Copper Sulfate Supplementation Alleviates Molybdenosis in the Tibetan Gazelles in the Qinghai Lake Basin
Abstract
:1. Introduction
2. Materials and Methods
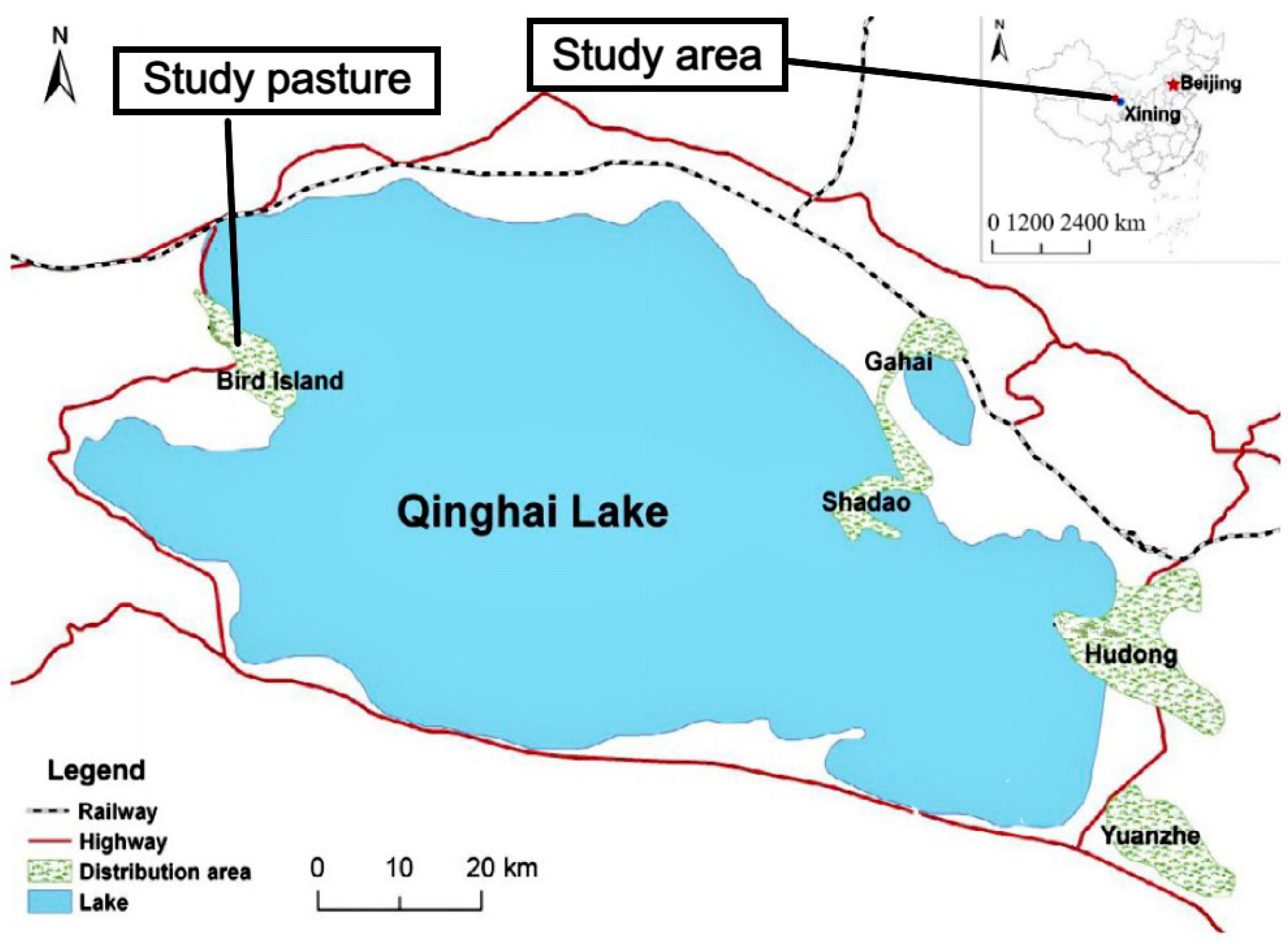
2.1. Study Pasture
2.2. Experimental Design
2.3. Sample Collection
2.4. Determination of Samples
2.5. Statistical Analyses
3. Results
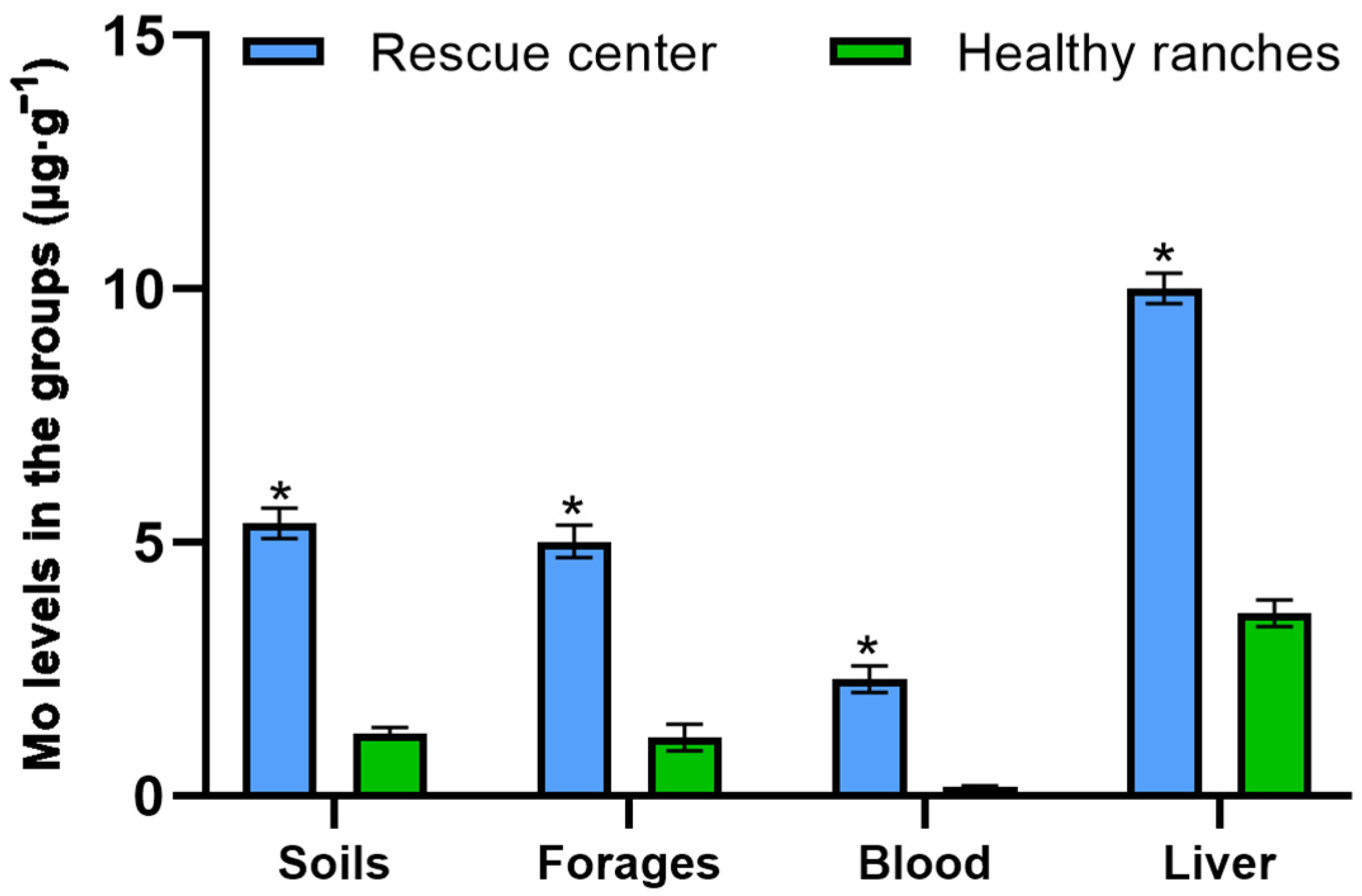
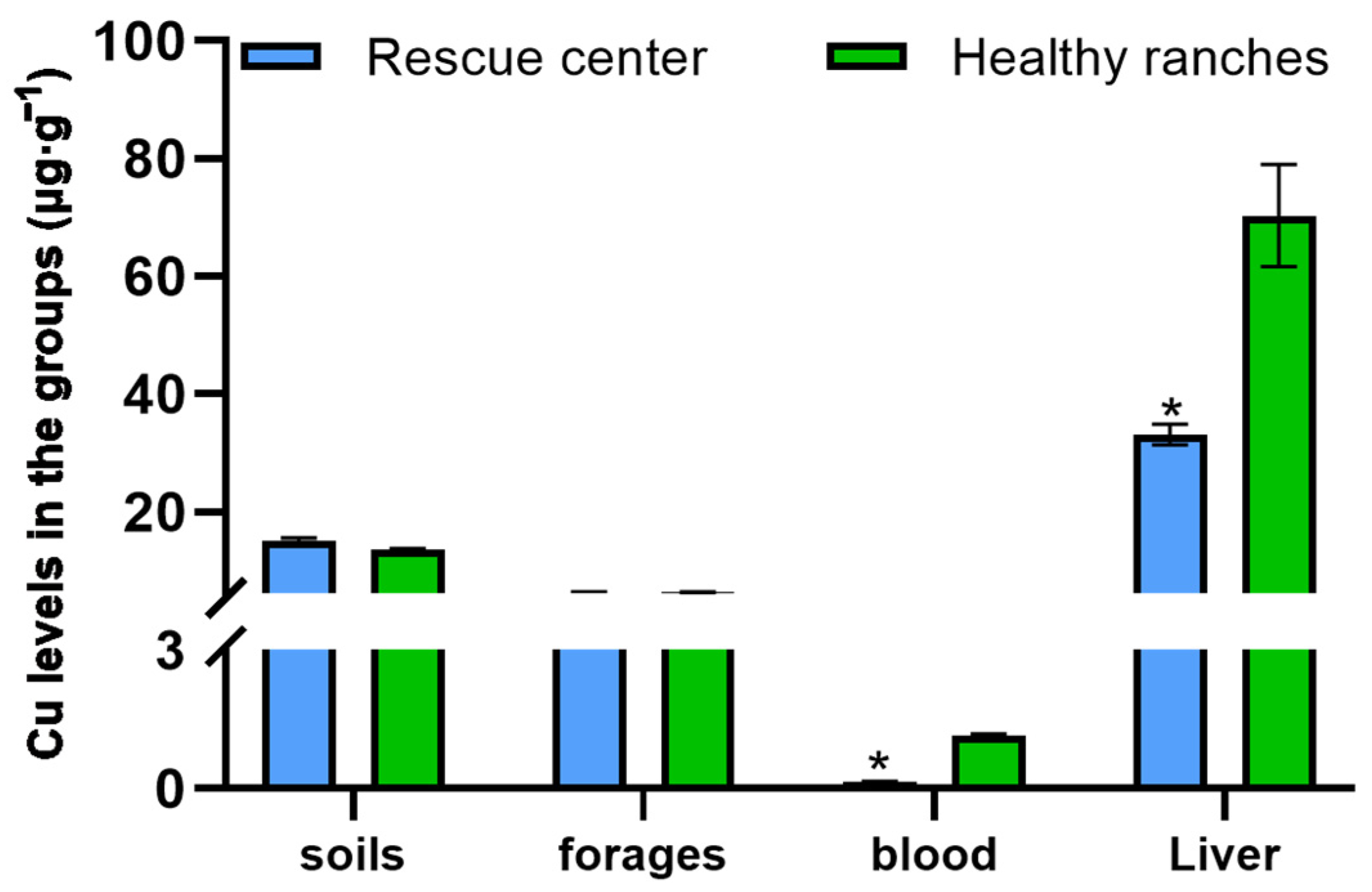
3.1. The Mineral Concentrations in Each Group
3.2. The Physiological Parameters in the Blood of the P. picticaudata
3.3. The Biochemical Parameters in the Blood of the P. picticaudata
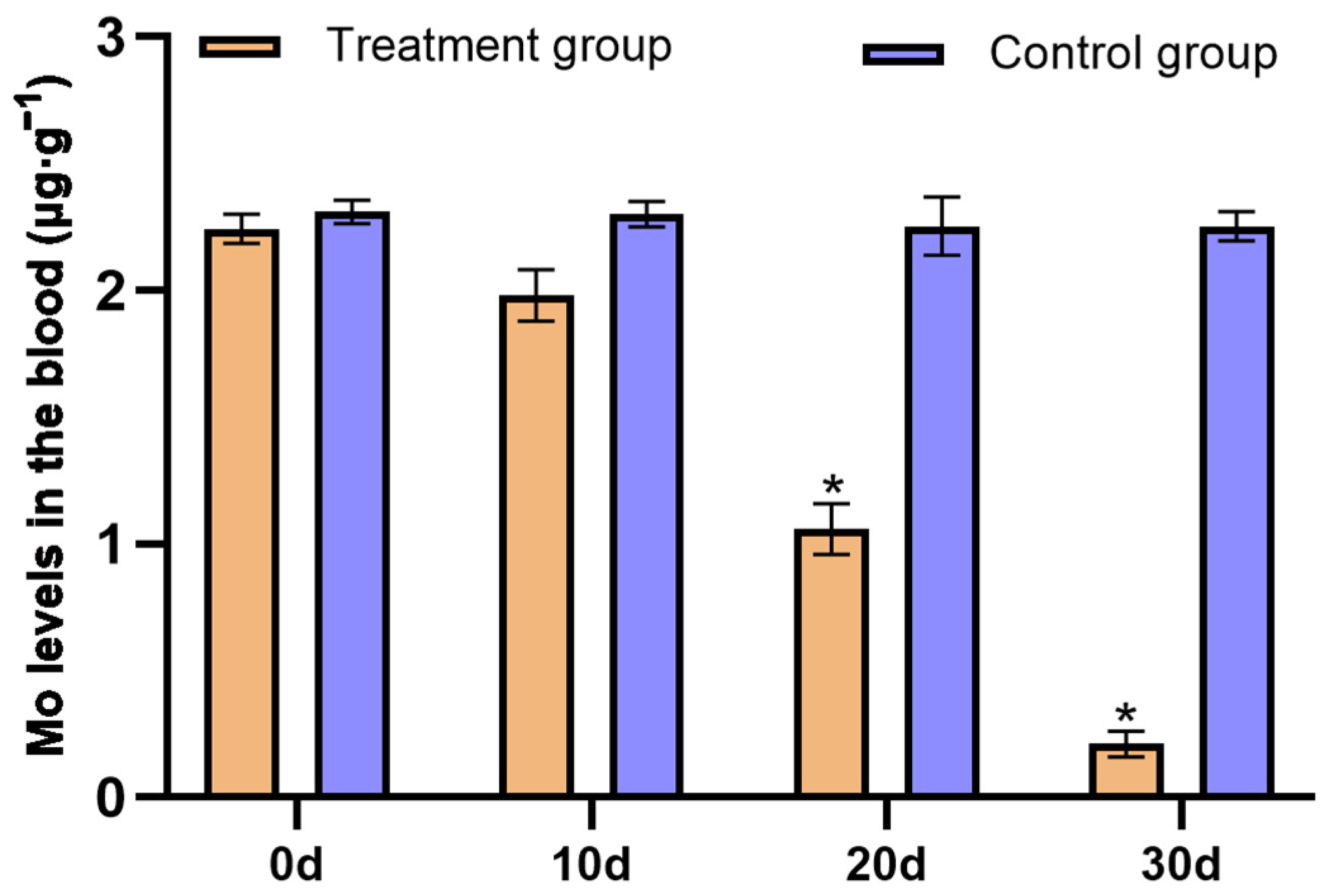
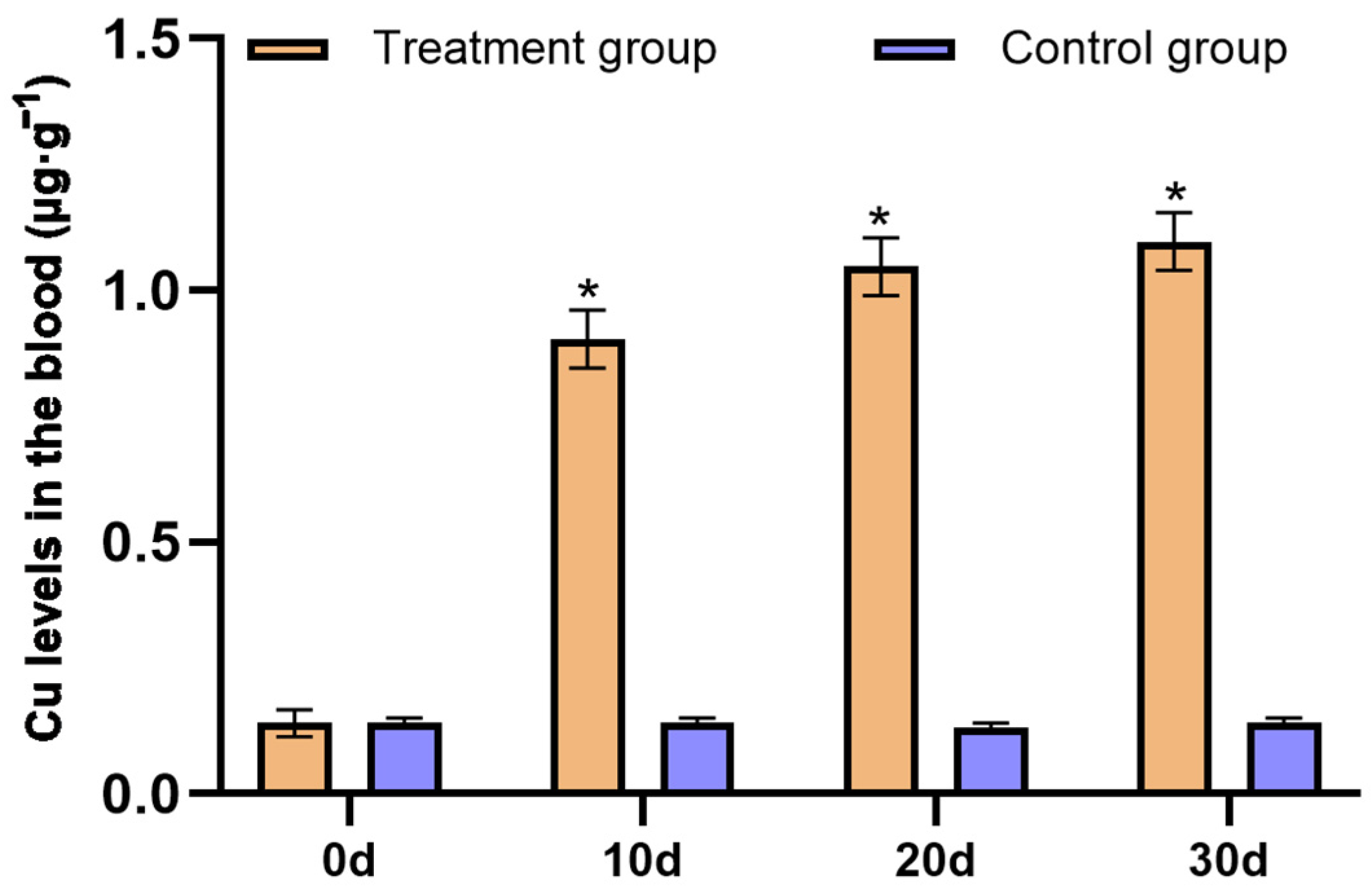
3.4. Effects of Replenishing CuSO4 on the P. picticaudata
4. Discussion
4.1. Effects on P. picticaudata in a High Molybdenum Environment
4.2. Supplying CuSO4 to Affected P. picticaudata
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Z.; Li, D.; Wang, Z. Population declines of Przewalski’s gazelle around Qinghai Lake, China. Oryx 2000, 34, 129–135. [Google Scholar] [CrossRef]
- Chi, Y.K.; Huo, B.; Shen, X.Y. Distribution characteristics of selenium nutrition on the natural habitat of Przewalski’s gazelle. Pol. J. Environ. Stud. 2019, 29, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.W.; Zhao, K.; Liu, F.Y.; Shen, X.Y. Studies of high molybdenum-induced copper deprivation in P. Przewalskii on the Qinghai Lake pasture in China. Appl. Sci. 2021, 11, 5071. [Google Scholar] [CrossRef]
- Shen, X.Y.; Jiang, Z.G. Serum biochemical values and mineral contents of tissues in Przewalski’s and Tibetan gazelles. Afr. J. Biotech. 2012, 11, 718–723. [Google Scholar]
- Shen, X.Y.; Huo, B.; Wu, T.; Song, C.J.; Chi, Y.K. iTRAQ-based proteomic analysis to identify molecular mechanisms of the selenium deficiency response in the Przewalski’s gazelle. J. Proteom. 2019, 203, 103389. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Jiang, Z.G.; Ping, X.G.; Cai, J.; You, Z.Q.; Li, W.C.; Wu, Y.L. Current status and conservation of the Endangered Przewalski’s gazelle Procapra przewalskii, endemic to the Qinghai-Tibetan Plateau, China. Oryx 2012, 46, 145–153. [Google Scholar] [CrossRef]
- Poor, E.E.; Jakes, A.; Loucks, C.; Suitor, M. Modeling fence location and density at a regional scale for use in wildlife management. PLoS ONE 2014, 9, e83912. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.J.; Johnson, J.L.; Rajagopalan, K.V. Molecular basis of the biological function of molybdenum. Developmental patterns of sulfite oxidase and xanthine oxidase in the rat. Arch. Biochem. Biophys. 1974, 164, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Presta, L.; Fondi, M.; Emiliani, G.; Fani, R. Molybdenum and biological systems (Molybdenum cofactors containing enzymes and pathways). Molybdenum Cofactors Their Role Evol. Metab. Pathw. 2015, 8, 21–31. [Google Scholar]
- Johnson, J.L.; Waud, W.R.; Cohen, H.J. Molecular basis of the biological function of molybdenum. J. Biol. Chem. 1974, 249, 5056–5061. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Zhao, J.; Mao, M.X.; Zhao, Z.Q.; Liu, F.J.; Wang, H.W. Disordered expression of tight junction proteins is involved in the Mo-induced intestinal microenvironment dysbiosis in sheep. Biol. Trace Elem. Res. 2023, 201, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Majak, W.; Steinke, D.; Mcgillivray, J.; Lysyk, T. Clinical signs in cattle grazing high molybdenum forage. J. Range Manag. 2004, 57, 269–274. [Google Scholar] [CrossRef]
- White, C.L.; Caldwalader, T.K.; Hoekstra, W.G.; Pope, A. Effects of copper and molybdenum supplements on the copper and selenium status of pregnant ewes and lambs. J. Anim. Sci. 1989, 67, 803–809. [Google Scholar] [CrossRef]
- Gooneratne, S.R.; Laarveld, B.; Pathirana, K.K.; Christensen, D.A. Effects of dietary Cu, Mo and S on urinary Cu and Zn excretion in Simmental and Angus cattle. Res. Vet. Sci. 2011, 91, e116–e120. [Google Scholar] [CrossRef]
- Van Ryssen, J.B.J.; Malsen, S.V.; Barrowman, P.R. Effect of dietary molybdenum and sulphur on the copper status of hypercu-protic sheep after withdrawal of dietary copper. S. Afr. J. Anim. Sci. 1986, 16, 77–82. [Google Scholar]
- Shen, X.Y.; Li, X.; Zhang, R.D. Studies of Unsteady Gait Disease of the Tibetan Gazelle (Procapra picticaudata). J. Wildl. Dis. 2010, 46, 560–563. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, Z. Climate change hastens the conservation urgency of an endangered ungulate. PLoS ONE 2011, 6, e22873. [Google Scholar] [CrossRef] [PubMed]
- Huo, B. Response Mechanism of Przewalski’s Gazelle (Procapra przewalskii) to Selenium Deprived Environment. Master’s Thesis, Southwest University of Science and Technology, Mianyang, China, 2020. [Google Scholar]
- Ward, G.M. Molybdenum toxicity and hypocuprosis in ruminants: A review. J. Anim. Sci. 1978, 46, 1078–1085. [Google Scholar] [CrossRef]
- Shen, X.Y.; Min, X.Y.; Zhang, S.H.; Song, C.J.; Xiong, K.N. Effect of heavy metal contamination in environment on antioxidant function in Wumeng semi-fine wool sheep in the Southwest China. Biol. Trace Elem. Res. 2020, 198, 505–514. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Cook, G.A.; Lesperance, A.L.; Bohman, V.R.; Jensen, E.H. Interrelationship of molybdenum and certain factors to the development of the molybdenum toxicity syndrome. J. Anim. Sci. 1966, 25, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Song, C.J.; Gan, S.Q.; Shen, X.Y. Effects of nano-copper poisoning on immune and antioxidant function in the Wumeng semi-fine wool sheep. Biol. Trace Elem. Res. 2020, 198, 515–520. [Google Scholar] [CrossRef]
- Mason, J. The relationship between copper, molybdenum and sulphur in ruminant and non-ruminant animals: A preview. Veter. Res. Commun. 1978, 2, 85–94. [Google Scholar] [CrossRef]
- Anke, M.; Holzinger, S.; Seifert, M.; Muller, R.; Schafer, U. The biological and toxicological importance of molybdenum in the environment and in the nutrition of plants, animals and man. Acta Biol. Hung. 2010, 39, 1–11. [Google Scholar]
- Cui, S.G.; Zhang, Y.L.; Guo, H.W.; Zhou, B.H.; Tian, E.J.; Zhao, J.; Lin, L.; Wang, H.W. Molybdenum-induced apoptosis of splenocytes and thymocytes and changes of peripheral blood in sheep. Biol. Trace Elem. Res. 2023, 201, 4389–4399. [Google Scholar] [CrossRef] [PubMed]
- Neilands, J.B.; Strong, F.M.; Elvehjem, C.A. Molybdenum in the nutrition of the rat. J. Biol. Chem. 1948, 172, 431–440. [Google Scholar] [CrossRef]
- Yatoo, M.I.; Saxena, A.; Deepa, P.M.; Habeab, B.P.; Dimri, U. Role of trace elements in animals: A review. Veter. World 2013, 6, 963–967. [Google Scholar] [CrossRef]
- Shen, X.Y.; Huo, B.; Gan, S.Q. Effects of nano-selenium on antioxidant capacityin Se-deprived Tibetan gazelle (Procapra picticaudata) in the Qinghai-Tibet plateau. Biol. Trace Elem. Res. 2021, 199, 981–988. [Google Scholar] [CrossRef]
- Qiu, J.; Zhou, P.; Shen, X.Y. Effects of Se-yeast on immune and antioxidant in the Se-deprived Pishan red sheep. Biol. Trace Elem. Res. 2021, 200, 2741–2749. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhou, P.; Shen, X.Y. Decreasing toxicity of heavy metal to the Altai Sheep by fertilized Nano-potassium molybdate. Pol. J. Environ. Stud. 2023, 32, 451–459. [Google Scholar] [CrossRef]
- Song, C.J.; Shen, X.Y. Effects of environmental zinc deficiency on antioxidant system function in Wumeng semi-fine wool sheep. Biol. Trace Elem. Res. 2020, 195, 110–116. [Google Scholar] [CrossRef]
- Huo, B.; Wu, T.; Song, C.J.; Shen, X.Y. Studies of selenium deficiency in the Wumeng semi-fine wool sheep. Biol. Trace Elem. Res. 2020, 194, 152–158. [Google Scholar] [CrossRef]
- Lucejko, M.; Flisiak, R. Effect of HCV core antigen and RNA clearance during the rapy with direct acting antivirals on hepatic stiffness measured with shear wave elastography in patients with chronic viral hepatitis C. Appl. Sci. 2018, 8, 198. [Google Scholar] [CrossRef]
- Ha, H.Y.; Alfulaij, N.; Berry, M.J.; Seale, L.A. From selenium absorption to selenoprotein degradation. Biol. Trace Elem. Res. 2019, 192, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Becana, M.; Moran, J.F.; Iturbe, O.T.I. Iron-dependent oxygen free radical generation in plants subjected to environmental stress: Toxicity and antioxidant protection. Plant Soil 1998, 201, 137–147. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Koppal, T.; Subramaniam, R.; Yatin, S. Vitamin E as an antioxidant free radical scavenger against amyloid beta-peptide-induced oxidative stress in neocortical synaptosomal membranes and hippocampal neurons in culture: Insights into Alzheimer’s disease. Rev. Neurosci. 1999, 10, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Komosinska, V.K.; Olczyk, K.; Kucharz, E.; Marcisz, C.; Kotulska, A. Free radical activity and antioxidant defense mechanisms in patients with hyperthyroidism due to Graves’ disease during the rapy. Clin. Chim. Acta 2000, 300, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Jakus, V. The role of free radicals, oxidative stress and antioxidant systems in diabetic vascular disease. Bratisl. Lek. Listy 2000, 101, 541–551. [Google Scholar]
- Xie, Z.Z.; Liu, Y.; Bian, J.S. Hydrogen sulfide and cellular redox homeostasis. Oxid. Med. Cell. Longev. 2016, 2016, 6043038. [Google Scholar] [CrossRef]
- Cavas, L.; Tarhan, L. Glutathione redox system, GSH-Px activity and lipid peroxidation (LPO) levels in tadpoles of ridibunda and viridis. Cell Biochem. Funct. 2010, 21, 75–79. [Google Scholar] [CrossRef]
- Cozza, G.; Rossetto, M.; Bosello-Travain, V.; Maiorino, M.; Roveri, A.; Toppo, S.; Zaccarin, M.; Zennaro, L.; Ursini, F. Glutathione peroxidase 4-catalyzed reduction of lipid hydroperoxides in membranes: The polar head of membrane phospholipids binds the enzyme and addresses the fatty acid hydroperoxide group toward the redox center. Free Rad. Biol. Med. 2017, 112, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Huo, B.; Shen, X.Y. Studies on antioxidant capacity in selenium-deprived the Choko yak in the Shouqu prairie. Biol. Trace Elem. Res. 2021, 199, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Li, J.J.; Wang, D.Q.; Li, G.Q.; Wang, G.L.; Lu, L.Z. Effects of heat stress on antioxidant defense system, inflammatory injury, and heat shock proteins of Muscovy and Pekin ducks: Evidence for differential thermal sensitivities. Cell Stress. Chaperon 2014, 19, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Kheradmand, A.; Gholami, M.; Saidi, S.H.; Mirhadi, S.A. Effect of royal jelly on testicular antioxidant enzymes activity, MDA level and spermatogenesis in rat experimental Varicocele model. Tissue Cell 2019, 57, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Çelebi, Ş. Effect of dietary vitamin E, selenium and their combination on concentration of selenium, MDA, and antioxidant enzyme activities in some tissues of laying hens. Pak. J. Zool. 2019, 51, 1155–1161. [Google Scholar] [CrossRef]
- Liu, G.Y.; Shen, X.Y. Study on Soil Selenium-Induced Copper Deficiency in Yudong Black Goats. Animals 2024, 14, 1481. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Shen, X.Y. Cadmium exposure affects serum metabolites and proteins in the male Guizhou black goat. Animals 2023, 13, 2705. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Shen, X.Y. Effects of cadmium on liver function and its metabolomics profile in the Guizhou black goat. Metabolites 2023, 13, 268. [Google Scholar] [CrossRef]
- Gong, J.B. Etiological analysis, diagnosis and prevention of molybdenum poisoning in cattle and sheep. Mod. Anim. Husb. Sci. Technol. 2020, 01, 124–126. [Google Scholar]
| Nutrient Components | Rescue Center | Healthy Ranches |
|---|---|---|
| DM | 94.04 ± 2.51 | 94.58 ± 2.39 |
| CP | 16.67 ± 0.79 | 16.42 ± 0.87 |
| EE | 4.33 ± 0.38 | 4.39 ± 0.35 |
| CF | 19.69 ± 1.52 | 19.77 ± 1.47 |
| CA | 9.52 ± 0.76 | 9.68 ± 0.73 |
| NFE | 44.13 ± 1.15 | 45.07 ± 1.21 |
| NDF | 44.61 ± 0.29 | 44.77 ± 0.39 |
| ADF | 26.73 ± 1.63 | 26.91 ± 1.68 |
| Parameters | Affected Animals | Healthy Animals |
|---|---|---|
| Hb (g·L−1) | 81.6 ± 7.59 * | 117 ± 7.60 |
| RBC (1012 L−1) | 6.54 ± 0.41 | 6.45 ± 0.53 |
| PCV (%) | 35.9 ± 3.67 * | 51.3 ± 7.30 |
| MCV (fL−1) | 45.5 ± 3.16 * | 53.9 ± 2.04 |
| MCH (pg) | 9.93 ± 1.44 * | 14.73 ± 0.84 |
| MCHC (%) | 22.9 ± 1.70 | 22.1 ± 1.67 |
| WBC (109 L−1) | 8.31 ± 0.59 | 8.03 ± 0.64 |
| Parameters | Affected Animals | Healthy Animals |
|---|---|---|
| Cp (mg·L−1) | 3.48 ± 11.69 * | 6.82 ± 11.31 * |
| ALB (g·L−1) | 20.1 ± 3.17 * | 30.4 ± 2.17 |
| ALP (U·L−1) | 941 ± 96 * | 635 ± 65 |
| ALT (U·L−1) | 45.1 ± 5.18 | 43.1 ± 3.24 |
| AST (U·L−1) | 127 ± 5.49 * | 84.40 ± 12.1 |
| CPK (U·L−1) | 386 ± 39 * | 250 ± 12 |
| CR (µmoL·L−1) | 64.5 ± 6.17 | 63.2 ± 3.46 |
| Chol (mmol·L−1) | 1.79 ± 0.12 | 1.72 ± 0.19 |
| GLB (g·L−1) | 14.9 ± 3.32 * | 24.6 ± 4.26 |
| LDH (U·L−1) | 536 ± 31 * | 420 ± 33 |
| GSH-Px (U·mL−1) | 134 ± 16 * | 329 ± 20 |
| SOD (U·mL−1) | 35.4 ± 3.37 * | 92.7 ± 8.85 |
| CAT (U·mL−1) | 2.96 ± 0.30 * | 13.31 ± 1.17 |
| T-AOC (U·mL−1) | 2.10 ± 0.08 * | 5.49 ± 0.31 |
| MDA (nmol·L−1) | 41.5 ± 2.68 * | 14.2 ± 1.28 |
| TP (g·L−1) | 37.1 ± 4.94 * | 54.3 ± 3.97 |
| Parameters | Treatment Group | Control Group |
|---|---|---|
| Hb (g·L−1) | 99.7 ± 8.61 * | 83.2 ± 7.47 |
| PCV (%) | 46.9 ± 3.52 * | 34.5 ± 3.38 |
| MCV (fL−1) | 48.5 ± 4.03 | 44.2 ± 3.21 |
| MCH (pg) | 12.9 ± 1.39 * | 9.7 ± 1.19 |
| Parameters | Treatment Group | Control Group |
|---|---|---|
| Cp (mg·L−1) | 5.21 ± 1.69 * | 3.72 ± 1.51 |
| ALB (g·L−1) | 28.2 ± 3.02 * | 20.3 ± 2.21 |
| ALP (U·L−1) | 679 ± 71 * | 933 ± 89 |
| AST (U·L−1) | 103 ± 6.41 * | 128 ± 5.31 |
| CPK (U·L−1) | 339 ± 28 * | 388 ± 24 |
| GLB (g·L−1) | 20.3 ± 3.17 * | 14.6 ± 3.14 |
| LDH (U·L−1) | 470 ± 27 * | 539 ± 27 |
| GSH-Px (U·mL−1) | 239 ± 28 * | 132 ± 17 |
| SOD (U·mL−1) | 60.1 ± 5.39 * | 36.2 ± 3.29 |
| CAT (U·mL−1) | 7.66 ± 1.31 * | 2.86 ± 0.29 |
| T-AOC (U·mL−1) | 4.11 ± 0.12 * | 2.17 ± 0.11 |
| MDA (nmol·L−1) | 20.4 ± 3.18 * | 42.3 ± 3.18 |
| TP (g·L−1) | 46.1 ± 5.64 * | 36.9 ± 4.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Shen, X. Copper Sulfate Supplementation Alleviates Molybdenosis in the Tibetan Gazelles in the Qinghai Lake Basin. Toxics 2024, 12, 546. https://doi.org/10.3390/toxics12080546
Liu G, Shen X. Copper Sulfate Supplementation Alleviates Molybdenosis in the Tibetan Gazelles in the Qinghai Lake Basin. Toxics. 2024; 12(8):546. https://doi.org/10.3390/toxics12080546
Chicago/Turabian StyleLiu, Guangyang, and Xiaoyun Shen. 2024. "Copper Sulfate Supplementation Alleviates Molybdenosis in the Tibetan Gazelles in the Qinghai Lake Basin" Toxics 12, no. 8: 546. https://doi.org/10.3390/toxics12080546
APA StyleLiu, G., & Shen, X. (2024). Copper Sulfate Supplementation Alleviates Molybdenosis in the Tibetan Gazelles in the Qinghai Lake Basin. Toxics, 12(8), 546. https://doi.org/10.3390/toxics12080546






