Per- and Polyfluoroalkyl Substances (PFASs) and Their Potential Effects on Female Reproductive Diseases
Abstract
1. Introduction
2. Search Strategy
3. Human Exposure to PFASs and Female Reproductive Disorders
3.1. Polycystic Ovary Syndrome
3.2. Endometriosis
3.3. Premature Ovarian Insufficiency
3.4. Diminished Ovarian Reserve
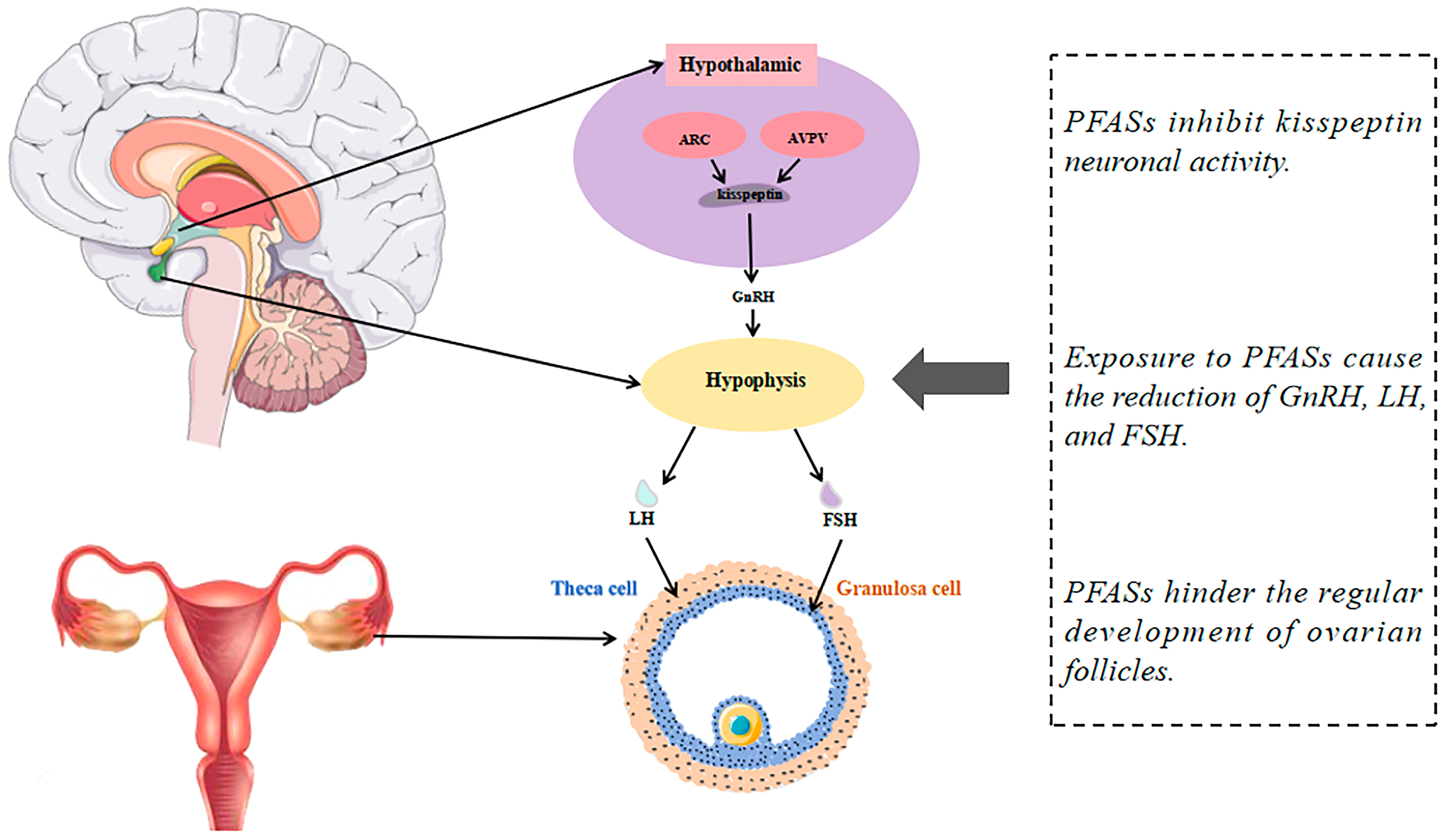
4. Mechanisms of PFASs’ Effects on Female Reproductive Health
4.1. PFAS Exposure Suppresses Kisspeptin Signaling and Impairs Reproductive Hormone Regulation
4.2. Disruption of Steroid Hormone Synthesis Gene Expression and Hormonal Interference by PFASs
4.3. PFASs as Estrogen Receptor Agonists and Antagonists Disrupt Estrogen Signaling and Reproductive Effects
4.4. Activation of the PPAR Signaling Pathway and Induction of Oxidative Stress by PFASs
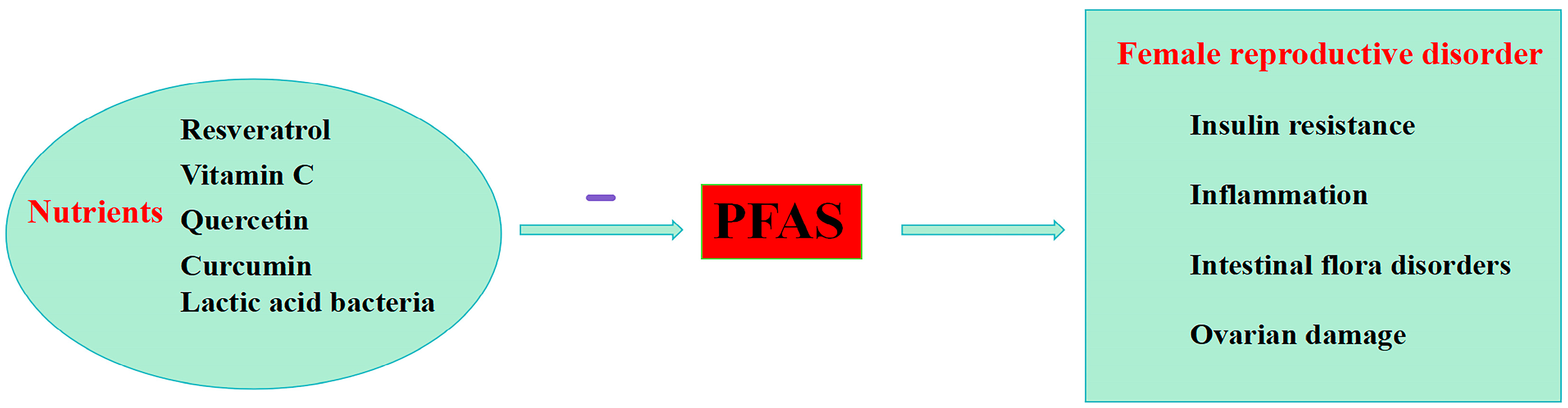
5. Nutritional Strategies to Reduce PFASs’ Effects in Female
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMH | Anti-mullerian hormone |
| ARC | Hypothalamic arcuate nucleus |
| AVPV | Anteroventral periventricular nucleus |
| AFC | Antral follicle count |
| Bcl-2/Bax | B-cell lymphoma 2/Bcl-2-associated X protein |
| BPA | Bisphenol A |
| C=F bonds | Carbon-fluorine bonds |
| COX-2 | Cyclooxygenase-2 |
| CYP17A1 | 17α-hydroxylase |
| CYP19A1 | Cytochrome P450 aromatase |
| DOR | Diminished ovarian reserve |
| E2 | Estradiol |
| EDCs | Endocrine-disrupting chemicals |
| ER | Estrogen receptor |
| FGF21 | Fibroblast growth factor 21 |
| FSH | Follicle-stimulating hormone |
| FT3 | Free triiodothyronine |
| FT4 | Free thyroxine |
| GenX | Hexafluoropropylene oxide dimer acid |
| GnRH | Gonadotropin-releasing hormone |
| HFPO-DA | Hexafluoropropylene oxide dimer acids |
| HPG axis | Hypothalamic-Pituitary-Gonadal Axis |
| Kiss1r | Kisspeptin receptor |
| LH | Luteinizing hormone |
| MtROS | Mitochondrial ROS |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NOR | Normal ovarian reserve |
| P450scc | Cholesterol side-chain cleavage enzyme |
| PCBs | Polychlorinated biphenyls |
| PCOS | Polycystic ovary syndrome |
| PFCAs | Perfluoroalkyl carboxylic acids |
| PFASs | Per- and polyfluoroalkyl substances |
| PFBA | Perfluorobutanoic acid |
| PFBS | Perfluorobutanesulfonic acid |
| PFDA | Perfluoroundecanoic acid |
| PFDoA | Perfluorododecanoic acid |
| PFDoDA | Perfluorododecanoic acid |
| PFHxA | Perfluorohexanoic acid |
| PFHxS | Perfluorohexane sulfonate |
| PFNA | Perfluorononanoic acid |
| PFOA | Perfluorooctanoic acid |
| PFOS | Perfluorooctane sulfonate |
| PFPeA | Perfluoropentanoic acid |
| PFUnDA | Perfluoroundecanoic acid |
| PFHxS | Perfluorohexane sulfonate |
| PFOS | Perfluorooctane sulfonate |
| PFSAs | Perfluoroalkane sulfonates |
| PFPeA | Perfluoropentanoic acid |
| POI | Premature ovarian insufficiency |
| P4 | Progesterone |
| PGE2 | Prostaglandin E2 |
| PPARs | Peroxisome proliferators-activated receptors |
| PRL | Prolactin |
| SCFAs | Short-chain fatty acids |
| T | Testosterone |
| T4 | Thyroxine |
| TSH | Thyroid-stimulating hormone |
| UCA | Urocanic acid |
| VEGF | Vascular endothelial growth factor |
| 17β-HSD | 17β-hydroxysteroid dehydrogenase |
| 3β-HSD | 3β-hydroxysteroid dehydrogenase |
| 6:2 Cl-PFESA | 6:2 chlorinated polyfluorinated ether sulfonate |
References
- Wallington, T.J.; Andersen, M.P.S.; Nielsen, O.J. The Case for a More Precise Definition of Regulated PFAS. Environ. Sci. Process. Impacts 2021, 23, 1834–1838. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; De Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; Van Leeuwen, S.P. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Maroli, A.; Tharayil, N.; Karanfil, T. The Overlooked Short- and Ultrashort-Chain Poly- and Perfluorinated Substances: A Review. Chemosphere 2019, 220, 866–882. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Buha Djordjevic, A.; Saso, L. PFAS Molecules: A Major Concern for the Human Health and the Environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.J.B.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.U.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J.; et al. Per- and Polyfluoroalkyl Substances in the Environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef] [PubMed]
- Podder, A.; Sadmani, A.H.M.A.; Reinhart, D.; Chang, N.-B.; Goel, R. Per and Poly-Fluoroalkyl Substances (PFAS) as a Contaminant of Emerging Concern in Surface Water: A Transboundary Review of Their Occurrences and Toxicity Effects. J. Hazard. Mater. 2021, 419, 126361. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Taniyasu, S.; Yamazaki, E.; Wu, R.; Lam, P.K.S.; Eun, H.; Yamashita, N. Fluorine Mass Balance Analysis and Per- and Polyfluoroalkyl Substances in the Atmosphere. J. Hazard. Mater. 2022, 435, 129025. [Google Scholar] [CrossRef]
- Bangma, J.; Guillette, T.C.; Bommarito, P.A.; Ng, C.; Reiner, J.L.; Lindstrom, A.B.; Strynar, M.J. Understanding the Dynamics of Physiological Changes, Protein Expression, and PFAS in Wildlife. Environ. Int. 2022, 159, 107037. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.O.; Armitage, J.M.; Bruton, T.A.; Dassuncao, C.; Heiger-Bernays, W.; Hu, X.C.; Kärrman, A.; Kelly, B.; Ng, C.; Robuck, A.; et al. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2021, 40, 631–657. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risk to Human Health Related to the Presence of Perfluorooctane Sulfonic Acid and Perfluorooctanoic Acid in Food. EFSA J. 2018, 16, e05194. [Google Scholar] [CrossRef]
- Giffard, N.G.; Gitlin, S.A.; Rardin, M.; Petali, J.M.; Chen, C.Y.; Romano, M.E. Occurrence and Risks of Per- and Polyfluoroalkyl Substances in Shellfish. Curr. Environ. Health Rep. 2022, 9, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Wee, S.Y.; Aris, A.Z. Environmental Impacts, Exposure Pathways, and Health Effects of PFOA and PFOS. Ecotoxicol. Environ. Saf. 2023, 267, 115663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-T.; Zeeshan, M.; Su, F.; Qian, Z.-M.; Dee Geiger, S.; Edward McMillin, S.; Wang, Z.-B.; Dong, P.-X.; Ou, Y.-Q.; Xiong, S.-M.; et al. Associations between Both Legacy and Alternative Per- and Polyfluoroalkyl Substances and Glucose-Homeostasis: The Isomers of C8 Health Project in China. Environ. Int. 2022, 158, 106913. [Google Scholar] [CrossRef] [PubMed]
- UNEP. Stockholm Convention on Persistent Organic Pollutants (POPs). 2021. Available online: https://chm.pops.int/TheConvention/POPsReviewCommittee/Meetings/POPRC16/Overview/tabid/8472/Default.aspx (accessed on 5 May 2024).
- UNEP. Stockholm Convention on Persistent Organic Pollutants (POPs). 2022. Available online: http://chm.pops.int/TheConvention/POPsReviewCommittee/Meetings/POPRC17/Overview/tabid/8900/Default.aspx (accessed on 5 May 2024).
- Brendel, S.; Fetter, É.; Staude, C.; Vierke, L.; Biegel-Engler, A. Short-Chain Perfluoroalkyl Acids: Environmental Concerns and a Regulatory Strategy under REACH. Environ. Sci. Eur. 2018, 30, 9. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef]
- Silva, A.B.P.; Carreiró, F.; Ramos, F.; Sanches-Silva, A. The Role of Endocrine Disruptors in Female Infertility. Mol. Biol. Rep. 2023, 50, 7069–7088. [Google Scholar] [CrossRef] [PubMed]
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and Poly-Fluoroalkyl Substances (PFAS) and Female Reproductive Outcomes: PFAS Elimination, Endocrine-Mediated Effects, and Disease. Toxicology 2022, 465, 153031. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and Infertility: Definition and Epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, W.; Wang, L.; Zhang, Y.; Zhang, Y.; Wang, M.; Wang, Y.; Li, P. A Review of Sources, Multimedia Distribution and Health Risks of Novel Fluorinated Alternatives. Ecotoxicol. Environ. Saf. 2019, 182, 109402. [Google Scholar] [CrossRef]
- Chambers, W.S.; Hopkins, J.G.; Richards, S.M. A Review of Per- and Polyfluorinated Alkyl Substance Impairment of Reproduction. Front. Toxicol. 2021, 3, 732436. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, Z.; Li, M.; Dong, H.; Li, J. Reproductive Toxicity of PFOA, PFOS and Their Substitutes: A Review Based on Epidemiological and Toxicological Evidence. Environ. Res. 2024, 250, 118485. [Google Scholar] [CrossRef] [PubMed]
- Roepke, T.A.; Sadlier, N.C. REPRODUCTIVE TOXICOLOGY: Impact of Endocrine Disruptors on Neurons Expressing GnRH or Kisspeptin and Pituitary Gonadotropins. Reproduction 2021, 162, F131–F145. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Harlow, S.D.; Randolph, J.F., Jr.; Loch-Caruso, R.; Park, S.K. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) and Their Effects on the Ovary. Hum. Reprod. Update 2020, 26, 724–752. [Google Scholar] [CrossRef] [PubMed]
- Grønnestad, R.; Johanson, S.M.; Müller, M.H.B.; Schlenk, D.; Tanabe, P.; Krøkje, Å.; Jaspers, V.L.B.; Jenssen, B.M.; Ræder, E.M.; Lyche, J.L.; et al. Effects of an Environmentally Relevant PFAS Mixture on Dopamine and Steroid Hormone Levels in Exposed Mice. Toxicol. Appl. Pharmacol. 2021, 428, 115670. [Google Scholar] [CrossRef] [PubMed]
- Dangudubiyyam, S.V.; Mishra, J.S.; Kumar, S. Perfluorooctane Sulfonic Acid Modulates Expression of Placental Steroidogenesis-Associated Genes and Hormone Levels in Pregnant Rats. Reprod. Toxicol. 2023, 118, 108390. [Google Scholar] [CrossRef] [PubMed]
- Fort, D.J.; Mathis, M.B.; Fort, C.E.; Fort, H.M.; Fort, T.D.; Guiney, P.D.; Weeks, J.A. Effect of Perfluorooctanesulfonate Exposure on Steroid Hormone Levels and Steroidogenic Enzyme Activities in Juvenile Silurana tropicalis. J. Appl. Toxicol. 2019, 39, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, D.L.; Blackwell, B.R.; Cavallin, J.E.; Collins, J.; Hoang, J.X.; Hofer, R.N.; Houck, K.A.; Jensen, K.M.; Kahl, M.D.; Kutsi, R.N.; et al. Verification of In Vivo Estrogenic Activity for Four Per- and Polyfluoroalkyl Substances (PFAS) Identified as Estrogen Receptor Agonists via New Approach Methodologies. Environ. Sci. Technol. 2023, 57, 3794–3803. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The Effects of Oxidative Stress on Female Reproduction: A Review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Duszewska, A.M.; Kucharski, M.; Tyczyński, P.; Smolarczyk, R. Oxidative Stress in Polycystic Ovary Syndrome. Reproduction 2022, 164, F145–F154. [Google Scholar] [CrossRef]
- Xi, Y.; Zhang, Y.; Zhu, S.; Luo, Y.; Xu, P.; Huang, Z. PPAR-Mediated Toxicology and Applied Pharmacology. Cells 2020, 9, 352. [Google Scholar] [CrossRef]
- Komar, C.M. Peroxisome Proliferator-Activated Receptors (PPARs) and Ovarian Function—Implications for Regulating Steroidogenesis, Differentiation, and Tissue Remodeling. Reprod. Biol. Endocrinol. 2005, 3, 41. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic Ovary Syndrome: Definition, Aetiology, Diagnosis and Treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.J.M.; Norman, R.J.; Teede, H. The Management of Anovulatory Infertility in Women with Polycystic Ovary Syndrome: An Analysis of the Evidence to Support the Development of Global WHO Guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef]
- Merkin, S.S.; Phy, J.L.; Sites, C.K.; Yang, D. Environmental Determinants of Polycystic Ovary Syndrome. Fertil. Steril. 2016, 106, 16–24. [Google Scholar] [CrossRef]
- Gautam, R.; Prambil, A.M.; Patel, A.K.; Arora, T. Emerging Pollutants in Etiology and Pathophysiology of Polycystic Ovary Syndrome. Reprod. Toxicol. 2023, 123, 108515. [Google Scholar] [CrossRef] [PubMed]
- Rumph, J.T.; Stephens, V.R.; Martin, J.L.; Brown, L.K.; Thomas, P.L.; Cooley, A.; Osteen, K.G.; Bruner-Tran, K.L. Uncovering Evidence: Associations between Environmental Contaminants and Disparities in Women’s Health. Int. J. Environ. Res. Public Health 2022, 19, 1257. [Google Scholar] [CrossRef] [PubMed]
- Vagi, S.J.; Azziz-Baumgartner, E.; Sjödin, A.; Calafat, A.M.; Dumesic, D.; Gonzalez, L.; Kato, K.; Silva, M.J.; Ye, X.; Azziz, R. Exploring the Potential Association between Brominated Diphenyl Ethers, Polychlorinated Biphenyls, Organochlorine Pesticides, Perfluorinated Compounds, Phthalates, and Bisphenol a in Polycystic Ovary Syndrome: A Case–Control Study. BMC Endocr. Disord. 2014, 14, 86. [Google Scholar] [CrossRef]
- Heffernan, A.L.; Cunningham, T.K.; Drage, D.S.; Aylward, L.L.; Thompson, K.; Vijayasarathy, S.; Mueller, J.F.; Atkin, S.L.; Sathyapalan, T. Perfluorinated Alkyl Acids in the Serum and Follicular Fluid of UK Women with and without Polycystic Ovarian Syndrome Undergoing Fertility Treatment and Associations with Hormonal and Metabolic Parameters. Int. J. Hyg. Environ. Health 2018, 221, 1068–1075. [Google Scholar] [CrossRef]
- Zhan, W.; Qiu, W.; Ao, Y.; Zhou, W.; Sun, Y.; Zhao, H.; Zhang, J. Environmental Exposure to Emerging Alternatives of Per- and Polyfluoroalkyl Substances and Polycystic Ovarian Syndrome in Women Diagnosed with Infertility: A Mixture Analysis. Environ. Health Perspect. 2023, 131, 057001. [Google Scholar] [CrossRef]
- Radke, E.G.; Christensen, K. Invited Perspective: Challenges in Evaluating the Effect of Per- and Polyfluoroalkyl Substance Mixtures on Polycystic Ovarian Syndrome. Environ. Health Perspect. 2023, 131, 051301. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, G.; Lin, Y.; Sun, F.; Zheng, L.; Yu, Y.; Xu, H. Association between Perfluoroalkyl Substances in Follicular Fluid and Polycystic Ovary Syndrome in Infertile Women. Toxics 2024, 12, 104. [Google Scholar] [CrossRef]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, Pathobiology, and Therapeutic Prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef] [PubMed]
- Louis, G.M.B.; Peterson, C.M.; Chen, Z.; Hediger, M.L.; Croughan, M.S.; Sundaram, R.; Stanford, J.B.; Fujimoto, V.Y.; Varner, M.W.; Giudice, L.C.; et al. Perfluorochemicals and Endometriosis: The ENDO Study. Epidemiology 2012, 23, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Raza, M.; Pollack, A.Z. Perfluoroalkyl Substances and Endometriosis in US Women in NHANES 2003–2006. Reprod. Toxicol. 2016, 65, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, R.; Jin, F.; Lou, H.; Mao, Y.; Zhu, W.; Zhou, W.; Zhang, P.; Zhang, J. Perfluoroalkyl Substances and Endometriosis-Related Infertility in Chinese Women. Environ. Int. 2017, 102, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.; Zhang, R.; Huo, X.; Zhu, W.; Zhang, J. Environmental Exposure to Legacy and Emerging Per- and Polyfluoroalkyl Substances and Endometriosis in Women of Childbearing Age. Sci. Total Environ. 2024, 907, 167838. [Google Scholar] [CrossRef] [PubMed]
- McGlacken-Byrne, S.M.; Conway, G.S. Premature Ovarian Insufficiency. Best Pract. Res. Clin. Obstet. Gynaecol. 2022, 81, 98–110. [Google Scholar] [CrossRef]
- Chon, S.J.; Umair, Z.; Yoon, M.-S. Premature Ovarian Insufficiency: Past, Present, and Future. Front. Cell Dev. Biol. 2021, 9, 672890. [Google Scholar] [CrossRef]
- Tsiligiannis, S.; Panay, N.; Stevenson, J.C. Premature Ovarian Insufficiency and Long-Term Health Consequences. Curr. Vasc. Pharmacol. 2019, 17, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Szeliga, A.; Calik-Ksepka, A.; Maciejewska-Jeske, M.; Grymowicz, M.; Smolarczyk, K.; Kostrzak, A.; Smolarczyk, R.; Rudnicka, E.; Meczekalski, B. Autoimmune Diseases in Patients with Premature Ovarian Insufficiency—Our Current State of Knowledge. Int. J. Mol. Sci. 2021, 22, 2594. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, Y.; Ma, J.; Ma, H.; Liang, X. Potential Factors Result in Diminished Ovarian Reserve: A Comprehensive Review. J. Ovarian Res. 2023, 16, 208. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Yan, W.; Zhou, T.; Shen, W.; Wang, T.; Li, M.; Zhou, S.; Wu, M.; Dai, J.; Huang, K.; et al. Endocrine Disrupting Chemicals Impact on Ovarian Aging: Evidence from Epidemiological and Experimental Evidence. Environ. Pollut. 2022, 305, 119269. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Xiang, W. Mechanisms of Ovarian Aging in Women: A Review. J. Ovarian Res. 2023, 16, 67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tan, R.; Pan, R.; Xiong, J.; Tian, Y.; Wu, J.; Chen, L. Association of Perfluoroalkyl and Polyfluoroalkyl Substances With Premature Ovarian Insufficiency in Chinese Women. J. Clin. Endocrinol. Metab. 2018, 103, 2543–2551. [Google Scholar] [CrossRef] [PubMed]
- Penzias, A.; Azziz, R.; Bendikson, K.; Falcone, T.; Hansen, K.; Hill, M.; Hurd, W.; Jindal, S.; Kalra, S.; Mersereau, J.; et al. Testing and Interpreting Measures of Ovarian Reserve: A Committee Opinion. Fertil. Steril. 2020, 114, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Wang, Y.; Zheng, L.; Lu, D.; Wu, J.; Wu, B.; Wu, Z.; Jiang, Z. Effect of Folic Acid Supplementation on Diminished Ovarian Reserve: Study Protocol of a Single-Centre, Open-Label, Randomised, Placebo-Controlled Clinical Trial. BMJ Open 2022, 12, e057689. [Google Scholar] [CrossRef] [PubMed]
- Björvang, R.D.; Hallberg, I.; Pikki, A.; Berglund, L.; Pedrelli, M.; Kiviranta, H.; Rantakokko, P.; Ruokojärvi, P.; Lindh, C.H.; Olovsson, M.; et al. Follicular Fluid and Blood Levels of Persistent Organic Pollutants and Reproductive Outcomes among Women Undergoing Assisted Reproductive Technologies. Environ. Res. 2022, 208, 112626. [Google Scholar] [CrossRef]
- Tian, T.; Hao, Y.; Wang, Y.; Xu, X.; Long, X.; Yan, L.; Zhao, Y.; Qiao, J. Mixed and Single Effects of Endocrine Disrupting Chemicals in Follicular Fluid on Likelihood of Diminished Ovarian Reserve: A Case-Control Study. Chemosphere 2023, 330, 138727. [Google Scholar] [CrossRef]
- Bellavia, A.; Zou, R.; Björvang, R.D.; Roos, K.; Sjunnesson, Y.; Hallberg, I.; Holte, J.; Pikki, A.; Lenters, V.; Portengen, L.; et al. Association between Chemical Mixtures and Female Fertility in Women Undergoing Assisted Reproduction in Sweden and Estonia. Environ. Res. 2023, 216 (Pt 1), 114447. [Google Scholar] [CrossRef]
- Shen, H.; Gao, M.; Li, Q.; Sun, H.; Jiang, Y.; Liu, L.; Wu, J.; Yu, X.; Jia, T.; Xin, Y.; et al. Effect of PFOA Exposure on Diminished Ovarian Reserve and Its Metabolism. Reprod. Biol. Endocrinol. 2023, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.; Zhuang, L.; Cui, W.; Lu, Q.; Yang, P.; Su, S.; Wang, B.; Zhang, G.; Chen, D. Per- and Polyfluoroalkyl Substances (PFAS) Exposure in Women Seeking in Vitro Fertilization-Embryo Transfer Treatment (IVF-ET) in China: Blood-Follicular Transfer and Associations with IVF-ET Outcomes. Sci. Total Environ. 2022, 838 Pt 3, 156323. [Google Scholar] [CrossRef]
- Xie, Q.; Kang, Y.; Zhang, C.; Xie, Y.; Wang, C.; Liu, J.; Yu, C.; Zhao, H.; Huang, D. The Role of Kisspeptin in the Control of the Hypothalamic-Pituitary-Gonadal Axis and Reproduction. Front. Endocrinol. 2022, 13, 925206. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Hu, J.; Huang, Z.; Yu, M.; Lu, C.; Wang, X.; Wu, D. Neonatal and Juvenile Exposure to Perfluorooctanoate (PFOA) and Perfluorooctane Sulfonate (PFOS): Advance Puberty Onset and Kisspeptin System Disturbance in Female Rats. Ecotoxicol. Environ. Saf. 2019, 167, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, Y.; Tang, C.; Cao, X.; Chang, F.; Chen, L. Impact of Perfluorooctane Sulfonate on Reproductive Ability of Female Mice through Suppression of Estrogen Receptor α-Activated Kisspeptin Neurons. Toxicol. Sci. 2018, 165, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, X.; Cao, X.; Xia, Y.; Zhou, R.; Chen, L. Chronic Exposure of Female Mice to an Environmental Level of Perfluorooctane Sulfonate Suppresses Estrogen Synthesis Through Reduced Histone H3K14 Acetylation of the StAR Promoter Leading to Deficits in Follicular Development and Ovulation. Toxicol. Sci. 2015, 148, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, T.; Galloway, T.S.; Melzer, D.; Holcroft, P.; Cipelli, R.; Pilling, L.C.; Mondal, D.; Luster, M.; Harries, L.W. Associations between PFOA, PFOS and Changes in the Expression of Genes Involved in Cholesterol Metabolism in Humans. Environ. Int. 2013, 57–58, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Choi, J.-S.; Park, J.-W. Transcriptional Changes in Steroidogenesis by Perfluoroalkyl Acids (PFOA and PFOS) Regulate the Synthesis of Sex Hormones in H295R Cells. Chemosphere 2016, 155, 436–443. [Google Scholar] [CrossRef]
- Yang, M.; Lee, Y.; Gao, L.; Chiu, K.; Meling, D.D.; Flaws, J.A.; Warner, G.R. Perfluorooctanoic Acid Disrupts Ovarian Steroidogenesis and Folliculogenesis in Adult Mice. Toxicol. Sci. 2022, 186, 260–268. [Google Scholar] [CrossRef]
- Chaparro-Ortega, A.; Betancourt, M.; Rosas, P.; Vázquez-Cuevas, F.G.; Chavira, R.; Bonilla, E.; Casas, E.; Ducolomb, Y. Endocrine Disruptor Effect of Perfluorooctane Sulfonic Acid (PFOS) and Perfluorooctanoic Acid (PFOA) on Porcine Ovarian Cell Steroidogenesis. Toxicol. Vitr. 2018, 46, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zeng, Z.; Liang, H.; Weng, X.; Yao, H.; Fu, Y.; Li, Y.; Chen, J.; Wei, X.; Jing, C. Association between Perfluoroalkyl and Polyfluoroalkyl Substances and Women’s Infertility, NHANES 2013–2016. Int. J. Environ. Res. Public Health 2022, 19, 15348. [Google Scholar] [CrossRef] [PubMed]
- Benninghoff, A.D.; Bisson, W.H.; Koch, D.C.; Ehresman, D.J.; Kolluri, S.K.; Williams, D.E. Estrogen-Like Activity of Perfluoroalkyl Acids In Vivo and Interaction with Human and Rainbow Trout Estrogen Receptors In Vitro. Toxicol. Sci. 2011, 120, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, H.; Feng, H.; Xue, Q.; Zhang, A.; Fu, J. Evaluation of the Estrogenic/Antiestrogenic Activities of Perfluoroalkyl Substances and Their Interactions with the Human Estrogen Receptor by Combining In Vitro Assays and In Silico Modeling. Environ. Sci. Technol. 2020, 54, 14514–14524. [Google Scholar] [CrossRef] [PubMed]
- Komar, C.M.; Braissant, O.; Wahli, W.; Curry, T.E. Expression and Localization of PPARs in the Rat Ovary During Follicular Development and the Periovulatory Period. Endocrinology 2001, 142, 4831–4838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tian, J.; Liu, W.; Zhou, J.; Zhang, Y.; Ding, L.; Sun, H.; Yan, G.; Sheng, X. Perfluorooctanoic Acid Exposure Leads to Defect in Follicular Development through Disrupting the Mitochondrial Electron Transport Chain in Granulosa Cells. Sci. Total Environ. 2023, 905, 166954. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Chen, L.; Qin, Y.; Xu, Y.; Tian, Y.; Chen, L. Exposure of Female Mice to Perfluorooctanoic Acid Suppresses Hypothalamic Kisspeptin-Reproductive Endocrine System through Enhanced Hepatic Fibroblast Growth Factor 21 Synthesis, Leading to Ovulation Failure and Prolonged Dioestrus. J. Neuroendocrinol. 2020, 32, e12848. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Bonato, M.; Corrà, F.; Bellio, M.; Guidolin, L.; Tallandini, L.; Irato, P.; Santovito, G. PFAS Environmental Pollution and Antioxidant Responses: An Overview of the Impact on Human Field. Int. J. Environ. Res. Public Health 2020, 17, 8020. [Google Scholar] [CrossRef]
- Hennig, B.; Petriello, M.C.; Gamble, M.V.; Surh, Y.-J.; Kresty, L.A.; Frank, N.; Rangkadilok, N.; Ruchirawat, M.; Suk, W.A. The Role of Nutrition in Influencing Mechanisms Involved in Environmentally Mediated Diseases. Rev. Environ. Health 2018, 33, 87–97. [Google Scholar] [CrossRef]
- Kamal, D.A.M.; Salamt, N.; Yusuf, A.N.M.; Kashim, M.I.A.M.; Mokhtar, M.H. Potential Health Benefits of Curcumin on Female Reproductive Disorders: A Review. Nutrients 2021, 13, 3126. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, H.Y.; Jeon, J.D.; Kho, Y.; Kim, S.-K.; Park, M.-S.; Hong, Y.-C. The Modifying Effect of Vitamin C on the Association between Perfluorinated Compounds and Insulin Resistance in the Korean Elderly: A Double-Blind, Randomized, Placebo-Controlled Crossover Trial. Eur. J. Nutr. 2016, 55, 1011–1020. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Cui, X. Influence of Dietary Bioactive Compounds on the Bioavailability and Excretion of PFOA and Its Alternative HFPO-TA: Mechanism Exploration. Sci. Total Environ. 2023, 899, 165560. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Qiu, P.; Li, F.; Wang, M.; Zheng, J.; Wang, Q.; Xu, F.; Xiao, H. Lactic Acid Bacteria Alleviates Polycystic Ovarian Syndrome by Regulating Sex Hormone Related Gut Microbiota. Food Funct. 2018, 9, 87–95. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, S.; Mei, C.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Capabilities of Bio-Binding, Antioxidant and Intestinal Environmental Repair Jointly Determine the Ability of Lactic Acid Bacteria to Mitigate Perfluorooctane Sulfonate Toxicity. Environ. Int. 2022, 166, 107388. [Google Scholar] [CrossRef]

| Disease | Sample Size (Experimental Group/Controls) | Results | Ref. |
|---|---|---|---|
| PCOS | 52/50 | Those with PFOA and PFOS serum concentrations in the highest tertile were 6.9- and 5.8-fold more likely to develop PCOS compared to controls, respectively. | [40] |
| PCOS | 30/29 | The geometric mean concentration of PFOS was higher in the PCOS group than in the control group. | [41] |
| PCOS | 366/577 | Higher concentrations of 6:2 Cl-PFESA, HFPO-DA, PFDoA, and ∑3,4,5 m-PFOS enhanced the risk of PCOS, especially in obese/overweight women. | [42] |
| PCOS | 73/218 | Compared to non-PCOS women, PCOS women had higher concentrations of LH and T in their follicular fluid, and PFOA is directly involved in the pathogenesis of PCOS. | [44] |
| Endometriosis | 495/131 | PFOA and PFNA raise the risk of endometriosis in women. | [47] |
| Endometriosis | 54/699 | Women with endometriosis have more PFOA, PFOS, and PFNA in their blood than women who do not suffer from this condition. | [48] |
| Endometriosis | 157/178 | PFOA and PFNA raise the risk of endometriosis in women. | [49] |
| Endometriosis | 240/344 | Women with endometriosis have more PFOA, PFOS, and PFNA in their blood than women who are not suffering from this condition. | [50] |
| POI | 120/120 | High exposure to PFOA, PFOS, and PFHxS is associated with increased risk of POI. These also impact sex hormone and thyroid hormone levels. | [58] |
| DOR | 64/86 | PFUnDA and PFOA were significantly negatively correlated with OSI. | [62] |
| DOR | 185/148 | PFHxA was strongly associated with an increased risk of DOR. | [63] |
| DOR | 25/25 | The DOR group had higher PFOA exposure than the group with normal ovarian reserve function. | [64] |
| Experimental Subjects | Results | Ref. |
|---|---|---|
| Sprague–Dawley female rats | Neonatal and juvenile exposure to PFOA/PFOS in female rats accelerates puberty onset, increases estradiol and LH levels, disrupts estrous cycles, and downregulates Kisspeptin system gene expression. | [67] |
| Twelve-week-old female ICR mice | PFOS exposure suppresses ERα-induced activation of AVPV–Kisspeptin neurons, leading to prolonged diestrus, reduced corpora lutea, and diminished LH surge, ultimately impairing ovulation in female mice. | [68] |
| Twelve-week-old female ICR mice | Chronic low-dose PFOS exposure in adult female mice disrupts reproductive endocrine function by reducing the histone acetylation of StAR, leading to decreased estrogen biosynthesis, impaired follicular development, and ovulation failure. | [69] |
| H295R cell | PFOA and PFOS weakly antagonize ER transactivation, alter steroid hormone levels by inducing aromatase activity, and influence the transcription of genes involved in sex hormone and aldosterone synthesis. | [71] |
| Adult female mice | PFOA disrupts ovarian function in mice both in vitro and in vivo, causing alterations in hormone levels, steroidogenic gene expression, and folliculogenesis, suggesting a potential risk for premature ovarian failure. | [72] |
| Porcine theca and granulosa cells | In vitro analysis reveals that PFOS and PFOA disrupt steroidogenic secretion in porcine ovarian cells, inhibiting hormone secretion even under gonadotropic stimulation. | [73] |
| Mt Shasta strain juvenile rainbow trout | PFAAs exhibit estrogen-like activity in juvenile rainbow trout and bind to estrogen receptors in various species. | [75] |
| MCF-7 BUS and MVLN cells | Various PFASs exhibit both estrogenic and antiestrogenic effects. | [76] |
| Six-week-old ICR mice | PFOA exposure impairs follicular development in mice, increases granulosa cell mtROS and apoptosis, and results in reduced follicular reserve. | [78] |
| Twelve-week-old female ICR mice | Exposure to PFOA in mice leads to the down-regulation of the Kissin–reproductive endocrine system via enhanced PPARα-mediated hepatic FGF21 expression, potentially resulting in prolonged luteal phase and ovulation failure. | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, Y.; Feng, Y.; Shi, Y.; Xiao, J.; Liu, M.; Wang, K. Per- and Polyfluoroalkyl Substances (PFASs) and Their Potential Effects on Female Reproductive Diseases. Toxics 2024, 12, 539. https://doi.org/10.3390/toxics12080539
Yi Y, Feng Y, Shi Y, Xiao J, Liu M, Wang K. Per- and Polyfluoroalkyl Substances (PFASs) and Their Potential Effects on Female Reproductive Diseases. Toxics. 2024; 12(8):539. https://doi.org/10.3390/toxics12080539
Chicago/Turabian StyleYi, Yuqing, Yang Feng, Yuechen Shi, Jiaming Xiao, Ming Liu, and Ke Wang. 2024. "Per- and Polyfluoroalkyl Substances (PFASs) and Their Potential Effects on Female Reproductive Diseases" Toxics 12, no. 8: 539. https://doi.org/10.3390/toxics12080539
APA StyleYi, Y., Feng, Y., Shi, Y., Xiao, J., Liu, M., & Wang, K. (2024). Per- and Polyfluoroalkyl Substances (PFASs) and Their Potential Effects on Female Reproductive Diseases. Toxics, 12(8), 539. https://doi.org/10.3390/toxics12080539






