Stressful Effects of Individual and Combined Exposure to Low-Concentration Polylactic Acid Microplastics and Chromium on Marine Medaka Larvae (Oryzias melastigma)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Experimental Reagents
2.3. Maintenance of Marine Medaka Larvae
2.4. PLA Intake and Excretion Experiments
2.5. Exposure Experiment
2.6. Growth and Development, Intestinal Sectioning, and Biochemical Analysis
2.7. Behavioral Experiment
2.8. Statistical Analysis
3. Results
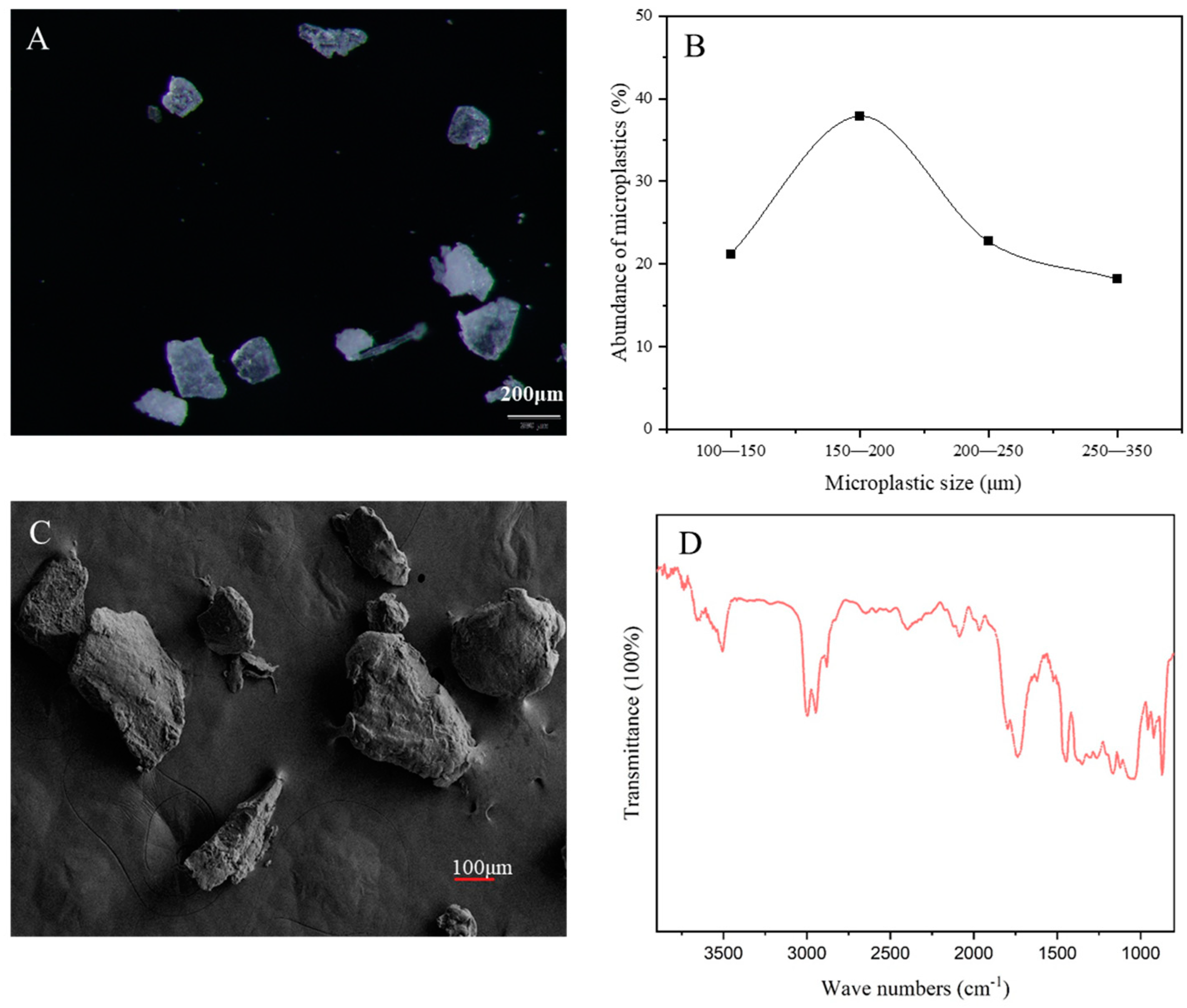
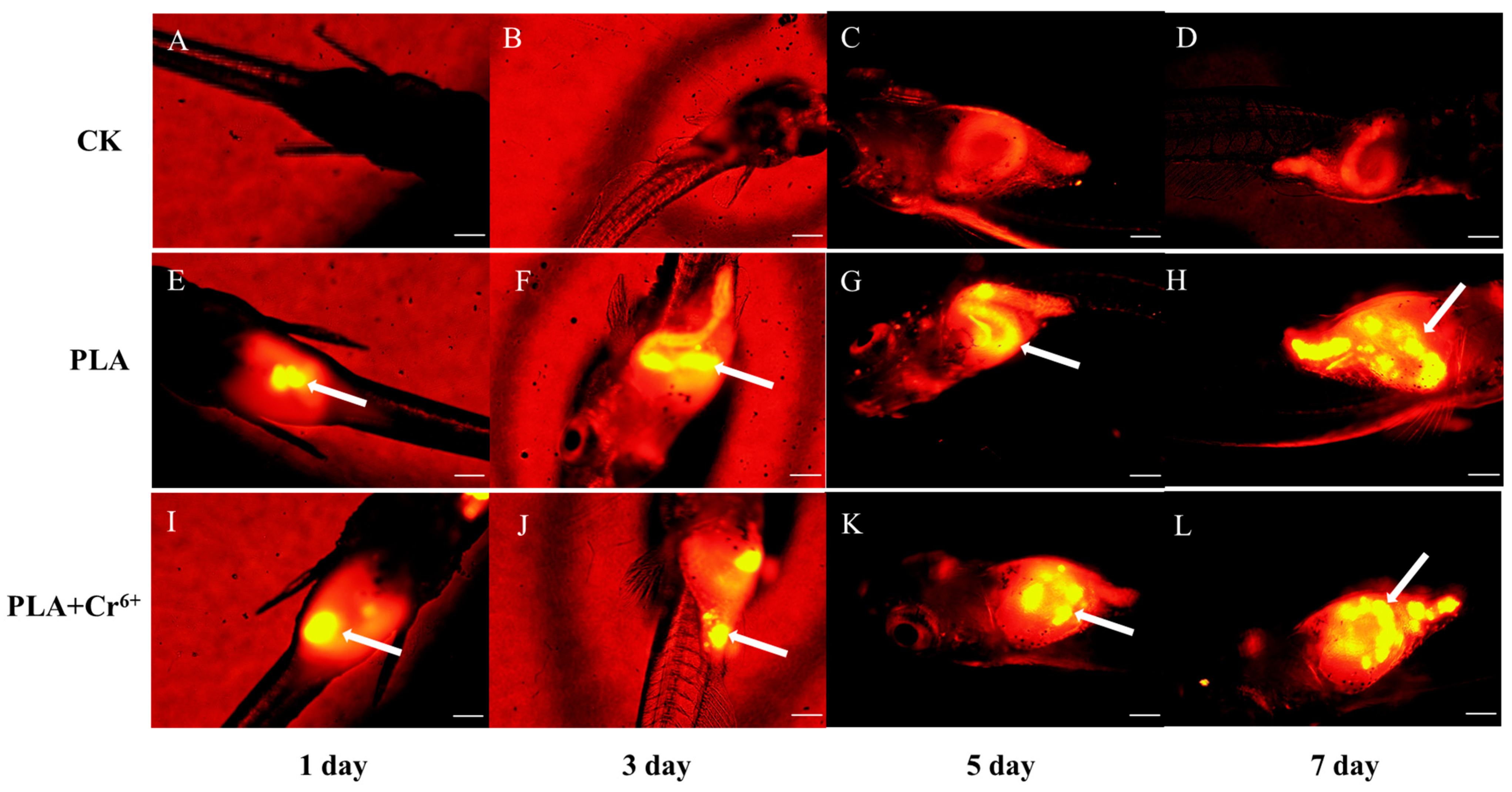
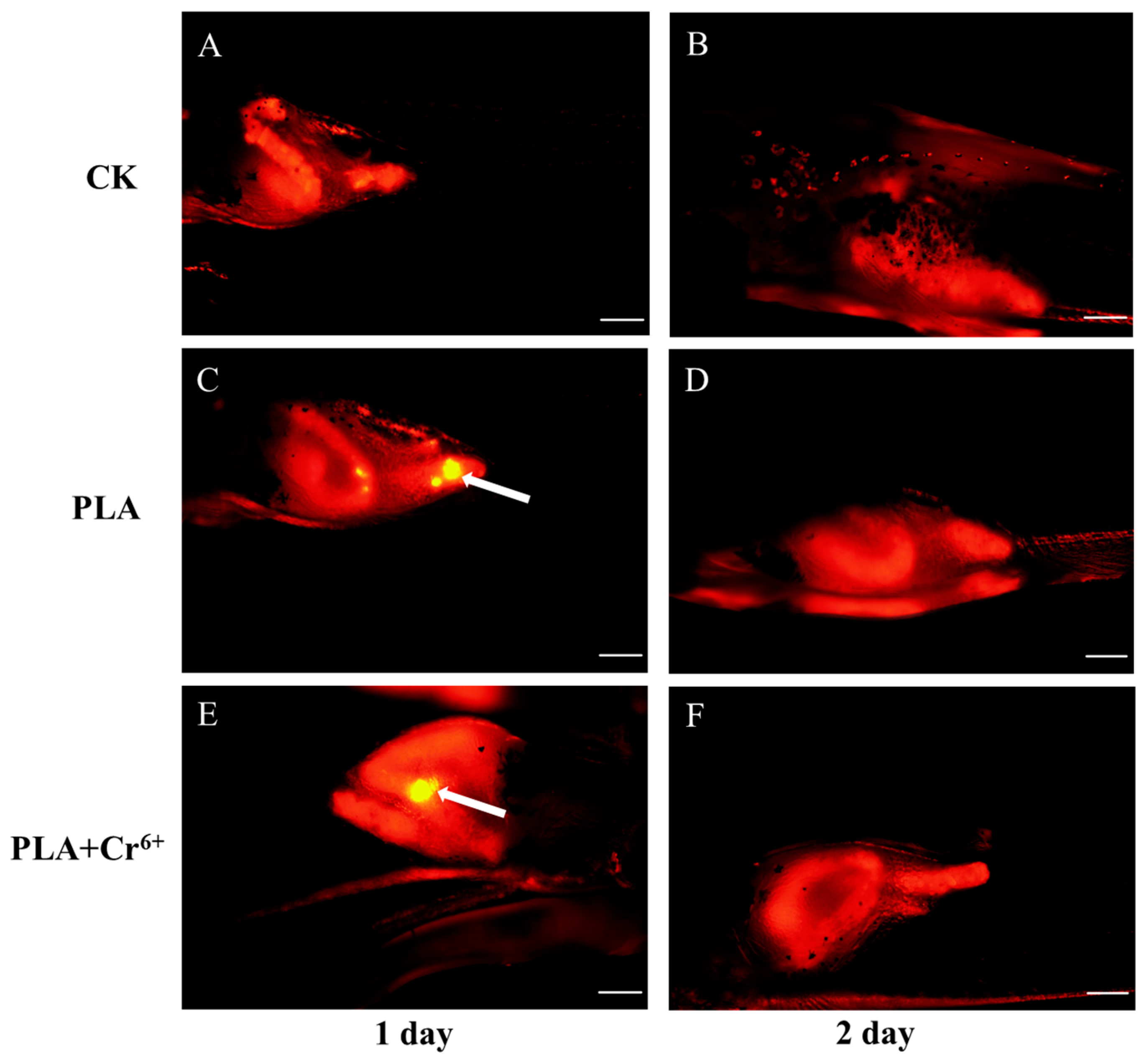
3.1. Intake and Excretion of PLA-MPs by Marine Medaka Larvae
3.2. Effects of Individual and Combined Exposure on the Growth of Marine Medaka Larvae
3.3. Effects of Individual and Combined Exposure on the Intestinal Tract of Marine Medaka Larvae
3.4. Effects of Individual and Combined Exposure on the Behavior of Marine Medaka Larvae
4. Discussion
4.1. Effects on Growth
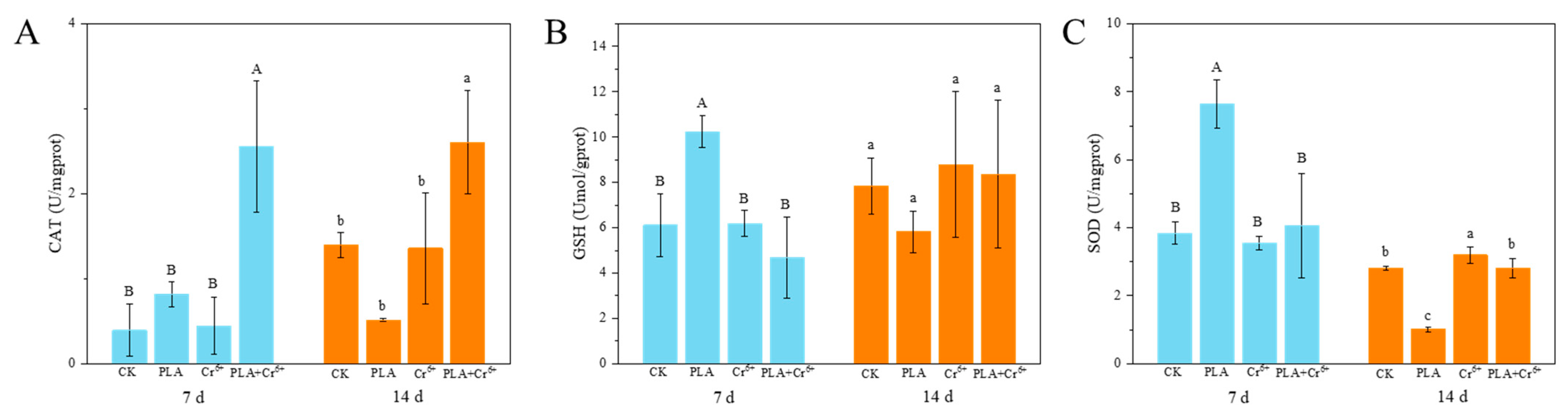
4.2. Intestinal Damage and Antioxidant Enzyme Activity
4.3. Influence of Locomotive Behavior
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, B.; Zhang, Z.; Wang, T.; Hu, H.; Qin, G.; Lu, T.; Hong, W.; Hu, J.; Penuelas, J.; Qian, H. Global distribution of marine microplastics and potential for biodegradation. J. Hazard. Mater. 2023, 451, 131198. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Wang, X.; Liu, K.; Li, D. Cross-oceanic distribution and origin of microplastics in the subsurface water of the South China Sea and Eastern Indian Ocean. Sci. Total Environ. 2022, 805, 150243. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Huda, A.N.M.S.; Niloy, M.N.H.; Chowdhury, G.W. Trophic transfer of microplastics in the aquatic ecosystem of Sundarbans mangrove forest, Bangladesh. Sci. Total Environ. 2022, 838, 155896. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, K.; Ding, D.; Liu, J.; Lin, Y. Size-dependent adverse effects of microplastics on intestinal microbiota and metabolic homeostasis in the marine medaka (Oryzias melastigma). Environ. Int. 2021, 151, 106452. [Google Scholar] [CrossRef]
- Thery, J.; Thery, J.; Bialais, C.; Kazour, M.; Moreau, M.; Dufour, D.; Benali, S.; Amara, R.; Monchy, S.; Raquez, J.M.; et al. A new method for microplastics identification in copepods. Front. Environ. Chem. 2022, 3, 905303. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, Q.; Xie, J.; Lin, Y.; Chen, Y.; Mao, K.; Qin, Y.; Diao, X. Microplastics in biota and surface seawater from tropical aquaculture area in Hainan, China. Gondwana Res. 2022, 108, 41–48. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Q.; Huang, Y.; Jiang, Y.; Li, J.; Michal, J.J.; Jiang, Z.; Xu, Y.; Lan, W. Long-term trends of microplastics in seawater and farmed oysters in the Maowei Sea, China. Environ. Pollut. 2021, 273, 116450. [Google Scholar] [CrossRef]
- Jeyavani, J.; Sibiya, A.; Gopi, N.; Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Riaz, M.N.; Palaniappan, B.; Govindarajan, M.; et al. Ingestion and impacts of water-borne polypropylene microplastics on Daphnia similis. Environ. Sci. Pollut. Res. 2022, 30, 13483–13494. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Akhtar, M.F.; Saleem, A.; Akhtar, B.; Sharif, A. Reproductive and metabolic toxic effects of polystyrene microplastics in adult female Wistar rats: A mechanistic study. Environ. Sci. Pollut. Res. 2023, 30, 63185–63199. [Google Scholar] [CrossRef]
- Duan, Z.; Cheng, H.; Duan, X.; Zhang, H.; Wang, Y.; Gong, Z.; Zhang, H.; Sun, H.; Wang, L. Diet preference of zebrafish (Danio rerio) for bio-based polylactic acid microplastics and induced intestinal damage and microbiota dysbiosis. J. Hazard. Mater. 2022, 429, 128332. [Google Scholar] [CrossRef]
- Pannetier, P.; Morin, B.; Bihanic, F.L.; Dubreil, L.; Clérandeau, C.; Chouvellon, F.; Arkel, K.V.; Danion, M.; Cachot, J. Environmental samples of microplastics induce significant toxic effects in fish larvae. Environ. Int. 2020, 134, 105047. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental impact of food packaging materials: A review of contemporary development from conventional plastics to polylactic acid based materials. Materials 2020, 13, 499413. [Google Scholar] [CrossRef]
- Ali, W.; Ali, H.; Gillani, S.; Zinck, P.; Souissi, S. Polylactic acid synthesis, biodegradability, conversion to microplastics and toxicity: A review. Environ. Chem. Lett. 2023, 21, 1761–1786. [Google Scholar] [CrossRef]
- Ribba, L.; Lopretti, M.; Oca-Vasquez, D.G.M.d.B.; Goyanes, S.; Vega-Baudrit, J.R. Biodegradable plastics in aquatic ecosystems: Latest findings, research gaps, and recommendations. Environ. Res. Lett. 2020, 17, 033003. [Google Scholar] [CrossRef]
- Wang, C.; Yu, J.; Lu, Y.; Hua, D.; Wang, X.; Zou, X. Biodegradable microplastics (BMPs): A new cause for concern? Environ. Sci. Pollut. Res. 2021, 28, 66511–66518. [Google Scholar] [CrossRef]
- Su, Y.; Cheng, Z.; Hou, Y.; Lin, S.; Gao, L.; Wang, Z.; Bao, R.; Peng, L. Biodegradable and conventional microplastics posed similar toxicity to marine algae Chlorella vulgaris. Aquat. Toxicol. 2022, 244, 106097. [Google Scholar] [CrossRef]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.; Alam, S.I.; Faggio, C. Effects of heavy metals on fish physiology—A review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef]
- Shaw, P.; Mondal, P.; Bandyopadhyay, A.; Chattopadhyay, A. Environmentally relevant concentration of chromium activates Nrf2 and alters transcription of related XME genes in liver of zebrafish. Chemosphere 2019, 214, 35–46. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, B.D.; Zhao, J.L.; Jiang, N.; Zhang, A.Z.; Wang, G.Q.; Li, M.Y. Amelioration of hexavalent chromium-induced bioaccumulation, oxidative stress, tight junction proteins and immune-related signaling factors by allium mongolicum regel flavonoids in Ctenopharyngodon idella. Fish. Shellfish Immun. 2020, 106, 993–1003. [Google Scholar] [CrossRef]
- Chaâbane, M.; Bejaoui, S.; Trabelsi, W.; Telahigue, K.; Chetoui, I.; Chalghaf, M.; Zeghal, N.; Cafsi, M.h.E.; Soudani, N. The potential toxic effects of hexavalent chromium on oxidative stress biomarkers and fatty acids profile in soft tissues of Venus verrucosa. Ecotoxicol. Environ. Saf. 2020, 196, 110562. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, H.; Hwang, U.K.; Kang, J.C.; Kang, Y.J.; Kim, K.I.; Kim, J.H. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ. Toxicol. Phar. 2019, 68, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gupta, S.K.; Bhushan, S.; Singh, N.P. Impacts of acute toxicity of arsenic (III) alone and with high temperature on stress biomarkers, immunological status and cellular metabolism in fish. Aquat. Toxicol. 2019, 214, 105233. [Google Scholar] [CrossRef]
- Fred-Ahmadu, O.H.; Ayejuyo, O.O.; Tenebe, I.T.; Benson, N.U. Occurrence and distribution of micro(meso)plastic-sorbed heavy metals and metalloids in sediments, Gulf of Guinea coast (SE Atlantic). Sci. Total Environ. 2022, 813, 152650. [Google Scholar] [CrossRef]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Lu, K.; Qiao, R.; An, H.; Zhang, Y. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio). Chemosphere 2018, 202, 514–520. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, Y.H.; Sayed, A.E.D.H.; Jeong, C.B.; Zhou, B.; Lee, J.S.; Byeon, E. Short- and long-term single and combined effects of microplastics and chromium on the freshwater water flea Daphnia magna. Aquat. Toxicol. 2022, 253, 106348. [Google Scholar] [CrossRef]
- Kutralam-Muniasamy, G.; Pérez-Guevara, F.; Martínez, I.E.; Shruti, V.C. Overview of microplastics pollution with heavy metals: Analytical methods, occurrence, transferrisks and call for standardization. J. Hazard. Mater. 2021, 415, 125755. [Google Scholar] [CrossRef]
- Han, Y.; Fu, M.; Wu, J.; Zhou, S.; Qiao, Z.; Peng, C.; Zhang, W.; Liu, F.; Ye, C.; Yang, J. Polylactic acid microplastics induce higher biotoxicity of decabromodiphenyl ethane on earthworms (Eisenia fetida) compared to polyethylene and polypropylene microplastics. Sci. Total Environ. 2023, 862, 160909. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Pedrouzo-Rodríguez, A.; Verdú, I.; Leganés, F.; Marco, E.; Rosal, R.; Fernández-Piñas, F. Microplastics as vectors of the antibiotics azithromycin and clarithromycin: Effects towards freshwater microalgae. Chemosphere 2021, 268, 128824. [Google Scholar] [CrossRef]
- Jang, F.H.; Wong, C.; Choo, J.; Sia, E.S.A.; Mujahid, A.; Müller, M. Increased transfer of trace metals and Vibrio sp. from biodegradable microplastics to catfish Clarias gariepinus. Environ. Pollut. 2022, 298, 118850. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lu, L.; Zheng, M.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma). Environ. Pollut. 2019, 254, 113024. [Google Scholar] [CrossRef]
- Kang, H.M.; Byeon, E.; Jeong, H.; Kim, M.S.; Chen, Q.; Lee, J.S. Different effects of nano- and microplastics on oxidative status and gut microbiota in the marine medaka Oryzias melastigma. J. Hazard. Mater. 2021, 405, 124207. [Google Scholar] [CrossRef]
- Huang, J.N.; Wen, B.; Xu, L.; Ma, H.C.; Li, X.X.; Gao, J.Z.; Chen, Z.Z. Micro/nano-plastics cause neurobehavioral toxicity in discus fish (Symphysodon aequifasciatus): Insight from brain-gut-microbiota axis. J. Hazard. Mater. 2022, 421, 126830. [Google Scholar] [CrossRef]
- Oliveira, J.P.J.; Estrela, F.N.; Rodrigues, A.S.L.; Guimaraes, A.T.B.; Rocha, T.L.; Malafaia, G. Behavioral and biochemical consequences of Danio rerio larvae exposure to polylactic acid bioplastic. J. Hazard. Mater. 2021, 404, 124152. [Google Scholar] [CrossRef]
- Guimarães, A.T.B.; Estrela, F.N.; Rodrigues, A.S.D.L.; Chagas, T.Q.; Pereira, P.S.; Silva, F.G.; Malafaia, G. Nanopolystyrene particles at environmentally relevant concentrations causes behavioral and biochemical changes in juvenile grass carp (Ctenopharyngodon idella). J. Hazard. Mater. 2021, 403, 123864. [Google Scholar] [CrossRef]
- Kim, J.; Haque, M.N.; Lee, S.; Lee, D.H.; Rhee, J.S. Exposure to environmentally relevant concentrations of polystyrene microplastics increases hexavalent chromium toxicity in aquatic animals. Toxics 2022, 10, 563. [Google Scholar] [CrossRef]
- Salerno, M.; Berlino, M.; Mangano, M.C.; Gianluca, S. Microplastics and the functional traits of fishes: A global meta-analysis. Glob. Chang. Biol. 2021, 27, 2645–2655. [Google Scholar] [CrossRef]
- Zimmermann, L.; Göttlich, S.; Oehlmann, J.; Wagner, M.; Völker, C. What are the drivers of microplastic toxicity? Comparing the toxicity of plastic chemicals and particles to Daphnia magna. Environ. Pollut. 2020, 267, 115392. [Google Scholar] [CrossRef]
- Castro-Castellon, A.T.; Horton, A.A.; Hughes, J.M.R.; Rampley, C.; Jeffers, E.S.; Bussi, G.; Whitehead, P. Ecotoxicity of microplastics to freshwater biota: Considering exposure and hazard across trophic levels. Sci. Total Environ. 2022, 816, 151638. [Google Scholar] [CrossRef]
- Jia, H.; Di, S.; Bei-bei, W.; Cheng-lian, F.; Hai-lei, S.; Ying, W.; Ning, Q. Ecological risk assessment and water quality standard evaluation of 10 typical metals in eight basins in China. China Environ. Sci. 2019, 39, 2970–2982. [Google Scholar] [CrossRef]
- Savoca, M.S.; Wohlfeil, M.E.; Ebeler, S.E.; Nevitt, G.A. Marine plastic debris emits a keystone infochemical for olfactory foraging seabirds. Sci. Adv. 2016, 2, e1600395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qiao, R.; Zhang, S.; Wang, G. Metabolomic profiling reveals the intestinal toxicity of different length of microplastic fibers on zebrafish (Danio rerio). J. Hazard. Mater. 2021, 403, 123663. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.L.; Yang, J.Y. Microplastic serves as a potential vector for Cr in an in-vitro human digestive model. Sci. Total Environ. 2020, 703, 134805. [Google Scholar] [CrossRef] [PubMed]
- Xue-chun, S.; Shuang-qing, H.; Qi, Z.; Kai-lin, G.; Meng-ru, F.; Wei, Z.; Cheng, P. Research progress on biotoxicological effects and mechanism of polylactic acid microplastics and their combined pollution. China Environ. Sci. 2023, 43, 935–945. [Google Scholar] [CrossRef]
- Zheng, T.; Yuan, D.; Liu, C. Molecular toxicity of nanoplastics involving in oxidative stress and desoxyribonucleic acid damage. J. Mol. Recognit. 2019, 32, e2804. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.B.; Kang, H.M.; Lee, M.C.; Kim, D.H.; Han, J.; Hwang, D.S.; Souissi, S.; Lee, S.J.; Shin, K.H.; Park, H.G.; et al. Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana. Sci. Rep. 2017, 7, 41323. [Google Scholar] [CrossRef] [PubMed]
- Godoy, V.; Martínez-Férez, A.; Martín-Lara, M.Á.; Vellido-Pérez, J.A.; Calero, M.; Blázquez, G. Microplastics as vectors of chromium and lead during dynamic simulation of the human gastrointestinal tract. Sustainability 2020, 12, 4792. [Google Scholar] [CrossRef]
- Chagas, T.Q.; Freitas, Í.N.; Montalvão, M.F.; Nobrega, R.H.; Machado, M.R.F.; Charlie-Silva, I.; Araújo, A.P.D.C.; Guimarães, A.T.B.; Alvarez, T.G.D.S.; Malafaia, G. Multiple endpoints of polylactic acid biomicroplastic toxicity in adult zebrafish (Danio rerio). Chemosphere 2021, 277, 130279. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Hentschel, B.T.; Teh, S.J. Long-term sorption of metals is similar among plastic types: Implications for plastic debris in aquatic environments. PLoS ONE 2014, 9, e85433. [Google Scholar] [CrossRef]
- Varg, J.E.; Bergvall, C.; Svanback, R. The stressful effects of microplastics associated with chromium (VI) on the microbiota of daphnia magna. Front. Environ. Sci. 2022, 10, 875512. [Google Scholar] [CrossRef]
- Zhang, Q.; Pardo, M.; Rudich, Y.; Kaplan-Ashiri, I.; Wong, J.P.S.; Davis, A.Y.; Black, M.S.; Weber, R.J. Chemical composition and toxicity of particles emitted from a consumer-level 3D printer using various materials. Environ. Sci. Technol. 2019, 53, 12054–12061. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Ma, Y.; Li, Q.; Chen, N.; Wen, S. Stressful Effects of Individual and Combined Exposure to Low-Concentration Polylactic Acid Microplastics and Chromium on Marine Medaka Larvae (Oryzias melastigma). Toxics 2024, 12, 594. https://doi.org/10.3390/toxics12080594
Yin Y, Ma Y, Li Q, Chen N, Wen S. Stressful Effects of Individual and Combined Exposure to Low-Concentration Polylactic Acid Microplastics and Chromium on Marine Medaka Larvae (Oryzias melastigma). Toxics. 2024; 12(8):594. https://doi.org/10.3390/toxics12080594
Chicago/Turabian StyleYin, Yuan, Yini Ma, Qiang Li, Nan Chen, and Shaobai Wen. 2024. "Stressful Effects of Individual and Combined Exposure to Low-Concentration Polylactic Acid Microplastics and Chromium on Marine Medaka Larvae (Oryzias melastigma)" Toxics 12, no. 8: 594. https://doi.org/10.3390/toxics12080594
APA StyleYin, Y., Ma, Y., Li, Q., Chen, N., & Wen, S. (2024). Stressful Effects of Individual and Combined Exposure to Low-Concentration Polylactic Acid Microplastics and Chromium on Marine Medaka Larvae (Oryzias melastigma). Toxics, 12(8), 594. https://doi.org/10.3390/toxics12080594




