (0.89,1.56) | 0.547 |
| Tu | ||||||
| Model 1 | 0.98 (0.92, 1.04) | 1.00 (reference) | 1.13 (0.90, 1.42) | 0.86 (0.69, 1.06) | 1.00 (0.80, 1.25) | 0.511 |
| Model 2 | 0.99 (0.92, 1.06) | 1.00 (reference) | 1.11 (0.87, 1.42) | 0.84 (0.66,1.06) | 1.01 (0.79, 1.29) | 0.602 |
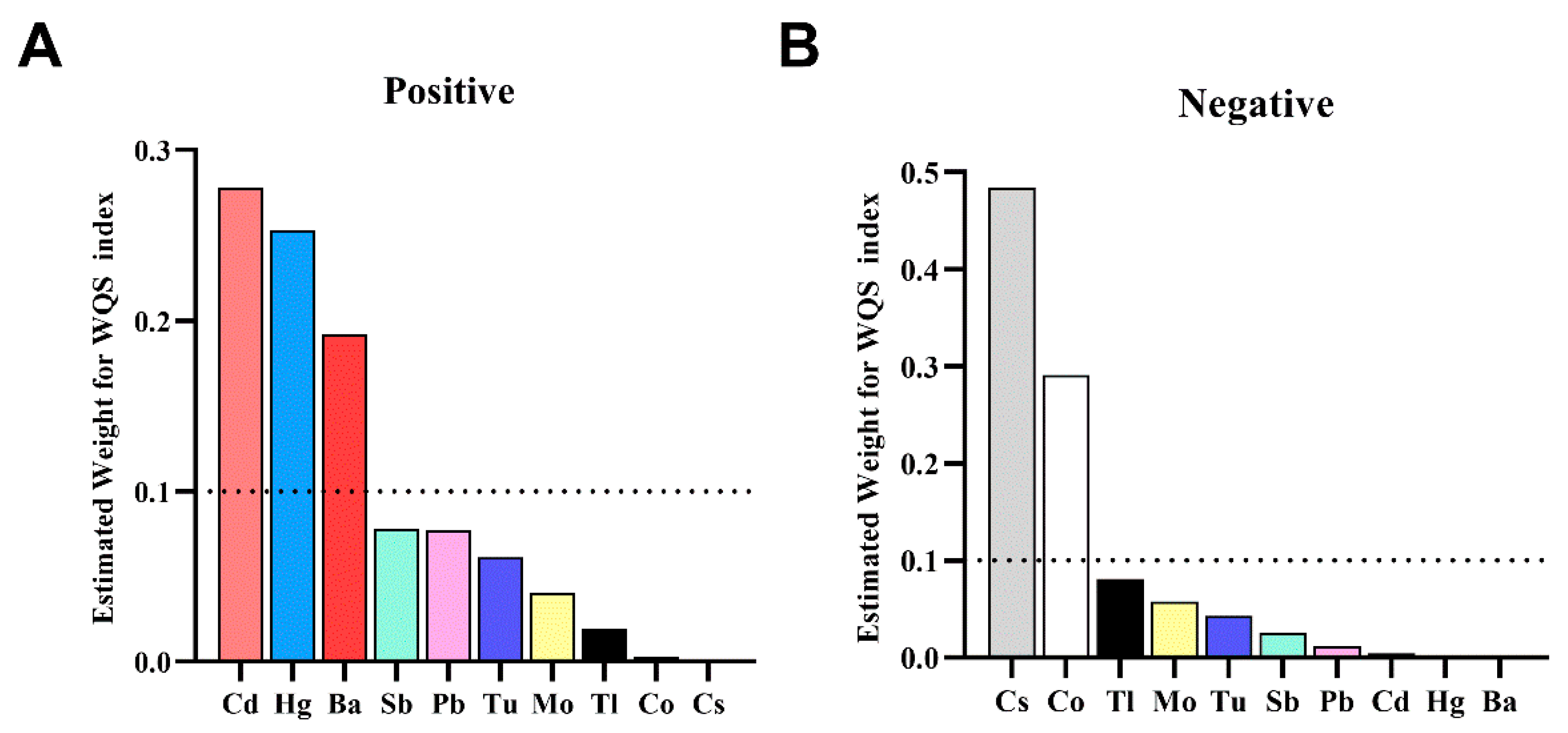
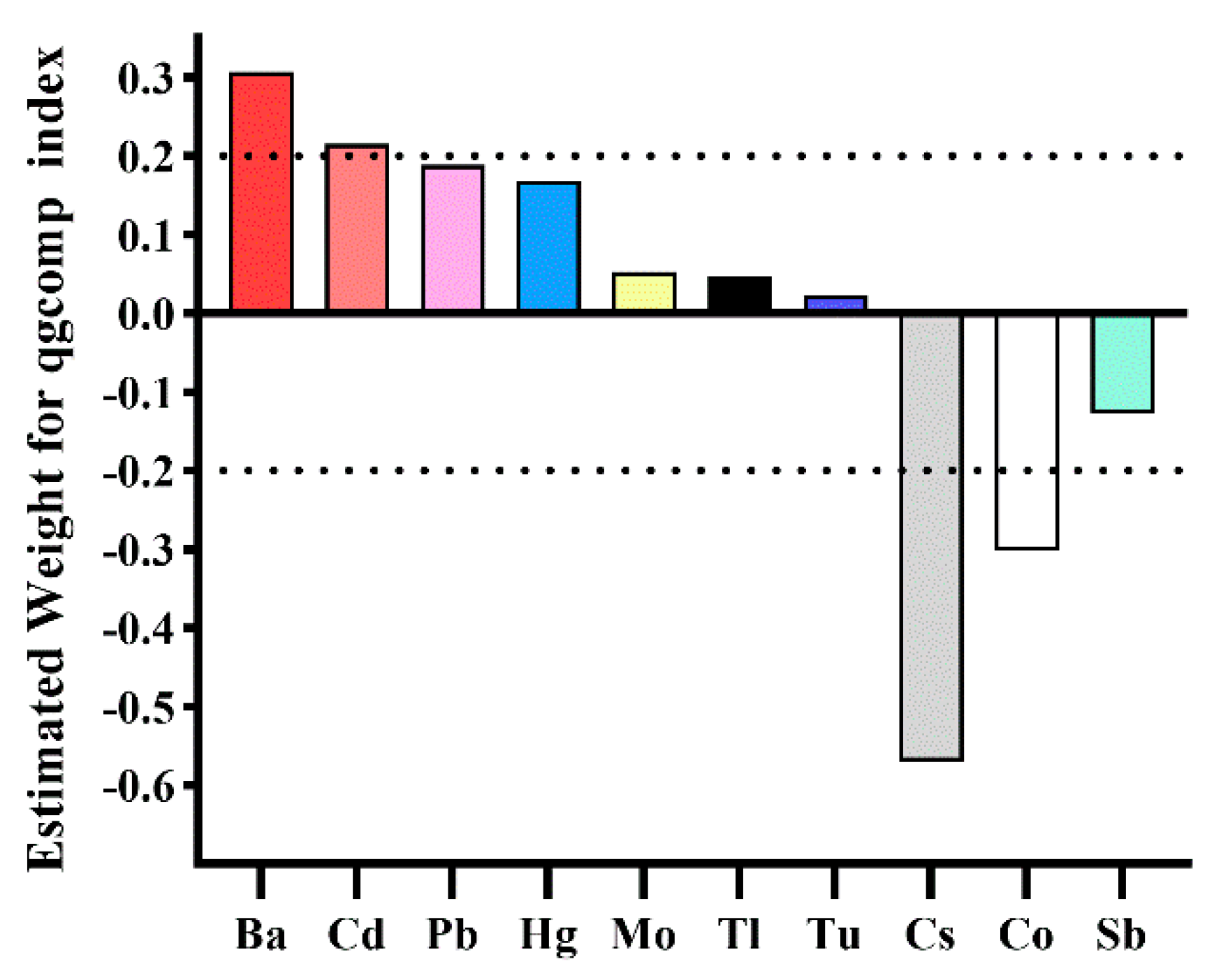
| Metal | Multivariate Logistic Regression * | WQS (Positive Direction) | WQS (Negative Direction) | qgcomp | BKMR (PIP) |
|---|---|---|---|---|---|
| Ba | + | 0.192 | 0.000 | 0.307 (+) | 1.000 |
| Cd | + | 0.278 | 0.004 | 0.215 (+) | 0.869 |
| Co | 0.003 | 0.291 | 0.302 (−) | 0.093 | |
| Cs | 0.000 | 0.484 | 0.570 (−) | 0.552 | |
| Hg | + | 0.253 | 0.001 | 0.168 (+) | 0.235 |
| Mo | 0.040 | 0.058 | 0.053 (+) | 0.792 | |
| Pb | + | 0.077 | 0.012 | 0.189 (+) | 0.119 |
| Sb | 0.078 | 0.026 | 0.128 (−) | 0.082 | |
| Tl | 0.019 | 0.081 | 0.047 (+) | 0.084 | |
| Tu | 0.061 | 0.043 | 0.022 (+) | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Cheng, B.-J.; Yang, P.-Y.; Wang, C.; Meng, K.; Li, T.-L.; Wang, J.; Liu, R. Associations of Urinary Heavy Metal Mixtures with High Remnant Cholesterol among US Adults: Evidence from the National Health and Nutrition Examination Survey (1998–2018). Toxics 2024, 12, 430. https://doi.org/10.3390/toxics12060430
Li H, Cheng B-J, Yang P-Y, Wang C, Meng K, Li T-L, Wang J, Liu R. Associations of Urinary Heavy Metal Mixtures with High Remnant Cholesterol among US Adults: Evidence from the National Health and Nutrition Examination Survey (1998–2018). Toxics. 2024; 12(6):430. https://doi.org/10.3390/toxics12060430
Chicago/Turabian StyleLi, Hui, Bei-Jing Cheng, Pei-Yan Yang, Chun Wang, Ke Meng, Tian-Lin Li, Jia Wang, and Ran Liu. 2024. "Associations of Urinary Heavy Metal Mixtures with High Remnant Cholesterol among US Adults: Evidence from the National Health and Nutrition Examination Survey (1998–2018)" Toxics 12, no. 6: 430. https://doi.org/10.3390/toxics12060430
APA StyleLi, H., Cheng, B.-J., Yang, P.-Y., Wang, C., Meng, K., Li, T.-L., Wang, J., & Liu, R. (2024). Associations of Urinary Heavy Metal Mixtures with High Remnant Cholesterol among US Adults: Evidence from the National Health and Nutrition Examination Survey (1998–2018). Toxics, 12(6), 430. https://doi.org/10.3390/toxics12060430






