Plastic Pollution in Paradise: Analyzing Plastic Litter on Malta’s Beaches and Assessing the Release of Potentially Toxic Elements
Abstract
1. Introduction
2. Materials and Methods
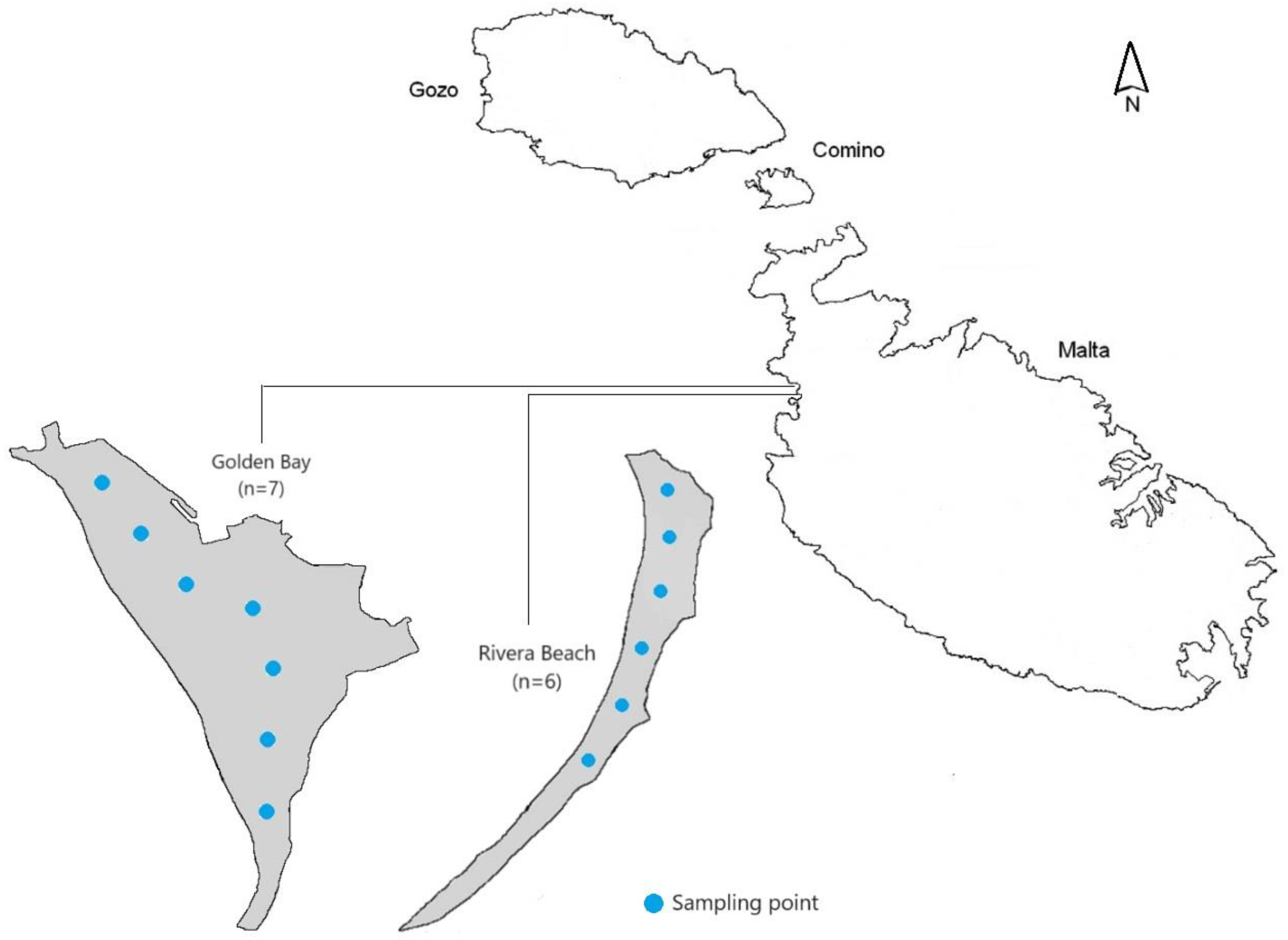
2.1. Study Area
2.2. Sampling
2.3. Sample Processing
2.4. Potentially Toxic Elements
2.5. Statistical Analysis
3. Results and Discussion
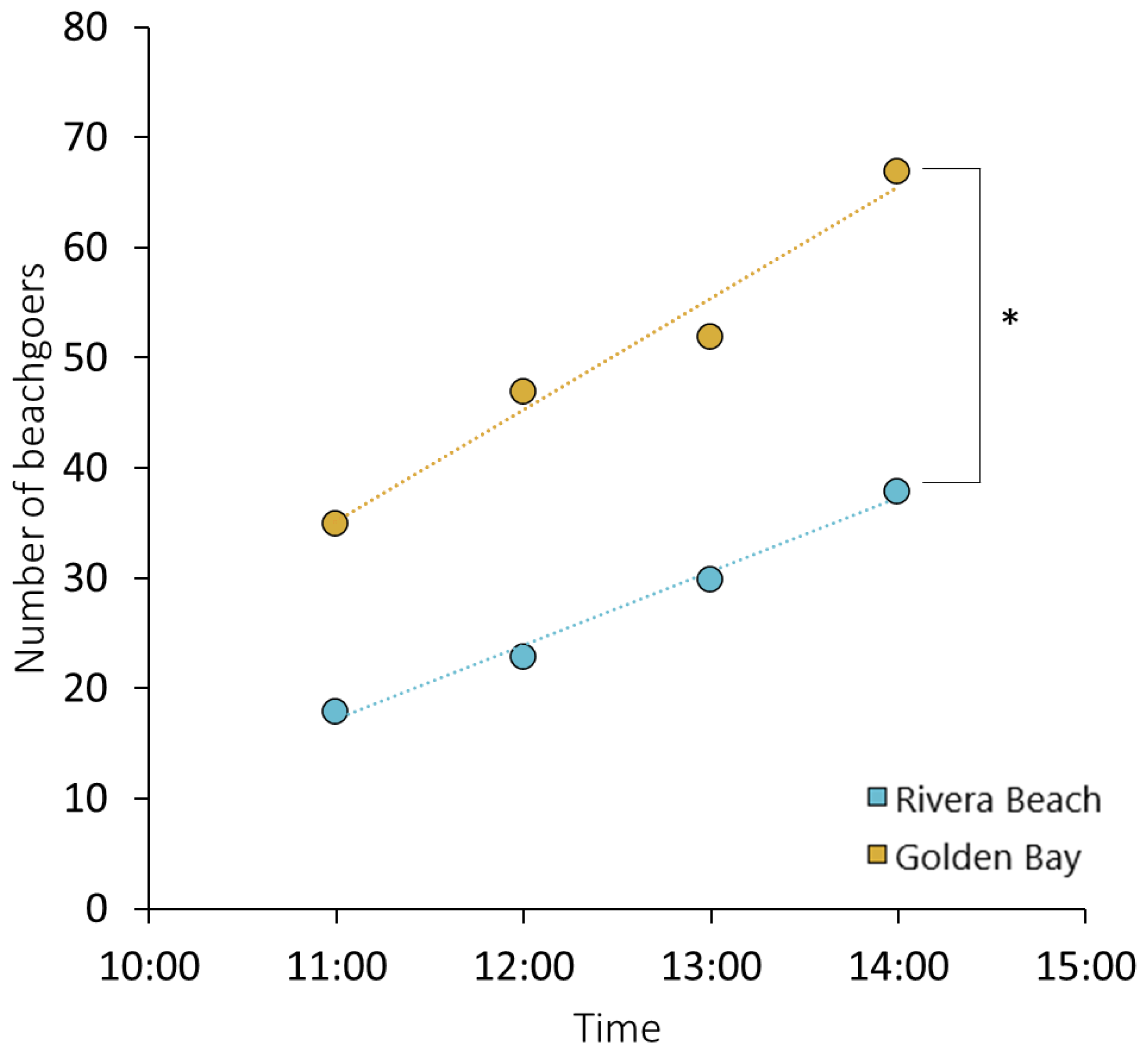
3.1. Variations in Plastic Litter Quantities
3.2. Dominance of Microplastic and Debris Breakdown
3.3. Supremacy of PE and PP on the Beaches of Malta
3.4. Composition of Plastic Litter Shapes
3.5. Color Distribution and Ecological Insights
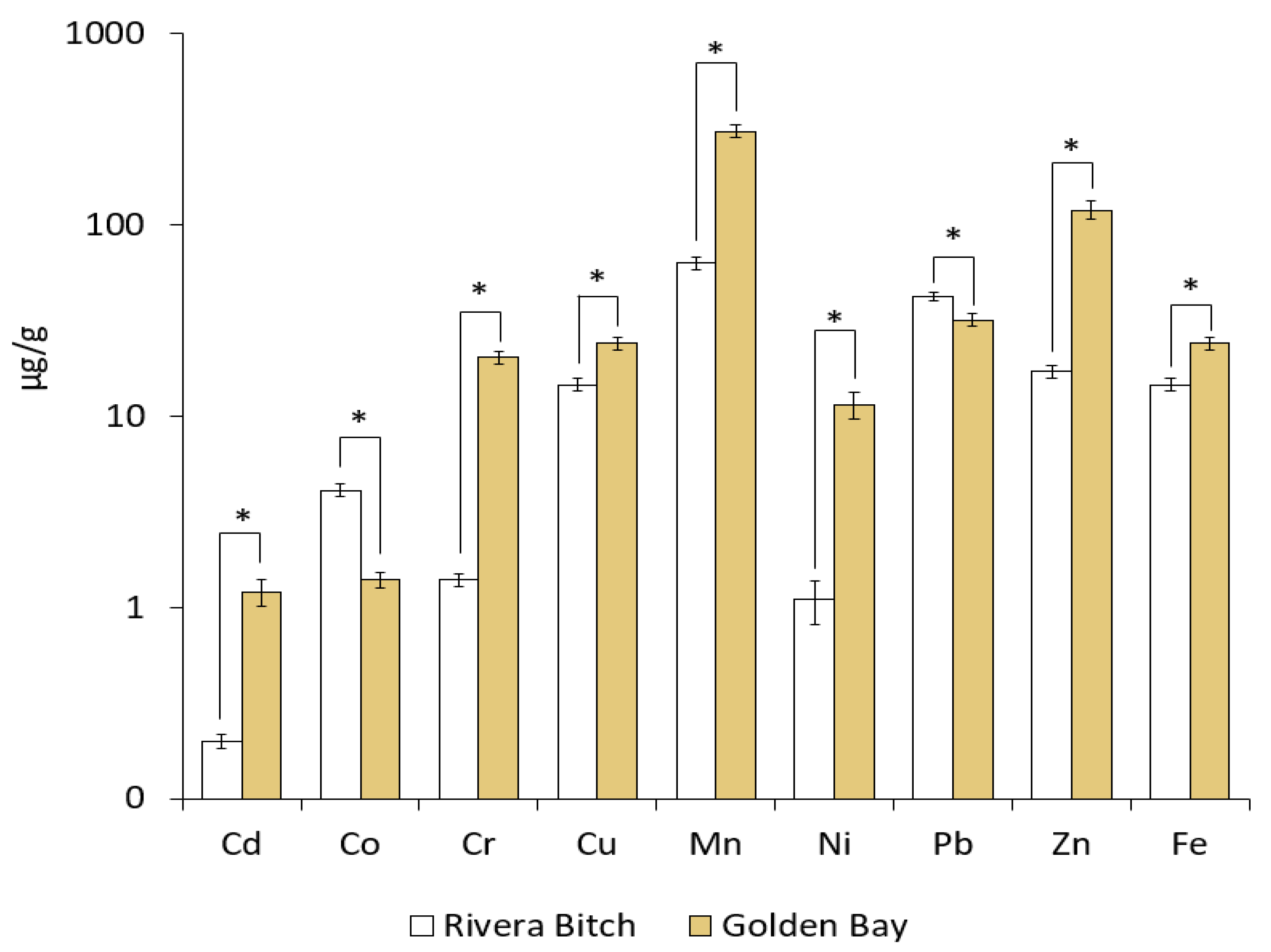
3.6. Potentially Toxic Element Contents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Attard, S. The Evolution of Malta’s tourism product over recent years. Cent. Bank Malta Q. Rev. 2018, 4, 41–55. [Google Scholar]
- Briguglio, L.; Avellino, M. Overtourism, Environmental Degradation and Governance in Small Islands with Special Reference to Malta. In Shaping the Future of Small Islands: Roadmap for Sustainable Development; Palgrave Macmillan: Singapore, 2021; pp. 301–322. [Google Scholar]
- PlasticsEurope. Plastics—The Facts 2022. An Analysis of European Plastics Production, Demand and Waste Data for 2018; PlasticsEurope; Association of Plastic Manufacturers: Brussels, Belgium, 2023. [Google Scholar]
- Anfuso, G.; Lynch, K.; Williams, A.T.; Perales, J.A.; Pereira da Silva, J.A.; Nogueira Mendes, R.; Veiga, J. Comments on marine litter in oceans, seas and beaches: Characteristics and impacts. Ann. Mar. Biol. Res. 2015, 2, 1–4. [Google Scholar]
- Urban-Malinga, B.; Zalewski, M.; Jakubowska, A.; Wodzinowski, T.; Malinga, M.; Pałys, B.; Dąbrowska, A. Microplastics on sandy beaches of the southern Baltic Sea. Mar. Pollut. Bull. 2020, 155, 111170. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, S.; Song, Y.K.; Hong, S.H.; Jang, Y.C.; Jang, M.; Shim, W.J. Relationships among the abundances of plastic debris in different size classes on beaches in South Korea. Mar. Pollut. Bull. 2013, 77, 349–354. [Google Scholar] [CrossRef]
- Vlachogianni, T.; Skocir, M.; Constantin, P.; Labbe, C.; Orthodoxou, D.; Pesmatzoglou, I.; Scoullos, M. Plastic pollution on the Mediterranean coastline: Generating fit-for-purpose data to support decision-making via a participatory-science initiative. Sci. Total Environ. 2020, 711, 135058. [Google Scholar] [CrossRef]
- Asensio-Montesinos, F.; Anfuso, G.; Williams, A.T. Beach litter distribution along the western Mediterranean coast of Spain. Mar. Pollut. Bull. 2019, 141, 119–126. [Google Scholar] [CrossRef]
- Nachite, D.; Maziane, F.; Anfuso, G.; Williams, A.T. Spatial and temporal variations of litter at the Mediterranean beaches of Morocco mainly due to beach users. Ocean Coast. Manag. 2019, 179, 104846. [Google Scholar] [CrossRef]
- Watkins, E.; Brink, P.; Withana, S.; Mutafoglu, K.; Schweitzer, J.P.; Russi, D.; Kettunen, M. Marine Litter: Socio Economic Study; A Report by IEEP for UNEP; Institute for European Environmental Policy: London, UK, 2017. [Google Scholar]
- Zielinski, S.; Botero, C.M.; Yanes, A. To clean or not to clean? A critical review of beach cleaning methods and impacts. Mar. Pollut. Bull. 2019, 139, 390–401. [Google Scholar]
- Cydzik-Kwiatkowska, A.; Grzyb, M.; Jachimowicz, P. Metatranscriptome analysis of bisphenol A-exposed aerobic granular sludge. Energies 2021, 14, 3263. [Google Scholar] [CrossRef]
- Díaz-Mendoza, C.; Mouthon-Bello, J.; Pérez-Herrera, N.L. Plastics and microplastics, effects on marine coastal areas: A review. Environ. Sci. Pollut. Res. 2020, 27, 39913–39922. [Google Scholar] [CrossRef]
- Shah, S.B. Heavy metals in the marine environment—An overview. Heavy Met. Scler. Corals 2021, 1–26. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Foekema, E.; Kooi, M.; Mintenig, S.; Ossendorp, B.C.; Redondo-Hasselerharm, P.E.; Verschoor, A.; Van Wezel, A.P.; Scheffer, M. Risks of Plastic Debris: Unravelling Fact, Opinion, Perception, and Belief. Environ. Sci. Technol. 2017, 51, 11513–11519. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Pan, L.; Fang, G.; Wang, Y.; Wang, L.; Su, B.; Li, D.; Xiang, B. Potentially Toxic Element Pollution Levels and Risk Assessment of Soils and Sediments in the Upstream River, Miyun Reservoir. China Int. J. Environ. Res. Public Health 2018, 15, 2364. [Google Scholar] [CrossRef] [PubMed]
- Tolkou, A.K.; Toubanaki, D.K.; Kyzas, G.Z. Detection of Arsenic, Chromium, Cadmium, Lead, and Mercury in Fish: Effects on the Sustainable and Healthy Development of Aquatic Life and Human Consumers. Sustainability 2023, 15, 16242. [Google Scholar] [CrossRef]
- Frias, J. Sorption of Potentially Toxic Elements to Microplastics. In Handbook of Microplastics in the Environment; Springer International Publishing: Cham, Switzerland, 2022; pp. 625–640. [Google Scholar]
- Jin, P.; Zhang, J.; Wan, J.; Overmans, S.; Gao, G.; Ye, M.; Dai, X.; Zhao, J.; Xiao, M.; Xia, J. The Combined Effects of Ocean Acidification and Heavy Metals on Marine Organisms: A Meta-Analysis. Front. Mar. Sci. 2021, 8, 801889. [Google Scholar] [CrossRef]
- Vrinda, P.K.; Amal, R.; Abhirami, N.; Mini, D.A.; Kumar, V.J.R.; Devipriya, S.P. Co-exposure of microplastics and heavy metals in the marine environment and remediation techniques: A comprehensive review. Environ. Sci. Pollut. Res. 2023, 30, 114822–114843. [Google Scholar] [CrossRef] [PubMed]
- Arias-Andres, M.; Klümper, U.; Rojas-Jimenez, K.; Grossart, H.P. Microplastic pollution increases gene exchange in aquatic ecosystems. Environ. Pollut. 2018, 237, 253–261. [Google Scholar] [CrossRef]
- Castro, R.; da Silva, M.; de Araújo, F. Revisión de estudios microplásticos en ecosistemas acuáticos brasileños. Manejo Océan. La Costa 2018, 165, 385–400. [Google Scholar]
- Bottari, T.; Mghili, B.; Gunasekaran, K.; Mancuso, M. Impact of plastic pollution on marine biodiversity in Italy. Water 2024, 16, 519. [Google Scholar] [CrossRef]
- Grelaud, M.; Ziveri, P. The generation of marine litter in Mediterranean island beaches as an effect of tourism and its mitigation. Sci. Rep. 2020, 10, 20326. [Google Scholar] [CrossRef] [PubMed]
- González, D.; Hanke, G.; Tweehuysen, G.; Bellert, B.; Holzhauer, M.; Palatinus, A.; Oosterbaan, L. Riverine litter monitoring-options and recommendations. In MSFD GES TG Marine Litter Thematic Report; JRC Technical Report; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Ranjani, M.; Veerasingam, S.; Venkatachalapathy, R.; Mugilarasan, M.; Bagaev, A.; Mukhanov, V.; Vethamony, P. Assessment of potential ecological risk of microplastics in the coastal sediments of India: A meta-analysis. Mar. Pollut. Bull. 2021, 163, 111969. [Google Scholar] [CrossRef] [PubMed]
- Operacz, A.; Bigaj, A.; Hap, K.; Kotowski, T. The Effect of Sample Preparation and Measurement Techniques on Heavy Metals Concentrations in Soil: Case Study from Kraków, Poland, Europe. Appl. Sci. 2022, 12, 2137. [Google Scholar] [CrossRef]
- Rozman, U.; Klun, B.; Marolt, G.; Imperl, J.; Kalčíková, G. A study of the adsorption of titanium dioxide and zinc oxide nanoparticles on polyethylene microplastics and their desorption in aquatic media. Sci. Total Environ. 2023, 888, 164163. [Google Scholar] [CrossRef] [PubMed]
- Constant, M.; Kerhervé, P.; Mino-Vercellio-Verollet, M.; Dumontier, M.; Sànchez Vidal, A.; Canals, M.; Heussner, S. Beached microplastics in the Northwestern Mediterranean Sea. Mar. Pollut. Bull. 2019, 142, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Axiak, V.; Bezzina, M.; Lomax, C.; Deidun, A.; Edwards, K. First Full Investigation of Levels of Microplastics on Sandy Beaches in Malta; MEDCOAST: Winston-Salem, NC, USA, 2017. [Google Scholar]
- Barnes, D.K. Remote islands reveal rapid rise of southern hemisphere sea debris. Sci. World J. 2005, 5, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Munari, C.; Scoponi, M.; Mistri, M. Plastic debris in the Mediterranean Sea: Types, occurrence and distribution along Adriatic shorelines. Waste Manag. 2017, 67, 385–391. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thomson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Expósito, N.; Rovira, J.; Sierra, J.; Folch, J.; Schuhmacher, M. Microplastics levels, size, morphology and composition in marine water, sediments and sand beaches. Case study of Tarragona coast (western Mediterranean). Sci. Total Environ. 2021, 786, 147453. [Google Scholar] [CrossRef]
- Rocha-Santos, T.; Duarte, A.C. A critical overview of the analytical approaches to the occurrence, the fate and the behavior of microplastics in the environment. TrAC Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Edo, C.; Tamayo-Belda, M.; Martínez-Campos, S.; Martín-Betancor, K.; González-Pleiter, M.; Pulido-Reyes, G.; Rosal, R. Occurrence and identification of microplastics along a beach in the Biosphere Reserve of Lanzarote. Mar. Pollut. Bull. 2019, 143, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.A.; Corcoran, P.L. Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar. Pollut. Bull. 2010, 60, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Holmes, L. Occurrence, distribution and characteristics of beached plastic production pellets on the island of Malta (central Mediterranean). Mar. Pollut. Bull. 2011, 62, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Ter Halle, A.; Ladirat, L.; Gendre, X.; Goudounèche, D.; Pusineri, C.; Routaboul, C.; Tenailleau, C.; Duployer, B.; Perez, E. Understanding the fragmentation pattern of marine plastic debris. Environ. Sci. Technol. 2016, 50, 5668–5675. [Google Scholar] [CrossRef] [PubMed]
- Minor, E.C.; Lin, R.; Burrows, A.; Cooney, E.M.; Grosshuesch, S.; Lafrancois, B. An analysis of microlitter and microplastics from Lake Superior beach sand and surface-water. Sci. Total Environ. 2020, 744, 140824. [Google Scholar] [CrossRef] [PubMed]
- Van der Hal, N.; Ariel, A.; Angel, D.L. Exceptionally high abundances of microplastics in the oligotrophic Israeli Mediterranean coastal waters. Mar. Pollut. Bull. 2017, 116, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Bolan, N.; Li, Y.; Ding, S.; Atugoda, T.; Vithanage, M.; Kirkham, M.B. Weathering of microplastics and interaction with other coexisting constituents in terrestrial and aquatic environments. Water Res. 2021, 196, 117011. [Google Scholar] [CrossRef]
- Liu, H.; Liu, K.; Fu, H.; Ji, R.; Qu, X. Sunlight mediated cadmium release from colored microplastics containing cadmium pigment in aqueous phase. Environ. Pollut. 2020, 263, 114484. [Google Scholar] [CrossRef]
- Ugwu, K.; Herrera, A.; Gómez, M. Microplastics in marine biota: A review. Mar. Pollut. Bull. 2021, 169, 112540. [Google Scholar] [CrossRef]
- Simon, M.; Hartmann, N.B.; Vollertsen, J. Accelerated weathering increases the release of toxic leachates from microplastic particles as demonstrated through altered toxicity to the green algae Raphidocelis subcapitata. Toxics 2021, 9, 185. [Google Scholar] [CrossRef] [PubMed]
- Carbery, M.; MacFarlane, G.R.; O’Connor, W.; Afrose, S.; Taylor, H.; Palanisami, T. Baseline analysis of metal(loid)s on microplastics collected from the Australian shoreline using citizen science. Mar. Pollut. Bull. 2020, 152, 110914. [Google Scholar] [CrossRef] [PubMed]
- Zatorski, W.; Brzozowski, Z.K.; Kolbrecki, A. New developments in chemical modification of fire-safe rigid polyurethane foams. Polym. Degrad. Stab. 2008, 93, 2071–2076. [Google Scholar] [CrossRef]
- Patterson, J.; Jeyasanta, K.I.; Sathish, N.; Edward, J.K.P.; Booth, A.M. Microplastic and heavy metal distributions in an Indian coral reef ecosystem. Sci. Total Environ. 2020, 744, 140706. [Google Scholar] [CrossRef]
- Filella, M.; Turner, A. Observational Study Unveils the Extensive Presence of Hazardous Elements in Beached Plastics from Lake Geneva. Front. Environ. Sci. 2018, 6, 1. [Google Scholar] [CrossRef]


| Elements | LOD (mg/L) | LOQ (mg/L) | Reference Value (mg/kg) | Measured Value (mg/kg) | AO (%) |
|---|---|---|---|---|---|
| Cd | 0.023 | 0.075 | 14.0 ± 1.4 | 13.5 ± 1.6 | 96.4 |
| Co | 0.046 | 0.063 | 66.5 ± 2.3 | 67.2 ± 1.9 | 101.1 |
| Cr | 0.072 | 0.219 | 138.0 ± 5.0 | 133 ± 3.1 | 96.4 |
| Cu | 0.008 | 0.027 | 46.9 ± 1.8 | 44.9 ± 2.3 | 95.7 |
| Mn | 0.061 | 0.176 | 112.0 ± 6.4 | 113.3 ± 4.3 | 101.2 |
| Ni | 0.056 | 0.171 | 94.0 ± 5.0 | 91.8 ± 1.7 | 97.7 |
| Pb | 0.020 | 0.060 | 51.3 ± 2.0 | 49.6 ± 2.4 | 96.7 |
| Zn | 0.066 | 0.220 | 270.0 ± 8.0 | 261.9 ± 6.5 | 97.0 |
| Fe | 0.051 | 0.098 | 320.0 ± 11.4 | 318.6 ± 8.7 | 99.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jachimowicz, P.; Klik, B.; Osińska, A.D. Plastic Pollution in Paradise: Analyzing Plastic Litter on Malta’s Beaches and Assessing the Release of Potentially Toxic Elements. Toxics 2024, 12, 568. https://doi.org/10.3390/toxics12080568
Jachimowicz P, Klik B, Osińska AD. Plastic Pollution in Paradise: Analyzing Plastic Litter on Malta’s Beaches and Assessing the Release of Potentially Toxic Elements. Toxics. 2024; 12(8):568. https://doi.org/10.3390/toxics12080568
Chicago/Turabian StyleJachimowicz, Piotr, Barbara Klik, and Adriana Dorota Osińska. 2024. "Plastic Pollution in Paradise: Analyzing Plastic Litter on Malta’s Beaches and Assessing the Release of Potentially Toxic Elements" Toxics 12, no. 8: 568. https://doi.org/10.3390/toxics12080568
APA StyleJachimowicz, P., Klik, B., & Osińska, A. D. (2024). Plastic Pollution in Paradise: Analyzing Plastic Litter on Malta’s Beaches and Assessing the Release of Potentially Toxic Elements. Toxics, 12(8), 568. https://doi.org/10.3390/toxics12080568










