Abstract
This study utilizes Mentha piperita (MI) for the first time to investigate the uptake and translocation of chlorpyrifos (CPF; 10 µg g−1) from soil, introducing a new approach to improve the efficacy of this technique, which includes using biosurfactants (Bacillus subtilis and Pseudomonas aeruginosa) at 107 CFU/mL to degrade CPF under greenhouse conditions. Moreover, antioxidant enzymes, including superoxide dismutase (SOD) and peroxidase (Prx), and oxidative stress due to hydrogen peroxide (H2O2) and malondialdehyde (MDA) in MI roots and leaves were evaluated under CPF stress. Our results demonstrated that amending soil with MI and B. subtilis followed by P. aeruginosa significantly reduced CPF levels in the soil (p > 0.05) and enhanced CPF concentrations in MI roots and leaves after 1, 3, 7, 10, and 14 days of the experiment. Furthermore, CPF showed its longest half-life (t1/2) in soil contaminated solely with CPF, lasting 15.36 days. Conversely, its shortest half-life occurred in soil contaminated with CPF and treated with MI along with B. subtilis, lasting 4.65 days. Soil contaminated with CPF and treated with MI and P. aeruginosa showed a half-life of 7.98 days. The half-life (t1/2) of CPF-contaminated soil with MI alone was 11.41 days. A batch equilibrium technique showed that B. subtilis is better than P. aeruginosa for eliminating CPF from soil in In vitro experiments. Notably, CPF-polluted soil treated with coadministration of MI and the tested bacteria improved the activities of SOD and Prx and reduced H2O2 and MDA compared with CPF-polluted soil treated with MI alone. Our findings demonstrated that using B. subtilis and P. aeruginosa as biosurfactants to augment phytoremediation represents a commendable strategy for enhancing the remediation of CPF contamination in affected sites while reducing the existence of harmful pesticide remnants in crop plants.
1. Introduction
Chlorpyrifos (CPF), which exhibits an extensive variety of insecticidal properties against economically significant agricultural pests, is extensively utilized as an organophosphate insecticide on a global scale [1]. However, CPF presents potential hazards to non-target organisms, and its remnants endure in the environment for an indeterminate duration [2]. Moreover, vegetables cultivated in polluted regions represent a substantial hazard to human well-being [3]. The alleged toxicity of this substance to human beings is purportedly associated with a range of clinical symptoms, including brain dysfunction, endocrine disturbance, and cardiovascular illness [4]. Moreover, the half-life (t1/2) of CPF and its products in soil shows that they are persistent; depending on the soil conditions, deterioration typically takes 111 to 350 days [5]. It exhibits moderate toxicity, with low water solubility (0.73 mg/L at 25 °C) and high soil absorption (organic carbon partition coefficient log Koc = 3.78 l/kg) [6]. The logarithm of the octanol–water partition coefficient (log Kow) for CPF is 5 [7]. The use of CPF in the United States is subject to limitations due to the presence of detrimental consequences [8]. In European Union nations, CPF has been banned due to its association with DNA damage triggered by oxidative stress or inhibition of topoisomerase II. These are regarded as molecular events linked to infant leukemia. Additionally, CPF has been classified as toxic for reproduction, posing risks to unborn children [9]. Nevertheless, its utilization remains prevalent in developing countries, such as Egypt, owing to its extensive applicability, cost-effectiveness, and notable efficacy [2]. Therefore, there is an urgent global need to reduce CPF residues in soil to eliminate their negative environmental impacts.
Several techniques, such as excavation, physical extraction, and in situ stabilization, are available to eliminate hazardous soil substances. Although they are highly effective, most of these methods have a significant cost [10]. Furthermore, besides these methods, the cultivation of plants can aid in the containment or mitigation of toxic pollutants. This process is commonly referred to as phytoremediation [10]. Phytoremediation involves utilizing plants to partially or significantly alleviate specific contaminants, including pesticides [11]. Phytoremediation is cost-effective, minimally disrupts soil structure, and enjoys greater public acceptance compared to alternative methods [12]. This method is currently extensively employed to cleanse organic or heavy metal pollutants in contaminated locations, such as agricultural lands, waste disposal sites, and polluted water bodies [13]. The absorption, storage, movement, and breakdown of small-scale pollutants by plants have been proposed as significant mechanisms in phytoremediation technology [14]. Phytoremediation leverages the unique capacity of plant root systems to selectively absorb contaminants and distribute them throughout the entire plant, aiding in their removal [15]. Several studies have focused on pesticide removal by phytoremediation [16,17,18]. Nevertheless, phytoremediation faces notable challenges, largely due to its time-consuming process and the inherent constraints of plants in effectively gathering significant amounts of pollutants while sustaining healthy growth under toxic conditions [19]. Additionally, plants may have limited ability to accumulate pollutants and can be highly susceptible to soil contamination levels that are too high [20]. To address this challenge, we employed bacteria capable of breaking down contaminants to prevent plants from experiencing the harmful effects of pollutants [21]. Utilizing plant–microbial strategies to enhance the phytoremediation process could prove effective, particularly in contamination cases with highly toxic and persistent organic pollutants [22]. Bacteria capable of degrading pollutants can aid plants in adapting to contaminated environments by detoxifying the soil by directly breaking organic contaminants into minerals [23]. Additionally, substances secreted by plants stimulate the proliferation and functionality of prospective bacteria that degrade pollutants in the vicinity of the root zone [24]. Bacillus sp. and Pseudomonas sp. are widely recognized bacterial groups employed in bioremediation processes [25,26]. These microbes gathered from polluted areas demonstrate enhanced capabilities in breaking down and utilizing pesticides [27]. Bacillus subtilis could biodegrade pendimethalin [28] and profenofos [29]. Another study showed that B. subtilis and P. aeruginosa isolated from the Beni Suef governorate, Egypt, enhanced the degradation of malathion [30]. Several research investigations have recorded the utilization of plant–microbe combinations for pollution remediation [11,31,32]. Nevertheless, there is still a scarcity of research focusing on pesticide removal using this method [33].
Mentha piperita (Lamiales: Lamiaceae; MI) is a fragrant herb commonly utilized in medicinal items, culinary preparations, beverages, teas, and cosmetic formulations [34]. Emerging from its root system, the plant exhibits rapid growth, with stems reaching heights of 40–130 cm. The stems, characterized by their red-purple hue, are extensively branched, while the elongated–oval leaves feature serrated edges and a light green coloration [35]. Azmat et al. [36] noted that roots play a significant role in accumulating compounds, as they are directly in contact with harmful chemicals in the soil and can carry these substances to the aboveground portions (leaves). Numerous research investigations have evaluated heavy metal remediation by MI [37,38,39,40]. To date, according to our knowledge, no data have been published about the role of MI in pesticide-polluted soil remediation.
Mounting evidence has suggested that the immoderate intracellular generation of reactive oxygen species (ROS) enables the determination of plant cell health [41]. Plants have developed different defense strategies to alleviate the harm induced by ROS. One such defense system is the enzymatic antioxidant mechanism, which comprises enzymes such as peroxidase (Prx) and superoxide dismutase (SOD). ROS generation is a popular event under stressful conditions and is influenced by enzymatic and nonenzymatic reactions [42]. Elevated ROS levels can activate antioxidant cascade pathways [43]. ROS-scavenging enzymes, such as SOD, promote the breakdown of hydrogen peroxide (H2O2) through dismutation [44]. Various other scavenging enzymes simultaneously convert H2O2 into water and oxygen [45]. Malondialdehyde (MDA) is generated as a final product when polyunsaturated fatty acids undergo decomposition during the peroxidation of membrane lipids. Its levels act as a marker for lipid peroxidation and oxidative harm [46]. The stress response to toxic substances typically involves ROS generation and lipid peroxidation [47].
This study hypothesized that the phytoremediation of M. piperita (MI) and microorganisms would be an excellent, inexpensive, and fast solution for cleaning polluted soil from CPF residue. Our findings aim to evaluate the potential of M. piperita (MI) and two bacterial strains (Bacillus subtilis and Pseudomonas aeruginosa) for the degradation and uptake of CPF-polluted soil under greenhouse conditions. Moreover, assessing the impact of CPF contamination on the enzymatic antioxidant system of MI roots and leaves involves analyzing the levels of SOD, Prx, MDA, and H2O2.
2. Materials and Methods
2.1. Insecticide and Bacteria
Chlorpyrifos (CPF) (97% purity) was obtained from the Agricultural Research Center, Egypt. The characteristics of CPF are outlined in Table 1. Bacillus subtilis subsp. subtilis AZFS3 (LC599401.1) and Pseudomonas aeruginosa KZFS4 (LC599404.1) were garnered from the Agricultural Microbiology Department, Faculty of Agriculture, Zagazig University, Egypt. Based on 16S rRNA gene sequencing, B. subtilis subsp. subtilis AZFS3 (LC599401.1) and P. aeruginosa KZFS4 (LC599404.1) were isolated from pesticide-contaminated soil, as presented in Fahmy et al. [48]. Moreover, antioxidant enzyme kits for Prx and SOD, as well as oxidative stress kits for MDA and H2O2, were sourced from the Biotechnology (SAE) Egyptian Co., Cairo, Egypt.

Table 1.
Physical and chemical properties of CPF, according to Mackay, Giesy, and Solomon [7].
2.2. Phytoremediation Experiment Setup and Procedures
A greenhouse study was conducted under ambient light, with temperatures ranging from 25 to 27 °C, and humidity was maintained between 66% and 69% to evaluate the effects of MI with and without the tested bacteria on the uptake of the CPF residue from polluted soil. The soil utilized in the research was obtained from a location in Giza (28.7666° N, 29.2321° E), Egypt. The clay loamy soil, sifted through a sieve, was allowed to air-dry (organic matter = 1.82%; pH = 7.1; electric conductivity = 2.28 S m−1). Subsequently, it was transferred into plastic pots. Each plastic pot was filled with 500 g of soil. The M. piperita L. (MI) seeds were purchased from a nearby market in Giza, Egypt. The experimental pots were organized in a randomized layout of five distinct treatments: (1) unpolluted soil plus MI without CPF (S+MI); (2) CPF-polluted soil without MI (S+CPF); (3) CPF-polluted soil plus MI (S+MI+CPF); (4) CPF-polluted soil plus MI and B. subtilis at 107 CFU/mL (S+MI+CPF+BS); (5) CPF-polluted soil plus MI with P. aeruginosa at 107 CFU/mL (S+MI+CPF+PA). Each pot was planted with eight MI seeds. The MI plants were carefully thinned after germination to maintain only one plant per pot. Nonsterilized soil was used in the experiment to investigate how B. subtilis and P. aeruginosa interact with the indigenous microbial community in degrading CPF-contaminated soil under field-like conditions. In treatments 4 and 5, the MI seeds were permitted to grow for two weeks following inoculation with the tested bacteria at a concentration of 107 CFU/mL (Tables S1 and S2). CPF was dissolved in 0.5 mL of acetone to obtain a concentration of 10 µg g−1. In the third week, treatments 2 to 5 received 10 µg g−1 of CPF. The MI plants were collected from the polluted soil for investigation at intervals of 1, 3, 7, 10, and 14 days following exposure to CPF. They were dissected, and their roots and leaves were separated. The MI roots underwent a two-minute wash under running tap water, followed by drying. The CPF residues were analyzed using 8 g of soil and 5 g of roots and leaves. The samples were preserved at −80 °C until subsequent analysis. In our study, a combined total of 90 pots were used for all treatments. Each treatment comprised 15 pots, with samples collected at five different time points, and three replicates were taken for each time point.
2.3. Biosurfactants Enhanced Recovery of CPF
In vitro research was conducted to assess the equilibrium adsorption isotherms of CPF, aiming to evaluate biosurfactants’ efficacy in enhancing CPF removal from polluted soil. Thus, 2 mL of two tested bacteria (B. subtilis and P. aeruginosa) at 107 CFU/mL was separately added to 100 mL flasks. The control in this experiment was distilled water. This experiment was conducted with three replicates. Subsequently, CPF was introduced into all conical flasks at a concentration of 10 µg mL−1. The total volume of all treatments in each flask was adjusted to 20 mL. Then, 1 g of soil was added to the above treatments, and the samples were incubated with shaking for 3 h. Subsequently, the resultant suspensions were maintained for 24 h at 27 °C. The suspensions underwent centrifugation for 10 min at 15,000 rpm, after which CPF concentration in the supernatants was assessed using HPLC to quantify the amount of CPF present.
2.4. CPF Residue Extraction and Analysis in Soil and IM
The extraction of CPF from the samples was conducted utilizing the QuEChERS method outlined in [49]. Briefly, 8 g of experimental soil and 5 g of roots and leaves were put into a 50 mL centrifuge tube, and then 10 mL of acetonitrile containing 1% acetic acid was added. The samples underwent vigorous shaking for one minute, followed by the addition of 6 g of MgSO4, 1.5 g of NaCl, and 1 g of sodium citrate tribasic dihydrate. Each tube was shaken immediately after the salt was added. The tubes were vigorously shaken for 1 min and then centrifuged at 4000 rpm for 5 min. A 1 mL portion of the supernatant was transferred to a clean-up tube containing C18, MgSO4, primary secondary amine (PSA), and graphitized carbon black (GCB). The tubes were agitated for 30 s and subsequently centrifuged at 4000 rpm for 5 min.
2.5. HPLC Analysis
The amount of CPF residues in the collected samples was analyzed using an HPLC fitted with a UV detector. The HPLC system eluted isocratically with a mobile phase consisting of water and acetonitrile (70% acetonitrile + 30% water with 0.1% acetic acid). The flow rate was controlled at 1.5 mL per minute, and the total duration of the run was 15 min. A C18 column (Chiralpak IB- 250 mm × 4.6 mm, with a particle size of 5 µm) programmed at 25 °C was used. The eluents were monitored via UV detection with a wavelength equal to 280 nm. The retention time for CPF was recorded as 8.30 min.
2.6. Accuracy and Precision
The effectiveness of the HPLC method was evaluated by examining various parameters, including the values of recovery and the limits of quantification (LOQ) and detection (LOD). The linearity demonstrated significance, with an outstanding correlation coefficient of R = 0.996. The LOQ value was 0.62 µg g−1 and the LOD value was 0.14 µg g−1. CPF recoveries were evaluated at the fortification levels of 0.05, 0.1, and 0.5 mg kg−1 across the soil, root, and leaf samples. Under the specified conditions, no interfering peaks were observed on the chromatogram. The average recovery ranges for the soil, roots, and leaves were as follows: 93.5–95.3%, 88.12–90.70%, and 87.14–92.41%, respectively.
2.7. Physiological Parameters
A total of 100 mg of roots and leaves was collected from treatments 1, 3, 4, and 5 after 1, 3, 7, 10, and 14 days of CPF exposure and homogenized at 4 °C with liquid nitrogen and 100 mM phosphate buffer (pH 7.0) involving 1 mM PMSF, 0.5% PVP, and 1 mM EDTA. The blend was centrifuged at 9000 rpm for 20 min. The enzyme extract’s supernatant was used to evaluate the enzyme’s activity. SOD, Prx, MDA, and H2O2 kits were purchased from Biotechnology (SAE) Egyptian Co., Egypt.
2.8. Data and Statistical Analysis
One-way ANOVA was conducted, and the mean values (mean ± standard deviation) were compared across all treatments. CoStat 6.311 CoHort Statistical Software (https://cohortsoftware.com/costat.html, accessed on 1 June 2024) was employed for the study objectives. The significance level was set at p ≤ 0.05.
According to Romeh [50], the quantity of CPF adsorbed was determined by subtracting the initial concentration of CPF from the concentration at equilibrium.
x/m = (C0 − Ce) V/W.
In this equation, x/m represents the concentration of CPF in the soil (µg g−1), C0 is the initial concentration of CPF (µg mL−1), Ce is the CPF concentration at equilibrium (µg mL−1), V denotes the solution volume, and W indicates the weight of the soil sample.
The determination of LOQ and LOD followed the equations according to European Commission guidelines for pesticide residue analytical methods [51]:
where So represents the standard deviation of the calibration line and b denotes the slope.
LOD = 3.3 S0/b and LOQ = 10 S0/b,
The degradation rate (K) and half-life value (t1/2) were determined using the equations described by Gomaa and Belal [52] as follows:
The degradation rate (K) = 2.303 × slope.
Half-life value (t1/2) = 0.693 K−1.
3. Results
3.1. Degradation and Translocation of CPF under Tested Bacteria
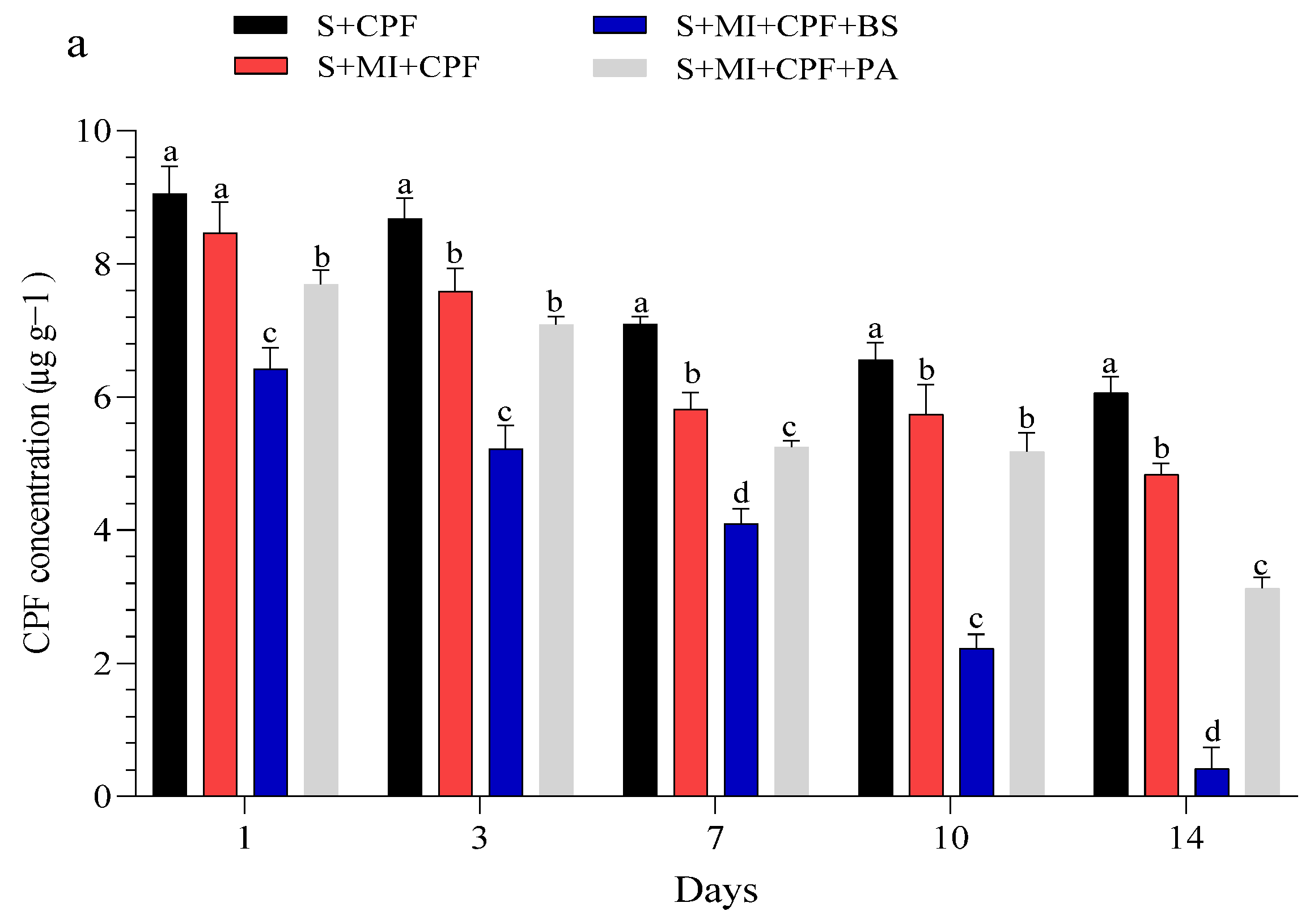
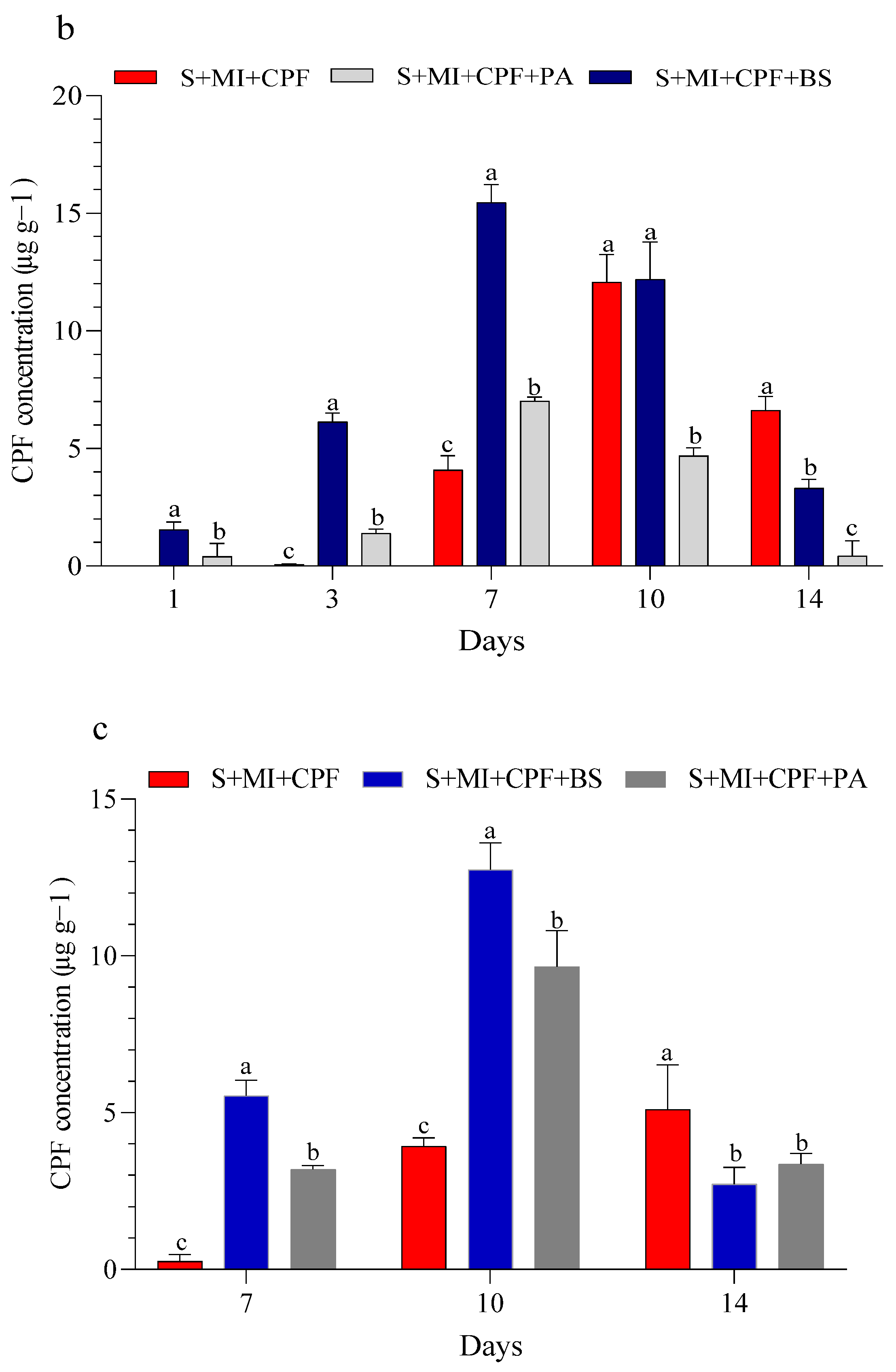
Figure 1 displays the CPF concentrations in the experimental soil and MI tissues throughout the study. Our findings indicated that all experimental groups effectively reduced a significant portion of CPF. Adding MI along with the tested bacteria to the soil reduced the CPF residues while simultaneously increasing the CPF concentrations in the roots and leaves of MI. B. subtilis and P. aeruginosa had a surfactant effect on the removal, absorption, and movement of CPF compared with MI alone. In the soil, the CPF concentration in the S+MI+CPF+BS treatment was in the range of 6.42–0.41 µg g−1 over the 14 days of the experiment, followed by the S+MI+CPF+PA treatment (7.69–3.12 µg g−1) and then 8.46–4.83 µg g−1 in the S+MI+CPF treatment compared with the S+CPF treatment (9.05–6.06 µg g−1) (Figure 1a). Concurrently, CPF remarkably accumulated in the MI roots to reach the highest levels after 7 days during the S+MI+CPF+BS treatment (15.46 µg g−1), followed by the S+MI+CPF+PA treatment (7.02 µg g−1) compared with the S+MI+CPF treatment. Conversely, no CPF concentration was observed in the leaves after 1 and 3 days of treatment. CPF moved into the MI leaves and reached 2.72 and 3.36 µg g−1 in the S+MI+CPF+BS and S+MI+CPF+PA treatments, respectively, after 14 days compared with the S+MI+CPF treatment (5.10 µg g−1) (Figure 1b,c).
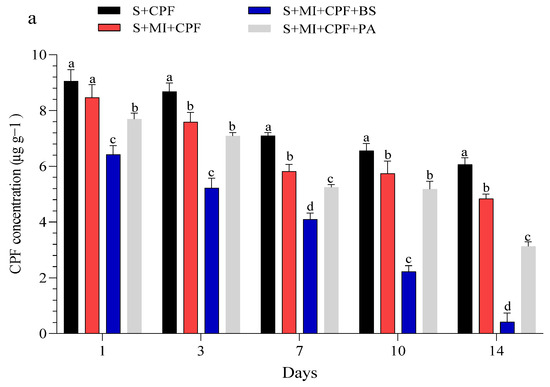
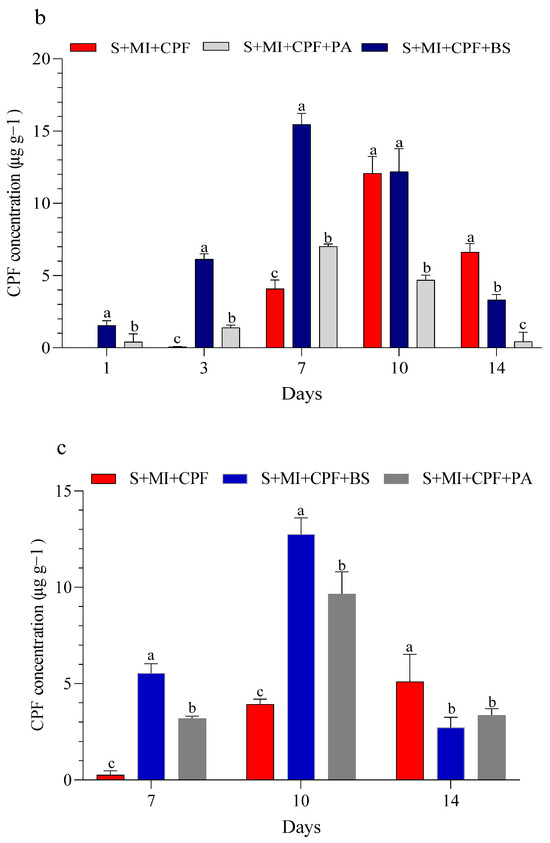
Figure 1.
Efficacy of the two tested bacteria (Bacillus subtilis and Pseudomonas aeruginosa) in the phytoremediation of chlorpyrifos (CPF)-polluted soil using Mentha piperita (MI) during the 14 days of the experiment. CPF in soil (a), CPF in roots (b), and CPF in leaves (c). Means and standard deviations of three replicates. Different letters on top of the bar indicate significant differences (p < 0.05). S+CPF, CPF-polluted soil without MI; S+MI+CPF, CPF-polluted soil plus MI; S+MI+CPF+BS, CPF-polluted soil plus MI and B. subtilis; S+MI+CPF+PA, CPF-polluted soil plus MI and P. aeruginosa.
3.2. Enhancing CPF Recovery in Soil
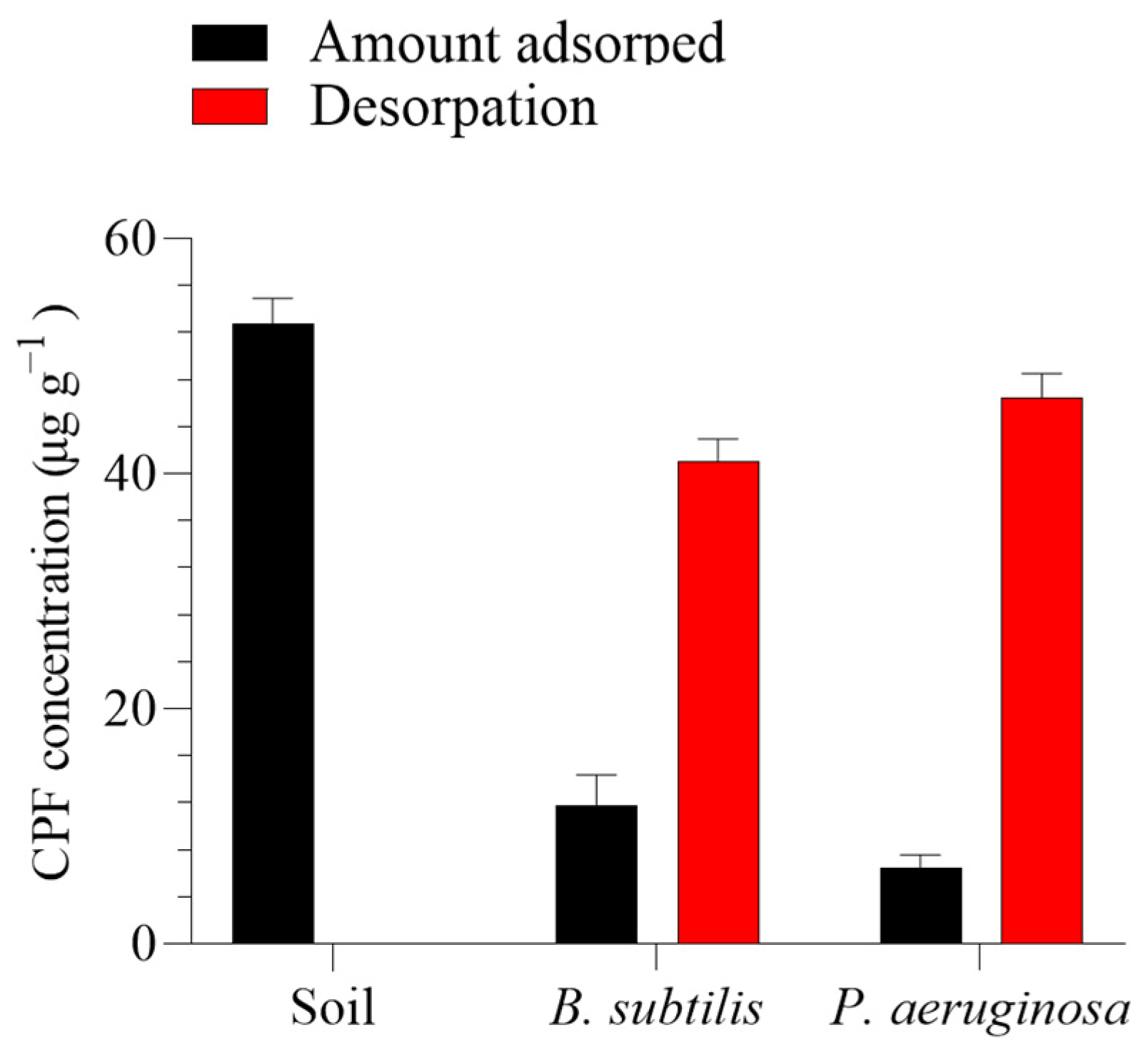
As shown in Figure 2, a batch equilibrium method was employed to assess the capacity of B. subtilis and P. aeruginosa to adsorb CPF from the polluted soil by comparing the degradation-improving biosurfactants with the control. B. subtilis was significantly effective (p > 0.05) in reducing CPF adsorption onto the soils, followed by P. aeruginosa. The amount of CPF adsorbed by B. subtilis and P. aeruginosa from the polluted soil was 11.83 and 6.44 µg g−1, respectively, after one day compared to the soil’s adsorption of 52.84 µg g−1.
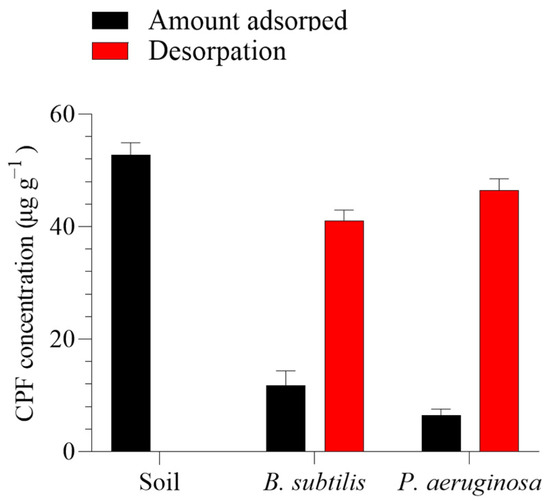
Figure 2.
In vitro efficacy of the two tested bacteria (Bacillus subtilis and Pseudomonas aeruginosa) in improving chlorpyrifos (CPF) recovery from soil over 24 h. Means and standard deviations of three replicates.
3.3. Reaction Rate Constants for CPF
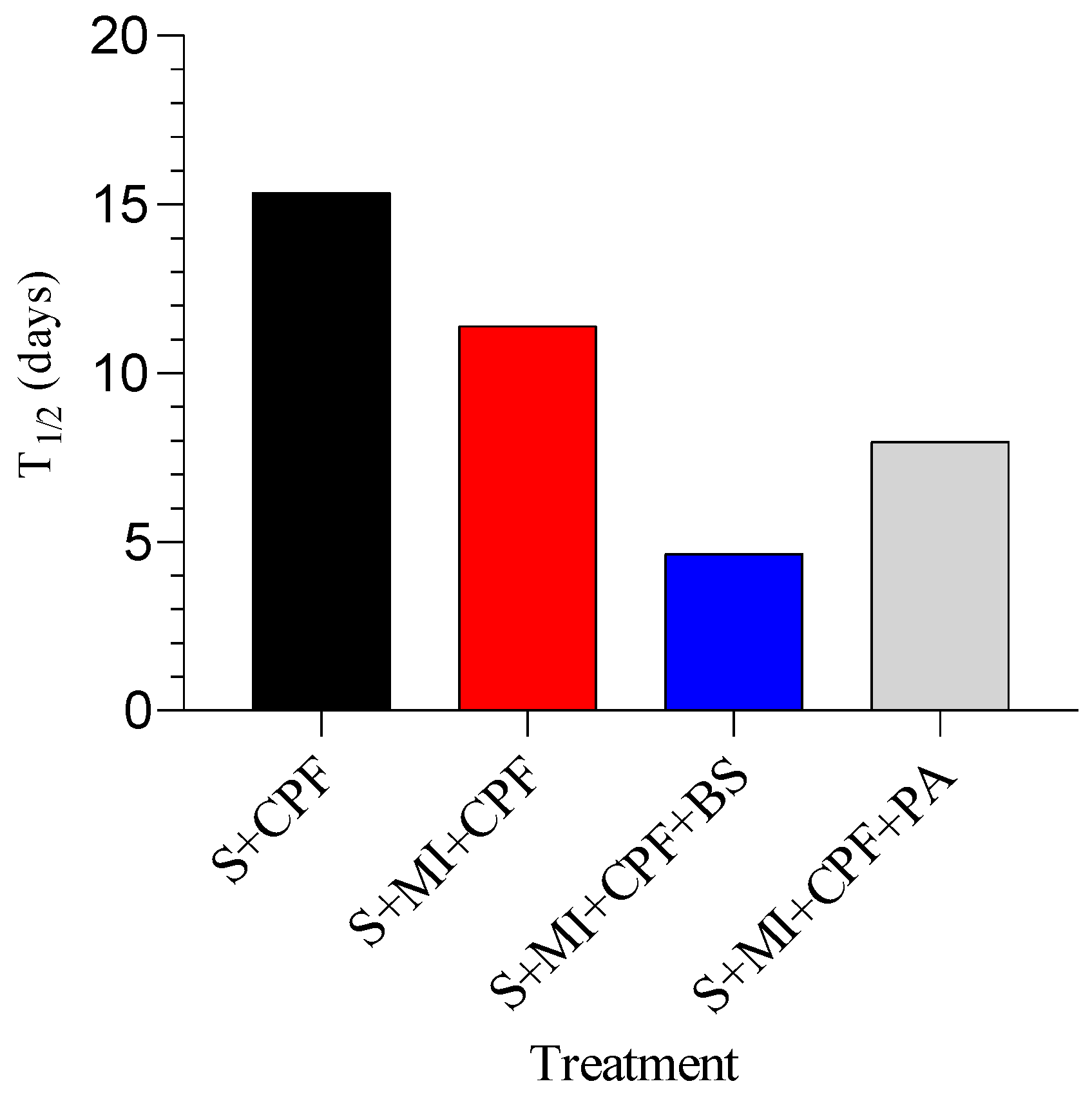
Using a first-order reaction, T1/2 of CPF in the soil inoculated with MI along with B. subtilis, MI inoculated with P. aeruginosa, and MI alone was found to be 4.65, 7.98, and 11.41 days, respectively, compared to 15.36 days for the soil alone (Figure 3).
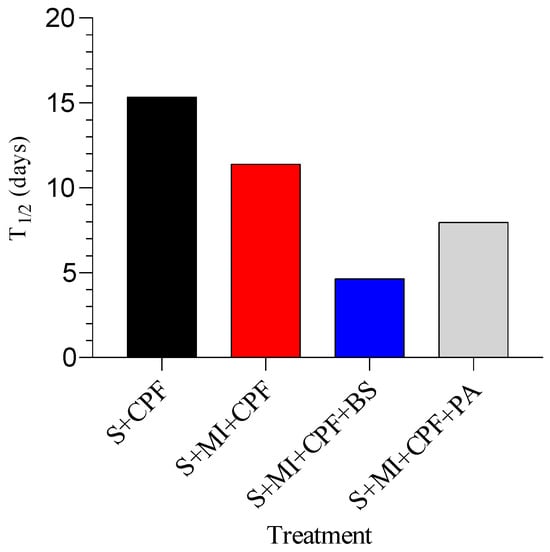
Figure 3.
The half-lives (t1/2) of chlorpyrifos (CPF) under different soil treatments. S+CPF, CPF-polluted soil without MI; S+MI+CPF, CPF-polluted soil plus MI; S+MI+CPF+BS, CPF-polluted soil plus MI and B. subtilis; S+MI+CPF+PA, CPF-polluted soil plus MI and P. aeruginosa.
3.4. Physiological Characteristics of Mentha Piperita
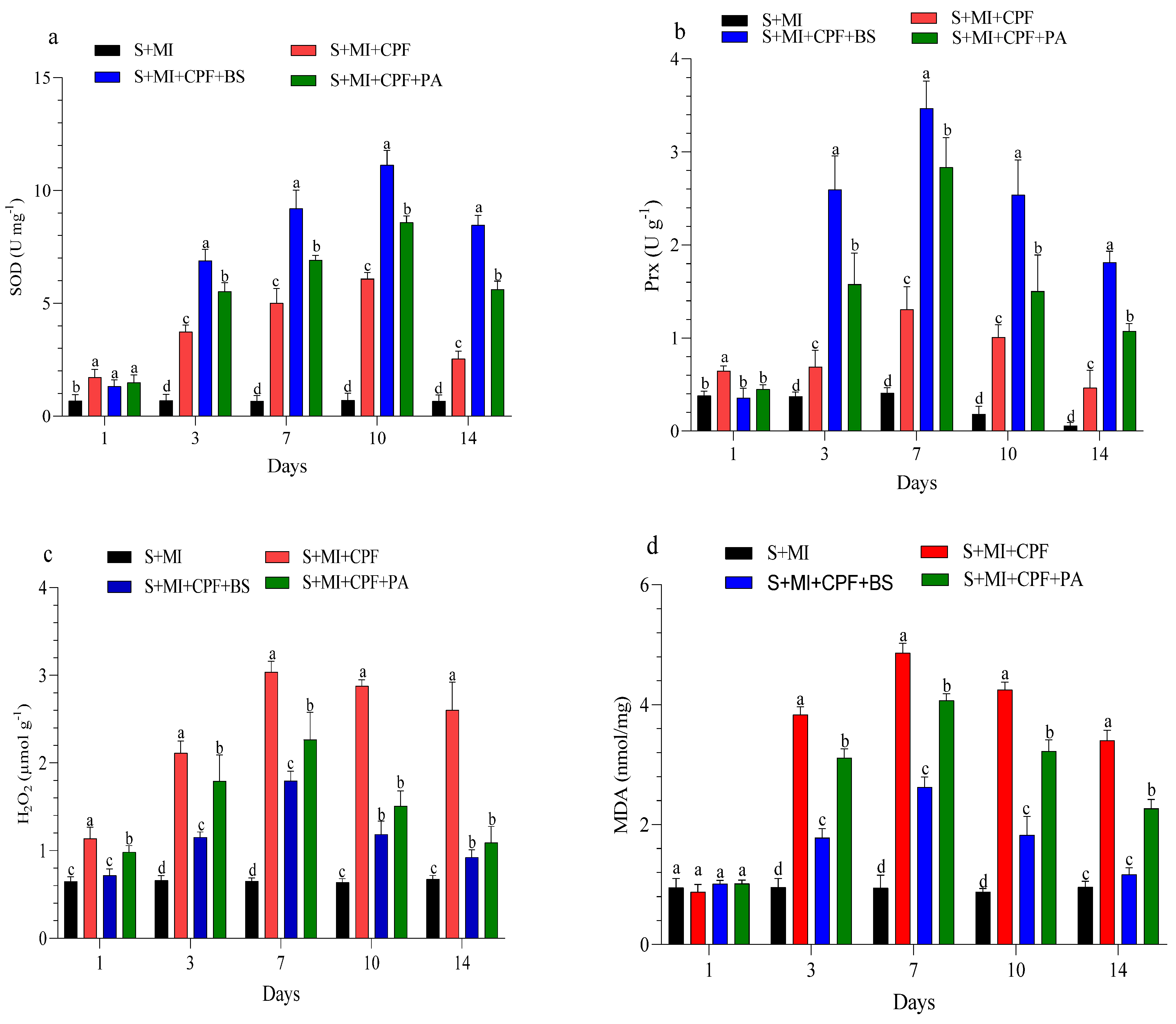
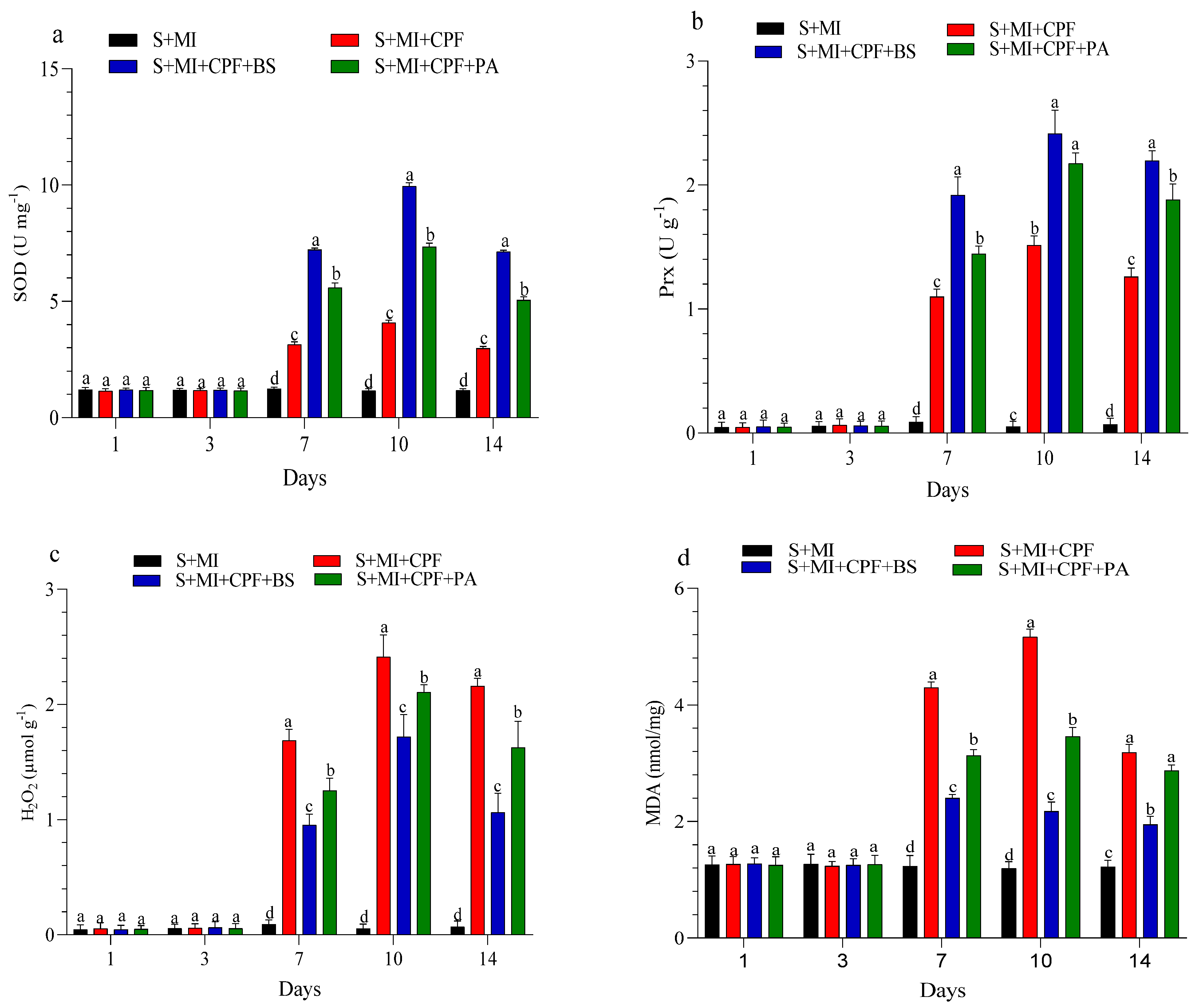
The changes in enzyme activity and oxidative enzymes in the roots and leaves of MI after 1, 3, 7, 10, and 14 days are shown in Figure 4 and Figure 5. MI amended with B. subtilis and P. aeruginosa under CPF stress in the roots and leaves significantly enhanced SOD and Prx activity compared with the S+MI+CPF treatment. SOD activity in the S+MI+CPF+BS and S+MI+CPF+PA treatments was achieved after 10 days of CPF exposure in the roots (11.1 and 8.58 U g−1) and leaves (9.95 and 7.34 U g−1) compared with S+MI+CPF alone (6.09 and 4.08 U g−1), respectively. The Prx activity in the roots under CPF stress after 7 and 14 days was recorded (p > 0.05) at 3.46 and 1.81 U g−1 in the S+MI+CPF+BS treatment and at 2.83 and 1.07 U g−1 in the S+MI+CPF+PA treatment compared with the S+MI+CPF treatment (1.30 and 0.46 U g−1), respectively. No significant difference was observed in the Prx activity of the MI leaves among all treatments after 1 and 3 days of CPF exposure. Meanwhile, the Prx activity of the MI leaves increased in the S+MI+CPF+BS treatment, followed by the S+MI+CPF+PA treatment compared with the S+MI+CPF treatment after 7 to 14 days of CPF exposure.
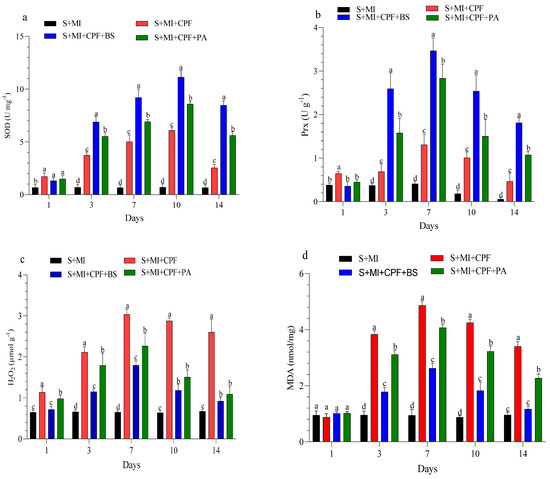
Figure 4.
Levels of superoxide dismutase (SOD) (a), peroxidase (Prx) (b), hydrogen peroxide (H2O2) (c), and malondialdehyde (MDA) (d) in Mentha piperita (MI) roots inoculated with Bacillus subtilis and Pseudomonas aeruginosa in soil contaminated with chlorpyrifos (CPF) through 1–14 days of exposure. Means and standard deviations of three replicates. Different letters on top of the bar indicate significant differences (p < 0.05). S+CPF, CPF-polluted soil without MI; S+MI+CPF, CPF-polluted soil plus MI; S+MI+CPF+BS, CPF-polluted soil plus MI and B. subtilis; S+MI+CPF+PA, CPF-polluted soil plus MI and P. aeruginosa.
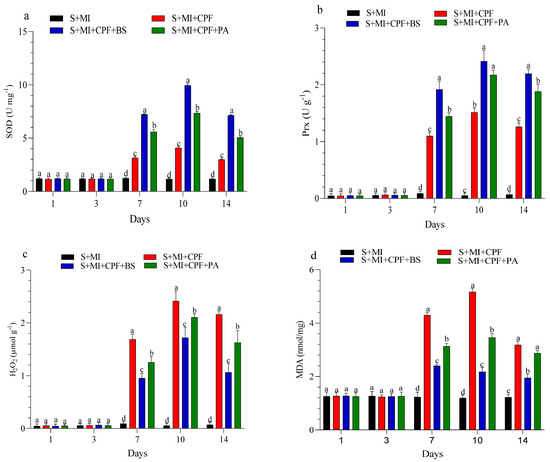
Figure 5.
Levels of superoxide dismutase (SOD) (a), peroxidase (Prx) (b), hydrogen peroxide (H2O2) (c), and malondialdehyde (MDA) (d) in Mentha piperita (MI) leaves inoculated with Bacillus subtilis and Pseudomonas aeruginosa in soil contaminated with chlorpyrifos (CPF) through 1–14 days of exposure. Means and standard deviations of three replicates. Different letters on top of the bar indicate significant differences (p < 0.05). S+CPF, CPF-polluted soil without MI; S+MI+CPF, CPF-polluted soil plus MI; S+MI+CPF+BS, CPF-polluted soil plus MI and B. subtilis; S+MI+CPF+PA, CPF-polluted soil plus MI and P. aeruginosa.
H2O2 content remarkably decreased (p > 0.05) in the MI roots and leaves amended with B. subtilis and P. aeruginosa compared with MI alone under CPF stress. H2O2 content reached the highest value after 7 days in the MI roots and after 10 days in the MI leaves during the S+MI+CPF treatment (3.03 and 2.41 µmol g−1) compared with the S+MI+CPF+BS treatment (1.79 and 1.72 µmol g−1) and the S+MI+CPF+PA treatment (2.26 and 2.10 µmol g−1), respectively. MDA content in the MI roots and leaves was 4.86 and 4.30 nmol/mg during the S+MI+CPF treatment, followed by the S+MI+CPF+PA treatment (4.07 and 3.13 nmol/mg) and then the S+MI+CPF+BA treatment (2.62 and 2.40 nmol/mg) after 7 days of exposure, respectively.
4. Discussion
Environmental pollution on a global scale is one of the foremost environmental challenges facing modern society [53]. Phytoremediation, an emerging technology, has garnered attention for its potential to remediate polluted soil due to its cost-effectiveness, aesthetic benefits, and long-term viability [54]. According to our findings, MI can absorb CPF from polluted soil, significantly improving CPF removal from the soil. This capability stems from the MI root system’s high capacity for binding and accumulating xenobiotics, thus safeguarding the plant against pollutant toxicity [55,56]. Also, analytical quantification demonstrates that MI is suitable for remediating heavily contaminated soils [57]. Our findings align with those of Dinu et al. [58], who showed that MI can effectively stabilize metals at the root level and tolerate metals when cultivated in a nutrient-rich substrate. Moreover, MI has been recognized as a hyperaccumulator plant and is recommended for remediation efforts targeting heavy metals [59,60]. Furthermore, some factors affect the pesticide sorbed to the soil and translocation in plant tissues, such as soil types, pesticide (lipophilicity or hydrophilic), water solubility, log Koc, and log Kow values [61]. Turgut [62] found that the absorption and movement of organic compounds relies on factors such as hydrophobicity (lipophilicity), solubility, polarity, molecular weight, plant species, and environmental conditions. Therefore, according to our study, the absorption of CPF in soil and its movement in MI roots and leaves may be because of the physical and chemical properties of CPF, which shows lipophilicity with a low water solubility of 0.73 mg/L, as well as high log Koc and log Kow values, of 3.78 and 5 l/kg, respectively [7]. Bouldin et al. [63] recorded that the main characteristic determining the movement of pesticides such as CPF within plants is their lipophilicity, which correlated with the Kow value. Notably, pesticides should have a log Kow ranging between 3.0 and 0.5 to achieve optimal uptake. Pesticides with lower log Kow values are frequently too hydrophilic to penetrate the cell membrane, while those with higher log Kow values tend to be highly hydrophobic and can adhere strongly to roots [63]. In addition, when pesticides have a high Koc value, they are strongly absorbed by soil, making it challenging for plants to uptake and transport them [61]. Chlorpyrifos (CPF) has been predicted to penetrate biomembranes and subsequently bind to the roots [64], with minimal uptake and translocation to the aboveground biomass of plants. Foliar absorption directly into the aboveground parts of plants is a significant pathway, particularly for volatile and semi-volatile compounds, in comparison to root uptake [65]. Nonetheless, the volatility of CPF in soil was notably reduced over extended exposure periods [66]; consequently, CPF residue tended to accumulate more in the roots than in the aerial parts of the plant. Interestingly, when CPF is tightly bound to soil, its availability for microbial degradation and plant absorption is reduced [67]. Additionally, CPF exhibits strong binding to soil and remains immobile to slightly mobile within soil due to its low water solubility and high Koc value [68].
Amending soil with MI plus B. subtilis and P. aeruginosa reduced CPF in the soil from 1 to 14 days of the experiment, accompanied by enhancement in the plant leaves and roots. Applying B. subtilis and P. aeruginosa to polluted soil using a batch equilibrium technique demonstrated more effective removal of CPF than soil alone. This effect was shown by the microbial degradation mechanism, which can be outlined in three phases [69]. Initially, the focus was on adsorption, as CPF adhered to the cell membrane surface in a dynamic equilibrium process, which was crucial. Next, the compound entered the cell membrane surface, and its penetration efficiency and rate were affected by its molecular structure. Lastly, the compound undergoes rapid enzymatic reactions within the membrane. As previously demonstrated by Gongora-Echeverria et al. [70], the bioremediation of bacteria proves effective in detoxifying accumulated pesticide residues in the environment. Furthermore, using B. subtilis culture holds promising potential for remedying agricultural soils contaminated with monocrotophos [71] and glyphosate [72]. Moreover, P. aeruginosa can degrade CPF, cypermethrin, and endosulfan [73,74]. Likewise, B. subtilis and P. aeruginosa have biodegradation properties that metabolize chlorantraniliprole-, flubendiamide-, and cypermethrin-contaminated soil [48,75,76]. Singh [77] advocated for the inoculation of soil with Pseudomonas sp., which accelerated CPF degradation in Australia. Interestingly, the success of bioaugmentation relies heavily on the inoculum density because introducing bacteria into the environment has a competitive advantage over native bacteria in exploiting space and nutrients [78]. The rate and efficiency of pesticide degradation are positively correlated with the inoculum density [79]: higher inoculum densities provide resilience against some environmental factors such as soil type and pH [80,81], and increasing the inoculum density can enhance the overall biodegradation capacity of the microbial community [82]. For example, the authors reported that a high inoculum density (>106 cells/g of soil) significantly influences the rate at which CPF degrades [80]. Singh et al. [83] demonstrated that Enterobacter sp. shows no degradation of CPF when introduced into soil at an inoculum density below 103 cells/g of soil. Moreover, the degradation rate of CPF in soil inoculated with Stenotrophomonas sp. was enhanced as soil pH increased from 4.3 to 7. However, there was no notable difference in the degradation rate between soil pH of 7 and 8.4 [84]. Also, introducing diazinon-degrading S. marcescens into soils accelerated the degradation of CPF, reducing its half-life (T1/2) by 20.7, 11.9, and 9.7 days in sandy, sandy loam, and silty soils, respectively, compared to soils without the inoculum [85]. Microbe-assisted phytoremediation holds significant promise for remediating soil polluted with pesticides [11,86]. MI does not possess the capability of degrading CPF; instead, it can only absorb CPF-polluted soil. However, soil microbes are essential for CPF degradation. In soil alone, the T1/2 value of CPF was recorded at 15.3 days. In contrast, in soil amended with B. subtilis, it was significantly shorter, at 4.65 days. The improvement in CPF dissipation within the phytoremediation system is attributed to the decrease in its half-life, likely resulting from CPF sorption to the plant metabolism, or improved degradation facilitated by the collaborative impact of plants and microorganisms in the root zone [11]. This study aligns with the findings of Malla et al. [87], who found that the T1/2 value for CPF degradation by Bacillus cereus in polluted soil was 1.26 days. Another study showed that the T1/2 of CPF was 3.01 days in Brassica oleracea and 1.35 days in Brassica nigra leaves [88].
Multiple findings have indicated that plants have developed a sophisticated antioxidant defense mechanism relying on SOD and Prx to combat free radicals’ action [89]. Enhanced stress tolerance in plants exposed to diverse stressors is linked to improved antioxidant enzyme activity [90]. Our findings showed that antioxidant activity (SOD and Prx) in the root and leaves of MI remarkably increased and oxidative stress (MDA and H2O2) decreased in the presence of the two tested bacteria compared with MI alone. An enhancement in the activity of SOD and Prx may be due to SOD enzymes playing a crucial role at the forefront of defense against ROS. Prx participates in the detoxification of H2O2. Notably, Prx can catalyze hydroxylic reactions as a secondary cyclic reaction, distinct from peroxidation reactions. Additionally, Prx participates in the breakdown of H2O2 [91]. The reduction in lipid peroxidation observed with B. subtilis and P. aeruginosa inoculation under CPF stress could be attributed to the increased synthesis of ROS-scavenging enzymes. Interestingly, xenobiotic-induced lipid peroxidation occurs because of the elimination of hydrogen from fatty acids by ROS, resulting in the generation of lipid radicals [92]. This initiates a reaction cascade, forming short-chain alkanes and acidic aldehydes, which disrupt the lipid structure [93]. These findings align with those of prior research demonstrating that bacterial inoculation enhances plant tolerance to xenobiotics by enhancing the antioxidative activity of various enzymes [94,95]. Gururani et al. [96] found Bacillus pumilus strain DH-11 and Bacillus firmus strain 40, which were isolated from the potato rhizosphere. These strains enhanced the zinc tolerance of potato plants by increasing the transcription levels of ROS-scavenging enzymes (Prx and SOD), thereby improving the plants’ tolerance to Zn. Furthermore, Martins et al. [97] showed that bacteria isolated from soil samples exhibit increased production of antioxidants in response to pesticide stress, such as acetochlor and metolachlor, suggesting that antioxidants serve as a mechanism for tolerance against oxidative stress. Helianthus annuus incubated with Bacillus sp. plus endosulfan decreased MDA and H2O2 production [98]. The inoculation of Bacillus siamensis in Triticum aestivum resulted in a reduction of cadmium toxicity, as evidenced by decreased MDA content and enhanced SOD levels [99]. Also, Bacillus subtilis decreased the MDA amount and enhanced Medicago sativa antioxidant enzyme activity under Cd stress [100]. Coadministration of PGPR and maize contaminated with Cd significantly reduced MDA and H2O2 content, restoring normal plant reactions [101].
5. Conclusions
Our study suggests that collaboration between plants and bacteria represents a promising new strategy for remediating chlorpyrifos (CPF)-contaminated soil. Mentha piperita (MI) can effectively extract CPF from polluted soil through its roots and move it to its leaves. Therefore, MI could be a promising model for mitigating CPF levels in contaminated soil. Moreover, Bacillus subtilis and Pseudomonas aeruginosa can improve the phytoremediation of CPF in polluted soil by serving as biosurfactants. Furthermore, antioxidant activity (SOD and Prx) was enhanced and oxidative stress (H2O2 and MDA) was reduced in the CPF-polluted soil treated with coadministration of MI and the tested bacteria compared with CPF-polluted soil treated with MI alone. Therefore, collaboration between phytoremediation and bacteria is suggested as a practical approach to expedite the elimination of pesticide residues from polluted soil, thereby ensuring the safety of both humans and non-target organisms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxics12060435/s1, Table S1: Total bacterial count of the soil before being treated with CPF. Table S2: Total bacterial count of soil during the time course of the experiments.
Author Contributions
Conceptualization, A.A.A.A. and E.E.A.; methodology, E.E.A.; software, Q.Z.; validation, A.A.A.A., M.A.F. and H.A.I.; formal analysis, S.I.Z.A.-W.; investigation, A.A.A.A.; resources, M.A.F.; data curation, M.M.; writing—original draft preparation, A.A.A.A.; writing—review and editing, A.A.A.A.; visualization, J.Y.; supervision, Q.Z.; project administration, J.Y.; funding acquisition, Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Our findings financial support from the National Key Research and Development Program of China (2021YFD1700803) and the Province Key Research and Development Program of Jiangsu, China (D21YFD17008).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data and materials are included in the manuscript.
Acknowledgments
The authors gratefully acknowledge financial support from the National Key Research and Development Program of China (2021YFD1700803) and the Province Key Research and Development Program of Jiangsu, China (D21YFD17008).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Van, E.; Jeanette, M.; Pan, P.; van Breukelen, F. Effects of chlorpyrifos and trichloropyridinol on HEK 293 human embryonic kidney cells. Chemosphere Environ. Toxicol. Risk Assess. 2018, 191, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Foong, S.Y.; Ma, N.L.; Lam, S.S.; Peng, W.; Low, F.; Lee, B.H.K.; Alstrup, A.K.O.; Sonne, C. A recent global review of hazardous chlorpyrifos pesticide in fruit and vegetables: Prevalence, remediation and actions needed. J. Hazard. Mater. 2020, 400, 123006. [Google Scholar] [CrossRef]
- Abd-Alrahman, S.H.; Mostafa, A.A. Mycoremediation of organophosphorous insecticide chlorpyrifos by fungal soil isolates. J. Pure Appl. Microbiol. 2014, 8, 2945–2951. [Google Scholar]
- Rahman, S.U.; Xuebin, Q.; Kamran, M.; Yasin, G.; Cheng, H.; Rehim, A.; Riaz, L.; Rizwan, M.; Ali, S.; Alsahli, A. Silicon elevated cadmium tolerance in wheat (Triticum aestivum L.) by endorsing nutrients uptake and antioxidative defense mechanisms in the leaves. Plant Physiol. Biochem. PPB 2021, 166, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Yang, L.; Wang, L.; Lv, J.; Gu, L.; Li, H.; Peng, W.; Yu, Q.; Ruan, H.; Li, Q.; et al. Environmental and Human Health Hazards from Chlorpyrifos, Pymetrozine and Avermectin Application in China under a Climate Change Scenario: A Comprehensive Review. Agriculture 2023, 13, 1683. [Google Scholar] [CrossRef]
- Liang, B.; Yang, C.; Gong, M.; Zhao, Y.; Zhu, C.; Jiang, J.; Li, S. Adsorption and degradation of triazophos, chlorpyrifos and their main hydrolytic metabolites in paddy soil from Chaohu Lake, China. J. Environ. Manag. 2011, 92, 2229–2234. [Google Scholar] [CrossRef] [PubMed]
- Mackay, D.; Giesy, J.P.; Solomon, K.R. Fate in the environment and long-range atmospheric transport of the organophosphorus insecticide, chlorpyrifos and its oxon. Rev. Environ. Contam. Toxicol. 2014, 231, 35–76. [Google Scholar] [CrossRef]
- Eaton, D.L.; Daroff, R.B.; Autrup, H.; Bridges, J.; Spencer, P.S. Review of the Toxicology of Chlorpyrifos with an Emphasis on Human Exposure and Neurodevelopment. Crit. Rev. Toxicol. 2008, 38 (Suppl. S2), 1–125. [Google Scholar] [CrossRef]
- Cilia, N.; Kandris, I. Training in the evaluation of pesticides (plant protection products and active substances) according to Regulation (EC) No 1107/2009. EFSA J. 2023, 21, e211007. [Google Scholar] [CrossRef]
- Rock, S.; Pivetz, B.; Madalinski, K.; Adams, N.; Wilson, T. Introduction to Phytoremediation; EPA/600/R-99/107 (NTIS PB2000-106690); U.S. Environmental Protection Agency: Washington, DC, USA, 2000. [Google Scholar]
- Aioub, A.A.A.; Li, Y.; Qie, X.; Zhang, X.; Hu, Z. Reduction of soil contamination by cypermethrin residues using phytoremediation with Plantago major and some surfactants. Environ. Sci. Eur. 2019, 2019, 26. [Google Scholar] [CrossRef]
- Liu, W.; Sun, J.; Ding, L.; Luo, Y.; Chen, M. Rhizobacteria (Pseudomona ssp. SB) assist phytoremediation of oily-sludge-contaminated soil by tall fescue (Testuca arundinacea L.). Plant Soil 2013, 371, 533–542. [Google Scholar] [CrossRef]
- Pandey, S.; Bhattacharya, T. Characterization and phytoremediation of heavy metals of flyash by native plant species: A case study of fly ash generated from Patratu Thermal Power Station, Jharkhand. In Proceedings of the RACEE-2016; Birla Institute of Technology: Mesra, India, 2016. [Google Scholar]
- Lv, T.; Zhang, Y.; Casas, M.E.; Carvalho, P.N.; Arias, C.A.; Bester, K.; Brix, H. Phytoremediation of imazalil and tebuconazole by four emergent wetland plant species in hydroponic medium. Chemosphere 2016, 148, 459–466. [Google Scholar] [CrossRef]
- Lubbu, M.A.; Taufikurahman, T. Effect of chromium phytoextraction to anticancer compound of Catharanthus roseus (L.) G. Don. In Proceedings of the International Conference on Universal Wellbeing, Kuala Lumpur, Malaysia, 5–6 December 2019. [Google Scholar]
- Aioub, A.A.A.; Zuo, Y.; Aioub, A.A.A.; Hu, Z. Biochemical and phytoremediation of Plantago major L. to protect tomato plants from the contamination of cypermethrin pesticide. Environ. Sci. Pollut. Res. 2021, 30, 7040–7055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Lu, Y.C.; Zhang, J.J.; Tan, L.R.; Yang, H. Accumulation and toxicological response of atrazine in rice crops. Ecotoxicol. Environ. Saf. 2014, 102, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A.; Lanza, G.R.; Gill, S.S.; Gill, R.; Newman, L. Phytoremediation: Management of Environmental Contaminants Volume-2; Springer International Publishing: Berlin, Germany, 2014. [Google Scholar] [CrossRef]
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of Cadmium: Physiological, Biochemical, and Molecular Mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Phaenark, C.; Pokethitiyook, P.; Kruatrachue, M.; Ngernsansaruay, C. Cd and Zn accumulation in plants from the Padaeng zinc mine area. Int. J. Phytoremediat. 2009, 11, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Ranjan, R.; Gupta, A.; Srivastava, P. Molecular basis of plant-microbe interaction in remediating organic pollutants. In Handbook of Bioremediation Physiological, Molecular and Biotechnological Interventions; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 603–623. [Google Scholar] [CrossRef]
- Kurashvili, M.; Adamia, G.; Ananiashvili, T.; Amiranasvili, L.; Varazi, T.; Pruidze, M.; Gordeziani, M.; Khatisashvili, G. Plants and Microorganisms for Phytoremediation of Soils Polluted with Organochlorine Pesticides. Int. J. Biol. Vet. Agric. Food Eng. 2014, 8, 382–384. [Google Scholar]
- Escalante-Espinosa, E.; Gallegos-Martinez, M.; Favela-Torres, E.; Gutierrez-Rojas, M. Improvement of the hydrocarbon phytoremediation rate by Cyperus laxus Lam. inoculated with a microbial consortium in a model system. Chemosphere 2005, 59, 405–413. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Oves, M. Erratum to: Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ. Chem. Lett. 2011, 10, 105–106. [Google Scholar] [CrossRef]
- Akbar, S.; Sultan, S. Soil bacteria showing a potential of chlorpyrifos degradation and plant growth enhancement. Braz. J. Microbiol. 2016, 47, 563–570. [Google Scholar] [CrossRef]
- Wasi, S.; Tabrez, S.; Ahmad, M. Use of Pseudomonas spp. for the bioremediation of environmental pollutants: A review. Environ. Monit. Assess. 2013, 185, 8147–8155. [Google Scholar] [PubMed]
- Ramadass, M.; Thiagarajan, P. Effective pesticide nano formulations and their bacterial degradation. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK; p. 022050. [CrossRef]
- Avarseji, Z.; Talie, F.; GholamaAlipour Alamdari, E.; Hoseini Tilan, M.S. Investigation of the biodegradability of pendimethalin by Bacillus subtilis, Pseudomonas fluorescens, and Escherichia coli. Adv. Environ. Technol. 2021, 7, 221–229. [Google Scholar]
- Salunkhe, V.P.; Sawant, I.S.; Banerjee, K.; Rajguru, Y.R.; Wadkar, P.N.; Oulkar, D.P.; Naik, D.G.; Sawant, S.D. Biodegradation of profenofos by Bacillus subtilis isolated from grapevines (Vitis vinifera). J. Agric. Food Chem. 2013, 61, 7195–7202. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.W.; Sabit, H.; Tawakkol, W. Biodegradation of malathion by Pseudomonas spp. and Bacillus spp. isolated from polluted sites in Egypt. Am.-Eurasian J. Agric. Environ. Sci. 2014; 14, 855–862. [Google Scholar]
- Mishra, A.; Mishra, S.P.; Arshi, A.; Agarwal, A.; Dwivedi, S.K. Plant-Microbe Interactions for Bioremediation and Phytoremediation of Environmental Pollutants and Agro-ecosystem Development; Springer: Singapore, 2020. [Google Scholar]
- Soldi, E.; Casey, C.; Murphy, B.R.; Hodkinson, T.R. Fungal Endophytes for Grass Based Bioremediation: An Endophytic Consortium Isolated from Agrostis stolonifera Stimulates the Growth of Festuca arundinacea in Lead Contaminated Soil. J. Fungi 2020, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Patra, D.D. Unraveling Plant-Rhizosphere-Microbe Interactions: An Overview. J. Indian Soc. Soil Sci. 2016, 64, S14–S26. [Google Scholar]
- Sustrikova, A.; Salamon, I. Essential oil of peppermint (Mentha × piperita L.) from fields in Eastern Slovakia. Hortic. Sci. 2004, 31, 31–36. [Google Scholar] [CrossRef]
- Dinu, C.; Vasile, G.; Tenea, A.; Stoica, C.; Stefania, G.; Serban, E.A. The influence of toxic metals As, Cd, Ni and Pb on nutrients accumulation in Mentha piperita. Rom. J. Ecol. Environ. Chem. 2021, 3, 141–152. [Google Scholar] [CrossRef]
- Azmat, R.; Haider, S.; Riaz, M. An inverse relation between Pb2+ and Ca2+ ions accumulation in phaseolus mungo and lens culinaris under Pb stress. Pak. J. Bot. 2009, 41, 2289–2295. [Google Scholar]
- Malinowska, E.; Jankowski, K. Copper and zinc concentrations of medicinal herbs and soil surrounding ponds on agricultural land. Landsc. Ecol. Eng. 2017, 13, 183–188. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, L.; Liu, Y.; Su, W.; Yan, J.; Xu, D. Effect of Biochar on the Growth, Photosynthesis, Antioxidant System and Cadmium Content of Mentha piperita ‘Chocolate’ and Mentha spicata in Cadmium-Contaminated Soil. Agronomy 2022, 12, 2737. [Google Scholar] [CrossRef]
- Yücel, E.; Yücel, M. Removal of Heavy Metal Cr (II), Ni (II), Cu (II), Zn (II), Cd (II), Pb (II) Ions from Aqueous Solution by Mentha piperita Extract. Langmuir 2020, 12, 21. [Google Scholar]
- Ugulu, I.; Khan, Z.I.; Rehman, S.; Ahmad, K.; Dogan, Y. Heavy Metal Content of Mentha piperita Samples Irrigated with Wastewater and Appraisal of Human Health Risk. In Conference of the Arabian Journal of Geosciences; Springer: Cham, Switzerland, 2019; pp. 173–176. [Google Scholar]
- Peixoto, F.; Alves-Fernandes, D.; Santos, D.; Fontaínhas-Fernandes, A. Toxicological effects of oxyfluorfen on oxidative stress enzymes in tilapia Oreochromis niloticus. Pestic. Biochem. Physiol. 2006, 85, 91–96. [Google Scholar] [CrossRef]
- MITHOFER. Biotic and heavy metal stress response in plants: Evidence for common signals. FEBS Lett. 2004, 566, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.K.; Rakwal, R.; Jwa, N.S.; Agrawal, V.P. Effects of signaling molecules, protein phosphatase inhibitors and blast pathogen (Magnaporthe grisea) on the mRNA level of a rice (Oryza sativa L.) phospholipid hydroperoxide glutathione peroxidase (OsPHGPX) gene in seedling leaves. Gene 2002, 283, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Aryadeep, R. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar]
- Mishra, S.; Imlay, J. Why do bacteria use so many enzymes to scavenge hydrogen peroxide? Arch. Biochem. Biophys. 2012, 525, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Liu, F.; Zhang, C.; Zhang, J.; Feng, H. Non-destructive determination of Malondialdehyde (MDA) distribution in oilseed rape leaves by laboratory scale NIR hyperspectral imaging. Sci. Rep. 2016, 6, 35393. [Google Scholar] [CrossRef] [PubMed]
- Maksymiec, W.; Krupa, Z. Effects of methyl jasmonate and excess copper on root and leaf growth. Biol. Plant. 2007, 51, 322–326. [Google Scholar] [CrossRef]
- Fahmy, M.A.; Salem, S.H.; Qattan, S.Y.A.; Abourehab, M.A.S.; Ashkan, M.F.; Al-Quwaie, D.A.; Abd El-Fattah, H.I.; Akl, B.A. Biodegradation of Chlorantraniliprole and Flubendiamide by Some Bacterial Strains Isolated from Different Polluted Sources. Processes 2022, 10, 2527. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Kok, A.d.; Hiemstra, M.; Bodegraven, P.V. Validation of a fast and easy method for the determination of residues from 229 pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. J. AOAC Int. 2005, 88, 595–614. [Google Scholar] [CrossRef]
- Romeh, A. Enhancing agents for phytoremediation of soil contaminated by cyanophos. Ecotoxicol. Environ. Saf. 2015, 117, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Pihlström, T.; Fernández-Alba, A.R.; Amate, C.F.; Poulsen, M.E.; Hardebusch, B.; Anastassiades, M.; Lippold, R.; Cabrera, L.C.; de Kok, A.; ORegan, F. Analytical quality control and method validation procedures for pesticide residues analysis in food and feed SANTE 11312/2021. Sante 2021, 11312, v2. [Google Scholar]
- Gomaa, E.A.; Belal, M.H. Determination of dimethoate residues in some vegetables and cotton plant. J. Plasma Phys. 1975, 22, 726–730. [Google Scholar]
- Gaur, N.; Sharma, S.; Yadav, N. Chapter 2—Environmental pollution. In Green Chemistry Approaches to Environmental Sustainability; Garg, V.K., Yadav, A., Mohan, C., Yadav, S., Kumari, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 23–41. [Google Scholar]
- Aioub, A.A.A.; Zuo, Y.; Li, Y.; Qie, X.; Zhang, X.; Essmat, N.; Wu, W.; Hu, Z. Transcriptome analysis of Plantago major as a phytoremediator to identify some genes related to cypermethrin detoxification. Environ. Sci. Pollut. Res. 2021, 28, 5101–5115. [Google Scholar] [CrossRef] [PubMed]
- Ražić, S.; Svetlana, Đ. Determination of chromium in Mentha piperita L. and soil by graphite furnace atomic absorption spectrometry after sequential extraction and microwave-assisted acid digestion to assess potential bioavailability. Chemosphere 2009, 78, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, J.; Yang, X.; Ren, L.; Wang, Y.; Ma, D.; Fan, P.; Wang, H.; Liu, L.; Dong, B. Effects of phenanthrene on the essential oil composition and leaf metabolome in peppermint plants (Mentha piperita L.). Ind. Crops Prod. 2022, 187, 115383. [Google Scholar] [CrossRef]
- Mohseni, A.; Reyhanitabar, A.; Najafi, N.; Oustan, S.; Bazargan, K. Phytoremediation potential and essential oil quality of peppermint grown in contaminated soils as affected by sludge and time. J. Agric. Sci. Technol. 2022, 24, 723–737. [Google Scholar]
- Dinu, C.; Gheorghe, S.; Tenea, A.G.; Stoica, C.; Vasile, G.-G.; Popescu, R.L.; Serban, E.A.; Pascu, L.F. Toxic Metals (As, Cd, Ni, Pb) impact in the most common medicinal plant (Mentha piperita). Int. J. Environ. Res. Public Health 2021, 18, 3904. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Chandra, M. Evaluation of phytoremediation potential of aromatic plants: A systematic review. J. Appl. Res. Med. Aromat. Plants 2022, 31, 100405. [Google Scholar] [CrossRef]
- Khair, K.U.; Farid, M.; Ashraf, U.; Zubair, M.; Rizwan, M.; Farid, S.; Ishaq, H.K.; Iftikhar, U.; Ali, S. Citric acid enhanced phytoextraction of nickel (Ni) and alleviate Mentha piperita (L.) from Ni-induced physiological and biochemical damages. Environ. Sci. Pollut. Res. Int. 2020, 27, 27010–27022. [Google Scholar] [CrossRef]
- Kerle, E.A.; Jenkins, J.J.; Vogue, P.A. Understanding Pesticide Persistence and Mobility for Groundwater and Surface Water Protection. 1994. Available online: https://ir.library.oregonstate.edu/concern/open_educational_resources/05741s066 (accessed on 1 June 2024).
- Turgut, C. Uptake and modeling of pesticides by roots and shoots of parrotfeather (Myriophyllum aquaticum) (5 pp). Environ. Sci. Pollut. Res. 2005, 12, 342–346. [Google Scholar]
- Bouldin, J.; Farris, J.; Moore, M.; Smith, S., Jr.; Cooper, C. Hydroponic uptake of atrazine and lambda-cyhalothrin in Juncus effusus and Ludwigia peploides. Chemosphere 2006, 65, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Trapp, S. Modelling uptake into roots and subsequent translocation of neutral and ionisable organic compounds. Pest Manag. Sci. Former. Pestic. Sci. 2000, 56, 767–778. [Google Scholar] [CrossRef]
- Wang, M.-J.; Jones, K.C. Behaviour and fate of chlorobenzenes (CBs) introduced into soil-plant systems by sewage sludge application: A review. Chemosphere 1994, 28, 1325–1360. [Google Scholar] [CrossRef]
- Racke, K.D. Environmental fate of chlorpyrifos. In Reviews of Environmental Contamination and Toxicology. Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 1993; Volume 131, pp. 1–150. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Bara, J.K.; Soni, R.; Shrivastava, K. Bioremediation of chlorpyrifos contaminated soil by microorganism. Int. J. Environ. Agric. Biotechnol. 2017, 2, 238833. [Google Scholar] [CrossRef]
- McCall, P.; Swann, R.; Laskowski, D.; Unger, S.; Vrona, S.; Dishburger, H. Estimation of chemical mobility in soil from liquid chromatographic retention times. Bull. Environ. Contam. Toxicol. 1980, 24, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Dar, M.A.; Nimesh, S.; López-Chuken, U.J.; Villarreal-Chiu, J.F. Microbial Degradation of Organophosphate Pesticides: A Review. Pedosphere 2018, 28, 190–208. [Google Scholar]
- Gongora-Echeverria, V.R.; Martin-Laurent, F.; Quintal-Franco, C.; Lorenzo-Flores, A.; Giacoman-Vallejos, G.; Ponce-Caballero, C. Dissipation and Adsorption of 2,4-D, Atrazine, Diazinon, and Glyphosate in an Agricultural Soil from Yucatan State, Mexico. Water Air Soil Pollut. 2019, 230, 131. [Google Scholar] [CrossRef]
- Acharya, K.; Shilpkar, P.; Shah, M.; Chellapandi, P. Biodegradation of insecticide monocrotophos by Bacillus subtilis KPA-1, isolated from agriculture soils. Appl. Biochem. Biotechnol. 2015, 175, 1789–1804. [Google Scholar] [CrossRef]
- Mousa, N.; Gatie, I.H.; Hasan, A. Biodegradation of (N-phosphonomethyl)glycine Utilizing Bacillus subtilis using different incubation periods. IOP Conf. Ser. Earth Environ. Sci. 2019, 388, 012080. [Google Scholar] [CrossRef]
- Chen, W.-J.; Zhang, W.; Lei, Q.; Chen, S.-F.; Huang, Y.; Bhatt, K.; Liao, L.; Zhou, X. Pseudomonas aeruginosa based concurrent degradation of beta-cypermethrin and metabolite 3-phenoxybenzaldehyde, and its bioremediation efficacy in contaminated soils. Environ. Res. 2023, 236, 116619. [Google Scholar] [CrossRef] [PubMed]
- Kharabsheh, H.A.; Han, S.; Allen, S.; Chao, S.L. Metabolism of chlorpyrifos by Pseudomonas aeruginosa increases toxicity in adult zebrafish (Danio rerio). Int. Biodeterior. Biodegrad. 2017, 121, 114–121. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.; Yan, Y. Isomerization and biodegradation of beta-cypermethrin by Pseudomonas aeruginosa CH7 with biosurfactant production. Bioresour. Technol. 2011, 102, 7139–7146. [Google Scholar] [CrossRef] [PubMed]
- Gangola, S.; Sharma, A.; Bhatt, P.; Khati, P.; Chaudhary, P. Presence of esterase and laccase in Bacillus subtilis facilitates biodegradation and detoxification of cypermethrin. Sci. Rep. 2018, 8, 12755. [Google Scholar] [CrossRef] [PubMed]
- Singh, B. Degradation of clodinafop propargyl by Pseudomonas sp. strain B2. Bull. Environ. Contam. Toxicol. 2013, 91, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Vogel, T.M. Bioaugmentation for Groundwater Remediation: An Overview. In Bioaugmentation for Groundwater Remediation; Springer: New York, NY, USA, 2013; pp. 1–37. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Li, S. Isolation of a chlorpyrifos-degrading bacterium, Sphingomonas sp. strain Dsp-2, and cloning of the mpd gene. Res. Microbiol. 2007, 158, 143–149. [Google Scholar] [CrossRef]
- Cycon, M.; Piotrowska-Seget, Z. Effect of selected pesticides on soil microflora involved in organic matter and nitrogen transformations: Pot experiment. Pol. J. Ecol. 2007, 55, 207–220. [Google Scholar]
- Reddy, G.S.; Reddy, B.; Tlou, M. Biodegradation of 2-hydroxyquinoxaline (2-HQ) by Bacillus sp. J. Hazard. Mater. 2014, 278, 100–107. [Google Scholar] [CrossRef]
- Singh, B.K.; Walker, A.; Morgan, J.A.W.; Wright, D.J. Biodegradation of chlorpyrifos by Enterobacter strain B-14 and its use in bioremediation of contaminated soils. Appl. Environ. Microbiol. 2004, 70, 4855–4863. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, Y.-H.; Zhang, B.-X.; Yang, C.-H.; Zhang, X. Isolation and characterization of a chlorpyrifos and 3, 5, 6-trichloro-2-pyridinol degrading bacterium. FEMS Microbiol. Lett. 2005, 251, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Cycoń, M.; Żmijowska, A.; Wójcik, M.; Piotrowska-Seget, Z. Biodegradation and bioremediation potential of diazinon-degrading Serratia marcescens to remove other organophosphorus pesticides from soils. J. Environ. Manag. 2013, 117, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, X.; Cheema, S.A.; Liu, W.; Shen, C. Beta-cyclodextrin enhanced phytoremediation of aged PCBs-contaminated soil from e-waste recycling area. J. Environ. Monit. Jem 2010, 12, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Malla, M.A.; Dubey, A.; Kumar, A.; Yadav, S.; Kumari, S. Modeling and optimization of chlorpyrifos and glyphosate biodegradation using RSM and ANN: Elucidating their degradation pathways by GC-MS based metabolomics. Ecotoxicol. Environ. Saf. 2023, 252, 114628. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Huang, H.; Chen, Z.; Wei, J.; Deng, C.; Tan, H.; Li, X. Representative commodity for six leafy vegetables based on the determination of six pesticide residues by gas chromatography. Akadémiai Kiadó 2019, 31, 49–56. [Google Scholar] [CrossRef]
- Prasad, S.M.; Dwivedi, R.; Zeeshan, M. Growth, photosynthetic electron transport, and antioxidant responses of young soybean seedlings to simultaneous exposure of nickel and UV-B stress. Photosynthetica 2005, 43, 177–185. [Google Scholar] [CrossRef]
- Fidalgo, F.; Freitas, R.; Ferreira, R.; Pessoa, A.M.; Teixeira, J. Solanum nigrum L. antioxidant defence system isozymes are regulated transcriptionally and posttranslationally in Cd-induced stress. Environ. Exp. Bot. 2011, 72, 312–319. [Google Scholar] [CrossRef]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide Dismutase and Stress Tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Mishra, V.; Srivastava, G.; Prasad, S.M. Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UV-B irradiation. Sci. Hortic. 2009, 120, 373–378. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Riaz, M.; Shahzad, S.M.; Imran, Q.; Ali, I. Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and salicylic acid (SA) in attenuation of chromium stress in maize plants. Plant Physiol. Biochem. 2016, 108, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Sonia, A.; Muhammad, A.; Aqeel, K.; Waheed, U.Y.; Nasim, A.A.; Basharat, R.; Muhammad, A.S. Combined effect of Bacillus fortis IAGS 223 and zinc oxide nanoparticles to alleviate cadmium phytotoxicity in Cucumis melo. Plant Physiol. Biochem. 2021, 158, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.; Upadhyaya, C.; Baskar, V.; Venkatesh, J.; Nookaraju, A.; Park, S.W. Plant Growth-Promoting Rhizobacteria Enhance Abiotic Stress Tolerance in Solanum tuberosum through Inducing Changes in the Expression of ROS-Scavenging Enzymes and Improved Photosynthetic Performance. J. Plant Growth Regul. 2012, 32, 245–258. [Google Scholar] [CrossRef]
- Martins, P.F.; Carvalho, G.; Gratão, P.L.; Dourado, M.N.; Pileggi, M.; Araújo, W.L.; Azevedo, R.A. Effects of the herbicides acetochlor and metolachlor on antioxidant enzymes in soil bacteria. Process Biochem. 2011, 46, 1186–1195. [Google Scholar] [CrossRef]
- Rani, R.; Kumar, V.; Usmani, Z.; Gupta, P.; Chandra, A. Influence of plant growth promoting rhizobacterial strains Paenibacillus sp. IITISM08, Bacillus sp. PRB77 and Bacillus sp. PRB101 using Helianthus annuus on degradation of endosulfan from contaminated soil. Chemosphere 2019, 225, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Awan, S.A.; Ilyas, N.; Khan, I.; Raza, M.A.; Rehman, A.U.; Rizwan, M.; Rastogi, A.; Tariq, R.; Brestic, M. Bacillus siamensis Reduces Cadmium Accumulation and Improves Growth and Antioxidant Defense System in Two Wheat (Triticum aestivum L.) Varieties. Plants 2020, 9, 878. [Google Scholar] [CrossRef]
- Li, Q.; Xing, Y.; Fu, X.; Ji, L.; Li, T.; Wang, J.; Chen, G.; Qi, Z.; Zhang, Q. Biochemical mechanisms of rhizospheric Bacillus subtilis-facilitated phytoextraction by alfalfa under cadmium stress—Microbial diversity and metabolomics analyses. Ecotoxicol. Environ. Saf. 2021, 212, 112016. [Google Scholar] [CrossRef]
- Tanwir, K.; Javed, M.T.; Abbas, S.; Shahid, M.; Akram, M.S.; Chaudhary, H.J.; Iqbal, M. Serratia sp. CP-13 alleviates Cd toxicity by morpho-physio-biochemical improvements, antioxidative potential and diminished Cd uptake in Zea mays L. cultivars differing in Cd tolerance. Ecotoxicol. Environ. Saf. 2021, 208, 111584. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).