The Effect of α-Fe2O3(0001) Surface Containing Hydroxyl Radicals and Ozone on the Formation Mechanism of Environmentally Persistent Free Radicals
Abstract
1. Introduction
2. Computational Details
3. Results
3.1. The Effect of α-Fe2O3(0001) Surface Containing ·OH and O3 on EPFRs Formation
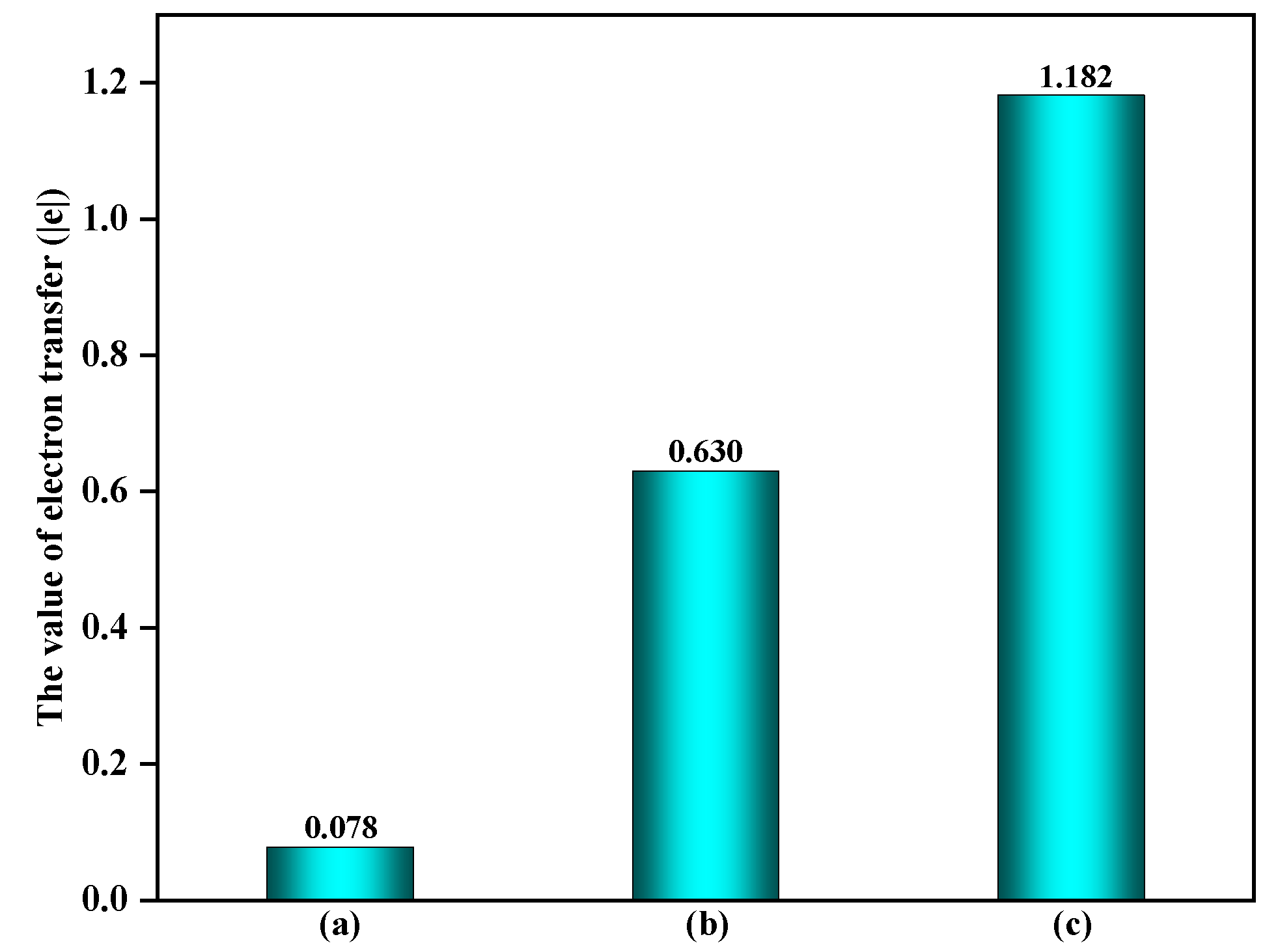
3.1.1. The Effect of α-Fe2O3(0001) Surface Containing ·OH on EPFRs Formation
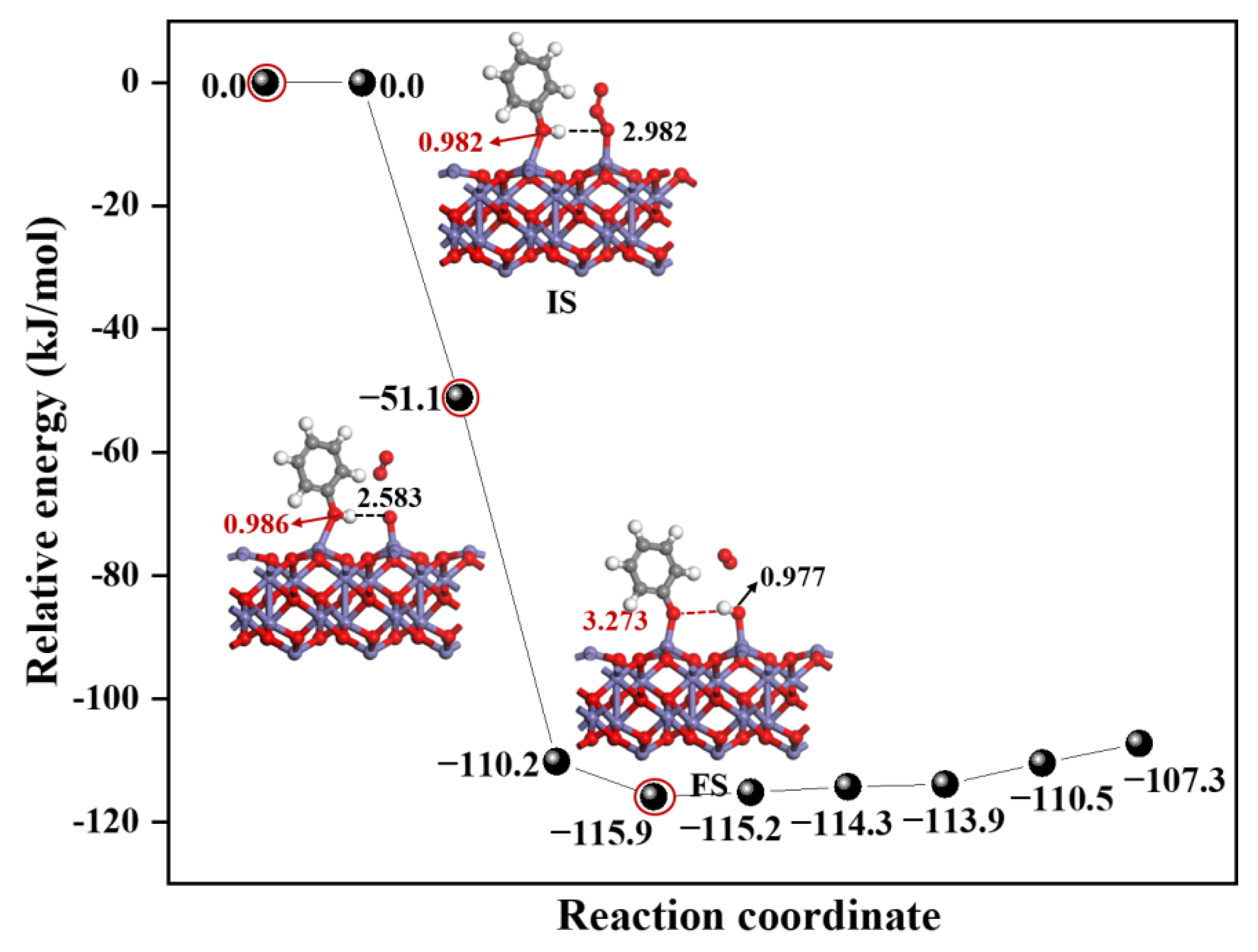
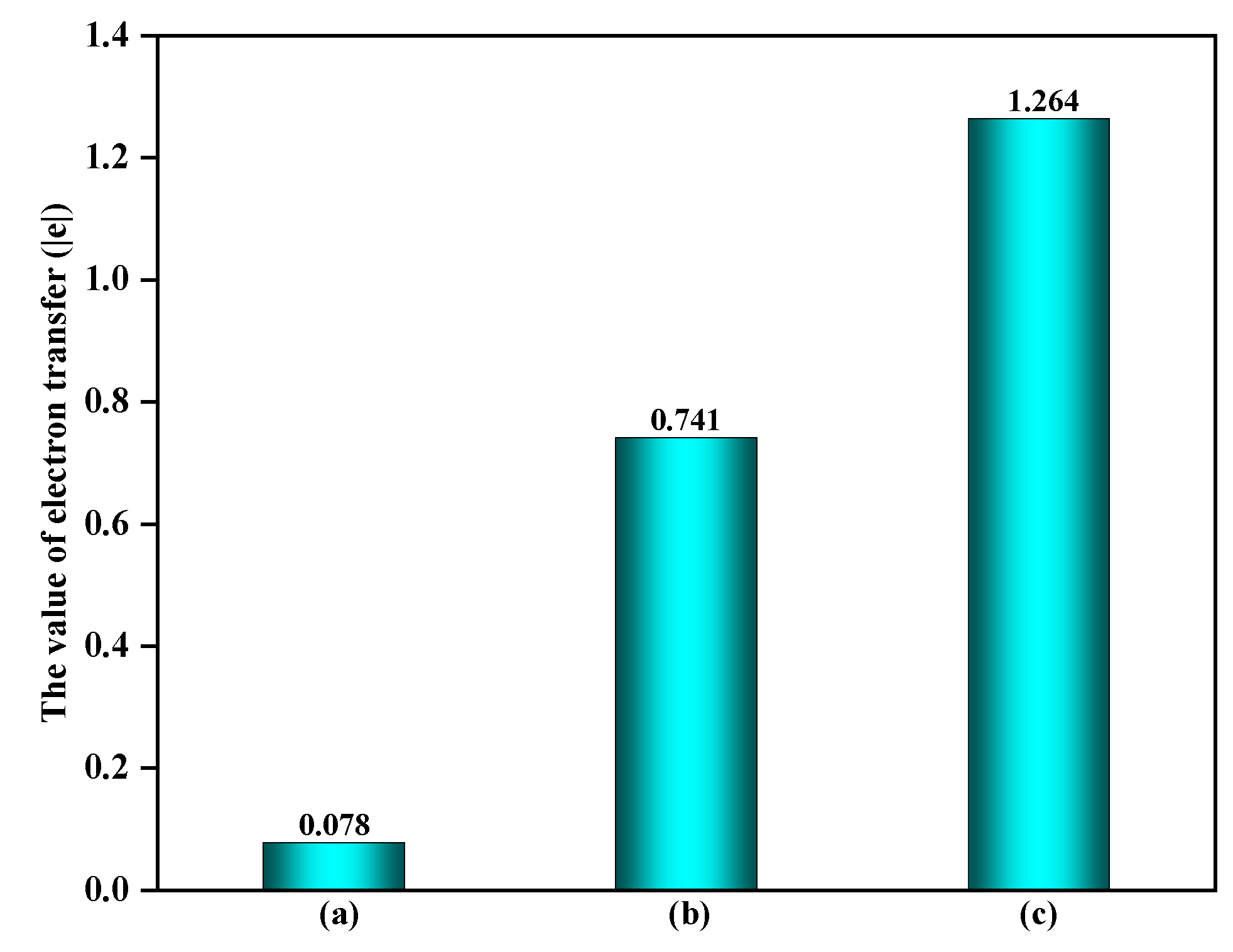
3.1.2. The Effect of α-Fe2O3(0001) Surface Containing O3 on EPFRs Formation
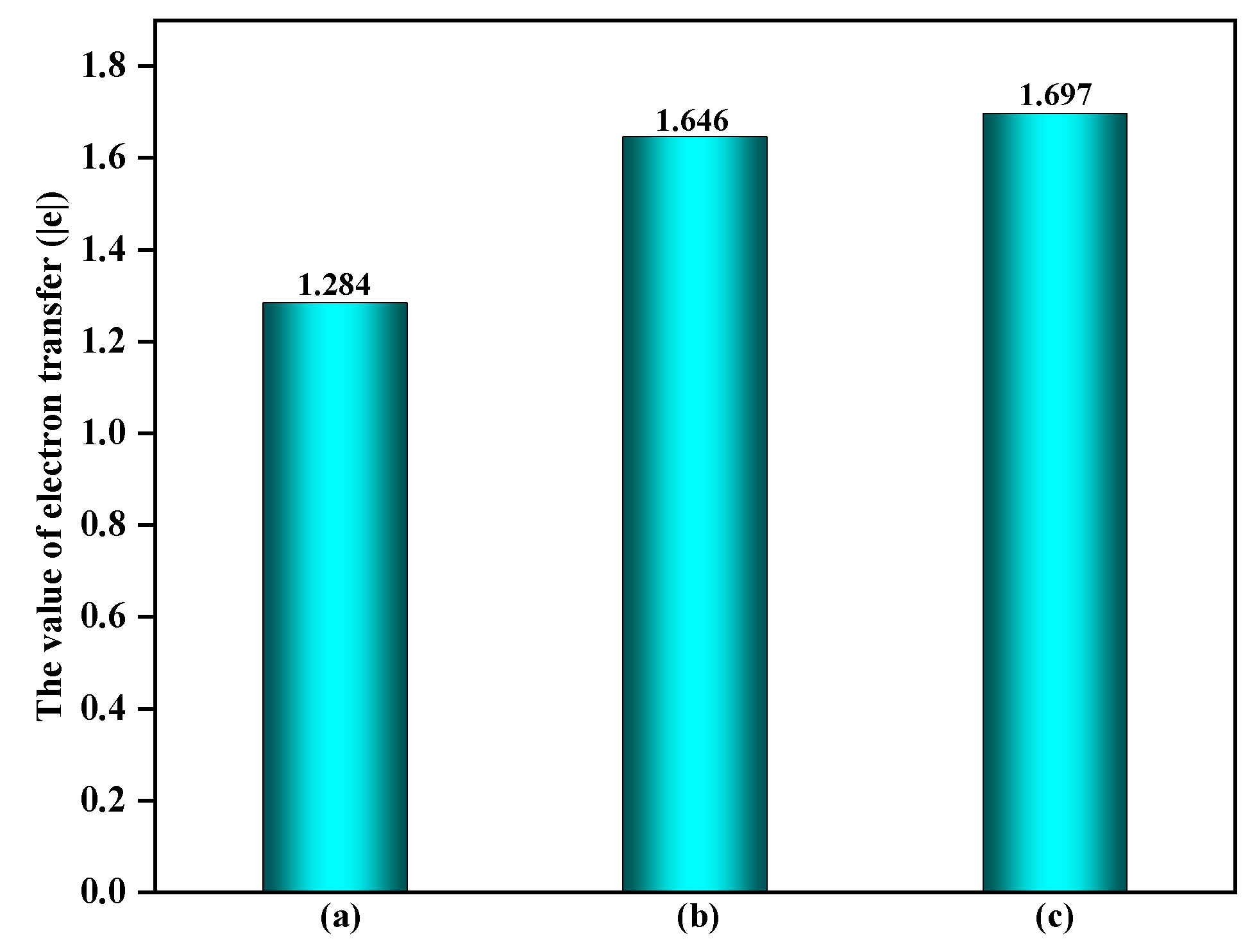
3.2. The Comparison of EPFRs Formation on the α-Fe2O3(0001) Surface Containing ·OH and O3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vejerano, E.P.; Rao, G.; Khachatryan, L.; Cormier, S.A.; Lomnicki, S. Environmentally Persistent Free Radicals: Insights on a New Class of Pollutants. Environ. Sci. Technol. 2018, 52, 2468–2481. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Li, H.; Lang, D.; Xing, B. Environmentally Persistent Free Radicals: Occurrence, Formation Mechanisms and Implications. Environ. Pollut. 2019, 248, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, P.; Miao, D.; Wu, L.; Qian, Y.; Chen, S.; Sharma, V.K.; Jia, H. FORMATION and Evolution of Solvent-Extracted and Nonextractable Environmentally Persistent Free Radicals in Fly Ash of Municipal Solid Waste Incinerators. Environ. Sci. Technol. 2019, 53, 10120–10130. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Li, S.; Wu, L.; Li, S.; Sharma, V.K.; Yan, B. Cytotoxic Free Radicals on Air-Borne Soot Particles Generated by Burning Wood or Low-Maturity Coals. Environ. Sci. Technol. 2020, 54, 5608–5618. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Pan, B.; Li, H.; Huang, Y.; Dong, X.; Ai, F.; Liu, L.; Wu, M.; Xing, B. The Overlooked Occurrence of Environmentally Persistent Free Radicals in an Area with Low-Rank Coal Burning, Xuanwei, China. Environ. Sci. Technol. 2018, 52, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wu, T.; Tang, Y.-T.; Chen, T.; Khachatryan, L.; Iyer, P.R.; Guo, D.; Chen, A.; Lyu, M.; Li, J.; et al. Environmentally Persistent Free Radicals in PM2.5: A Review. Waste Dispos. Sustain. Energy 2019, 1, 177–197. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.; Zhang, S.; Peng, C.; Zhang, L.; Yang, F.; Tian, M.; Huang, R.; Wang, Q.; Chen, Q.; Yao, X.; et al. Characteristics and Potential Exposure Risks of Environmentally Persistent Free Radicals in PM2.5 in the Three Gorges Reservoir Area, Southwestern China. Chemosphere 2020, 252, 126425. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.; Wang, M.; Sun, H.; Mu, Z.; Zhang, L.; Li, Y.; Chen, Q. Source Apportionment of Environmentally Persistent Free Radicals (EPFRs) in PM2.5 over Xi’an, China. Sci. Total Environ. 2019, 689, 193–202. [Google Scholar] [CrossRef]
- Khachatryan, L.; Vejerano, E.; Lomnicki, S.; Dellinger, B. Environmentally Persistent Free Radicals (EPFRs). 1. Generation of Reactive Oxygen Species in Aqueous Solutions. Environ. Sci. Technol. 2011, 45, 8559–8566. [Google Scholar] [CrossRef]
- Balakrishna, S.; Saravia, J.; Thevenot, P.; Ahlert, T.; Lominiki, S.; Dellinger, B.; Cormier, S.A. Environmentally Persistent Free Radicals Induce Airway Hyperresponsiveness in Neonatal Rat Lungs. Part. Fibre Toxicol. 2011, 8, 11. [Google Scholar] [CrossRef]
- Lord, K.; Moll, D.; Lindsey, J.K.; Mahne, S.; Raman, G.; Dugas, T.; Cormier, S.; Troxlair, D.; Lomnicki, S.; Dellinger, B.; et al. Environmentally Persistent Free Radicals Decrease Cardiac Function before and after Ischemia/Reperfusion Injury in Vivo. J. Recept. Signal Transduct. 2011, 31, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.I.; Saravia, J.; You, D.; Shrestha, B.; Jaligama, S.; Hebert, V.Y.; Dugas, T.R.; Cormier, S.A. Exposure to Combustion Generated Environmentally Persistent Free Radicals Enhances Severity of Influenza Virus Infection. Part. Fibre Toxicol. 2014, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, B.; Ding, L.; You, D.; Lomnicki, S.; Dellinger, B.; Cormier, S.A. In Vitro and in Vivo Assessment of Pulmonary Risk Associated with Exposure to Combustion Generated Fine Particles. Environ. Toxicol. Pharmacol. 2010, 29, 173–182. [Google Scholar] [CrossRef]
- Lomnicki, S.; Truong, H.; Vejerano, E.; Dellinger, B. Copper Oxide-Based Model of Persistent Free Radical Formation on Combustion-Derived Particulate Matter. Environ. Sci. Technol. 2008, 42, 4982–4988. [Google Scholar] [CrossRef]
- Vejerano, E.; Lomnicki, S.; Dellinger, B. Formation and Stabilization of Combustion-Generated Environmentally Persistent Free Radicals on an Fe(III)2O3 /Silica Surface. Environ. Sci. Technol. 2011, 45, 589–594. [Google Scholar] [CrossRef]
- Wang, J.; Domin, D.; Austin, B.; Zubarev, D.Y.; McClean, J.; Frenklach, M.; Cui, T.; Lester, W.A. A Diffusion Monte Carlo Study of the O−H Bond Dissociation of Phenol. J. Phys. Chem. A 2010, 114, 9832–9835. [Google Scholar] [CrossRef] [PubMed]
- Feld-Cook, E.E.; Bovenkamp-Langlois, L.; Lomnicki, S.M. Effect of Particulate Matter Mineral Composition on Environmentally Persistent Free Radical (EPFR) Formation. Environ. Sci. Technol. 2017, 51, 10396–10402. [Google Scholar] [CrossRef]
- Kiruri, L.W.; Khachatryan, L.; Dellinger, B.; Lomnicki, S. Effect of Copper Oxide Concentration on the Formation and Persistency of Environmentally Persistent Free Radicals (EPFRs) in Particulates. Environ. Sci. Technol. 2014, 48, 2212–2217. [Google Scholar] [CrossRef]
- Truong, H.; Lomnicki, S.; Dellinger, B. Potential for Misidentification of Environmentally Persistent Free Radicals as Molecular Pollutants in Particulate Matter. Environ. Sci. Technol. 2010, 44, 1933–1939. [Google Scholar] [CrossRef]
- Balakrishna, S.; Lomnicki, S.; McAvey, K.M.; Cole, R.B.; Dellinger, B.; Cormier, S.A. Environmentally Persistent Free Radicals Amplify Ultrafine Particle Mediated Cellular Oxidative Stress and Cytotoxicity. Part. Fibre Toxicol. 2009, 6, 11. [Google Scholar] [CrossRef]
- Liang, D.; Liu, J.; Bai, F.; Tu, K.; Wang, L.; Wang, Z.; He, T.; Zhang, X. Formation Mechanism of Environmentally Persistent Free Radicals during Soot Aging. Atmos. Environ. 2024, 333, 120663. [Google Scholar] [CrossRef]
- Wang, L.; Liang, D.; Liu, J.; Du, L.; Vejerano, E.; Zhang, X. Unexpected Catalytic Influence of Atmospheric Pollutants on the Formation of Environmentally Persistent Free Radicals. Chemosphere 2022, 303, 134854. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; He, S.; Xue, Q.; Liu, X.; Fu, J.; Xiao, K.; Zhang, A. First-Principles Study on the Heterogeneous Formation of Environmentally Persistent Free Radicals (EPFRs) over α-Fe2O3(0001) Surface: Effect of Oxygen Vacancy. J. Environ. Sci. 2024, 142, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Tong, S.; Wang, W.; Zhang, W.; Chen, M.; Peng, C.; Li, J.; Zhou, L.; Chen, Y.; Liu, M. Important Oxidants and Their Impact on the Environmental Effects of Aerosols. J. Phys. Chem. A 2021, 125, 3813–3825. [Google Scholar] [CrossRef] [PubMed]
- Dubowski, Y.; Vieceli, J.; Tobias, D.J.; Gomez, A.; Lin, A.; Nizkorodov, S.A.; McIntire, T.M.; Finlayson-Pitts, B.J. Interaction of Gas-Phase Ozone at 296 K with Unsaturated Self-Assembled Monolayers: A New Look at an Old System. J. Phys. Chem. A 2004, 108, 10473–10485. [Google Scholar] [CrossRef]
- Pillar-Little, E.A.; Camm, R.C.; Guzman, M.I. Catechol Oxidation by Ozone and Hydroxyl Radicals at the Air–Water Interface. Environ. Sci. Technol. 2014, 48, 14352–14360. [Google Scholar] [CrossRef]
- Woodill, L.A.; O’Neill, E.M.; Hinrichs, R.Z. Impacts of Surface Adsorbed Catechol on Tropospheric Aerosol Surrogates: Heterogeneous Ozonolysis and Its Effects on Water Uptake. J. Phys. Chem. A 2013, 117, 5620–5631. [Google Scholar] [CrossRef]
- Wang, C.; Li, W.; Zhang, Z.; Lei, D.; Che, G.; Gou, C.; Zhang, J.; Hao, Z. A Novel Iron Sulfide Phase with Remarkable Hydroxyl Radical Generation Capability for Contaminants Degradation. Water Res. 2024, 251, 121166. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, H.; Mu, Z.; Wang, Y.; Li, Y.; Zhang, L.; Wang, M.; Zhang, Z. Characteristics of Environmentally Persistent Free Radicals in PM2.5: Concentrations, Species and Sources in Xi’an, Northwestern China. Environ. Pollut. 2019, 247, 18–26. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- White, J.A.; Bird, D.M. Implementation of Gradient-Corrected Exchange-Correlation Potentials in Car-Parrinello Total-Energy Calculations. Phys. Rev. B 1994, 50, 4954–4957. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Mosey, N.J.; Liao, P.; Carter, E.A. Rotationally Invariant Ab Initio Evaluation of Coulomb and Exchange Parameters for DFT+U Calculations. J. Chem. Phys. 2008, 129, 014103. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A Climbing Image Nudged Elastic Band Method for Finding Saddle Points and Minimum Energy Paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Kiejna, A.; Pabisiak, T. Mixed Termination of Hematite (α-Fe2O3)(0001) Surface. J. Phys. Chem. C 2013, 117, 24339–24344. [Google Scholar] [CrossRef]
- Kokalj, A. Corrosion Inhibitors: Physisorbed or Chemisorbed? Corros. Sci. 2022, 196, 109939. [Google Scholar] [CrossRef]
- Pan, W.; Chang, J.; He, S.; Xue, Q.; Liu, X.; Fu, J.; Zhang, A. Major Influence of Hydroxyl and Nitrate Radicals on Air Pollution by Environmentally Persistent Free Radicals. Environ. Chem. Lett. 2021, 19, 4455–4461. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, R.; Liu, Z.; Wang, W.; Zhang, Q.; Wang, Q. Periodic DFT Calculation for the Formation of EPFRs from Phenol on γ-Al2O3(110): Site-Dependent Mechanism and the Role of Ambient Water. J. Environ. Chem. Eng. 2022, 10, 108386. [Google Scholar] [CrossRef]
- Sofia, D.; Giuliano, A.; Gioiella, F.; Barletta, D.; Poletto, M. Modeling of an Air Quality Monitoring Network with High Space-Time Resolution. Comput. Aided Chem. Eng. 2018, 43, 193–198. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, D.; Liu, J.; Wang, C.; Tu, K.; Wang, L.; Qiu, L.; Zhang, X.; Liu, L. The Effect of α-Fe2O3(0001) Surface Containing Hydroxyl Radicals and Ozone on the Formation Mechanism of Environmentally Persistent Free Radicals. Toxics 2024, 12, 582. https://doi.org/10.3390/toxics12080582
Liang D, Liu J, Wang C, Tu K, Wang L, Qiu L, Zhang X, Liu L. The Effect of α-Fe2O3(0001) Surface Containing Hydroxyl Radicals and Ozone on the Formation Mechanism of Environmentally Persistent Free Radicals. Toxics. 2024; 12(8):582. https://doi.org/10.3390/toxics12080582
Chicago/Turabian StyleLiang, Danli, Jiarong Liu, Chunlin Wang, Kaipeng Tu, Li Wang, Lili Qiu, Xiuhui Zhang, and Ling Liu. 2024. "The Effect of α-Fe2O3(0001) Surface Containing Hydroxyl Radicals and Ozone on the Formation Mechanism of Environmentally Persistent Free Radicals" Toxics 12, no. 8: 582. https://doi.org/10.3390/toxics12080582
APA StyleLiang, D., Liu, J., Wang, C., Tu, K., Wang, L., Qiu, L., Zhang, X., & Liu, L. (2024). The Effect of α-Fe2O3(0001) Surface Containing Hydroxyl Radicals and Ozone on the Formation Mechanism of Environmentally Persistent Free Radicals. Toxics, 12(8), 582. https://doi.org/10.3390/toxics12080582








