Effects of Strawberry Tree Water Leaf Extract and Arbutin on Biochemical Markers and DNA Integrity in Brain Cells of Lewis Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.2.1. Ethical Statement
2.2.2. Laboratory Animals
2.2.3. Treatment Protocol
2.3. Biochemical Analyses
2.3.1. Determination of Total Proteins
2.3.2. Lipid Peroxidation
2.3.3. Catalase Activity
2.3.4. Superoxide Dismutase Activity
2.4. Comet Assay
2.5. Statistical Analysis
3. Results and Discussion
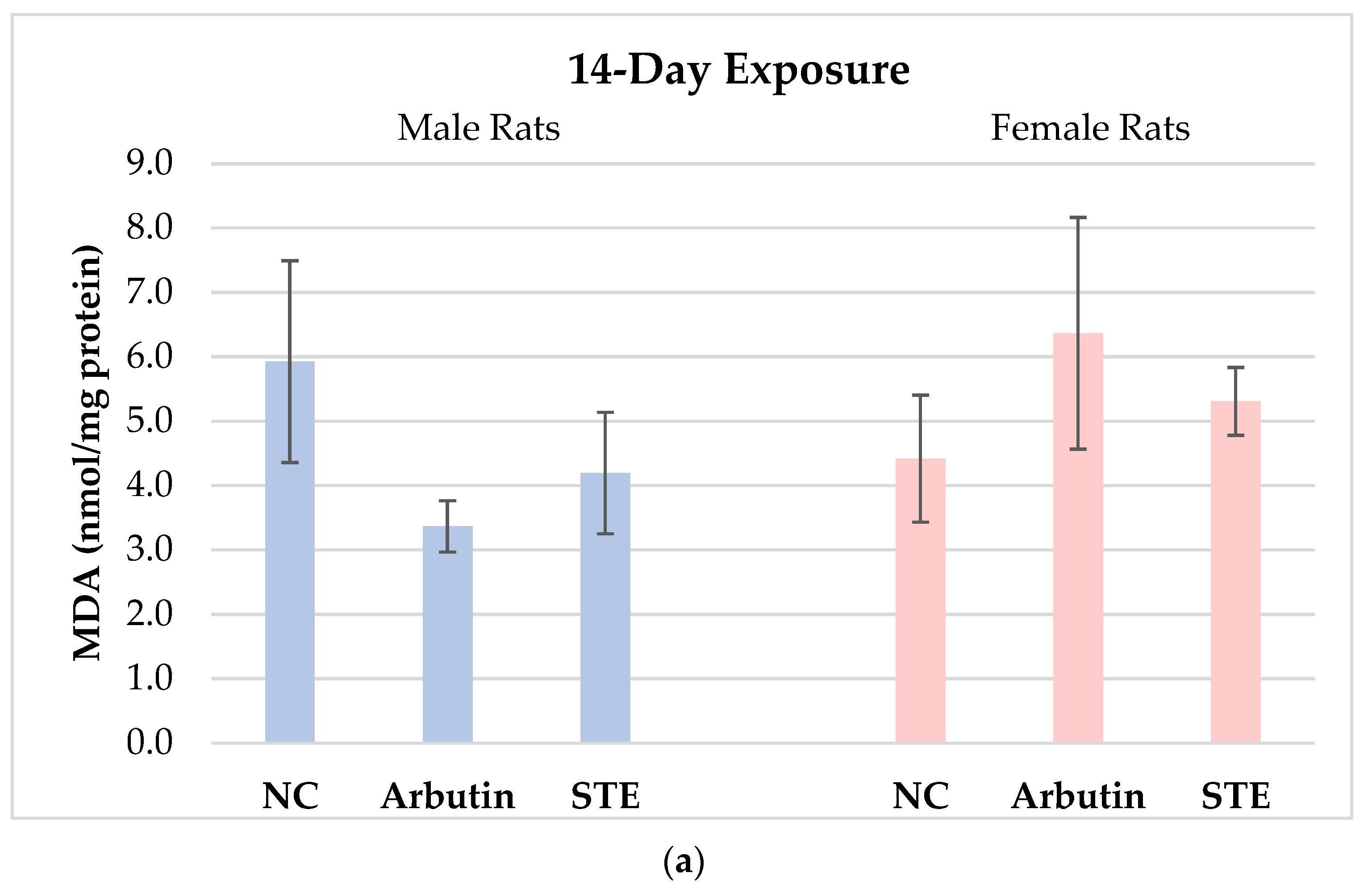
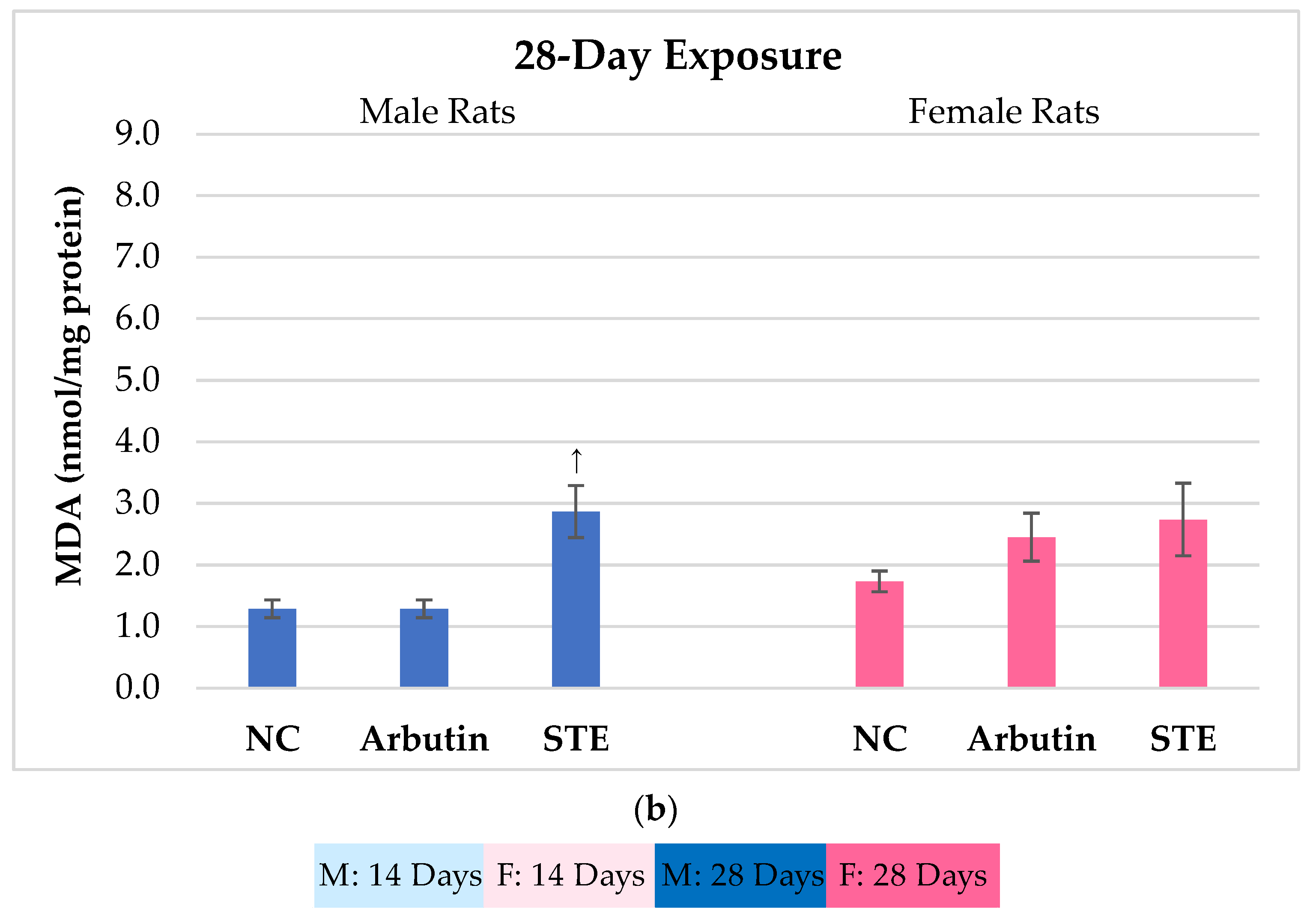
3.1. Lipid Peroxidation
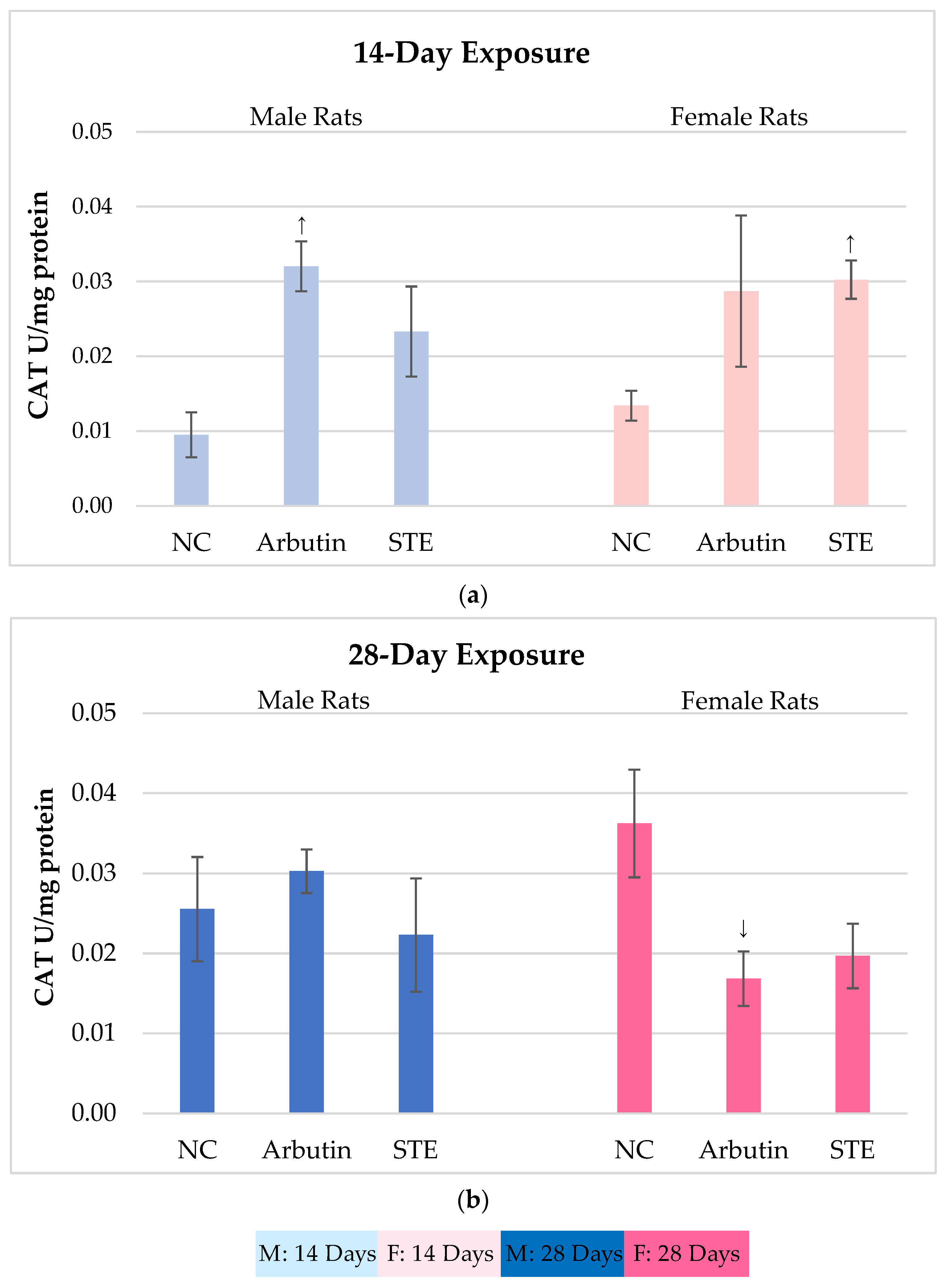
3.2. Catalase (CAT) Activity
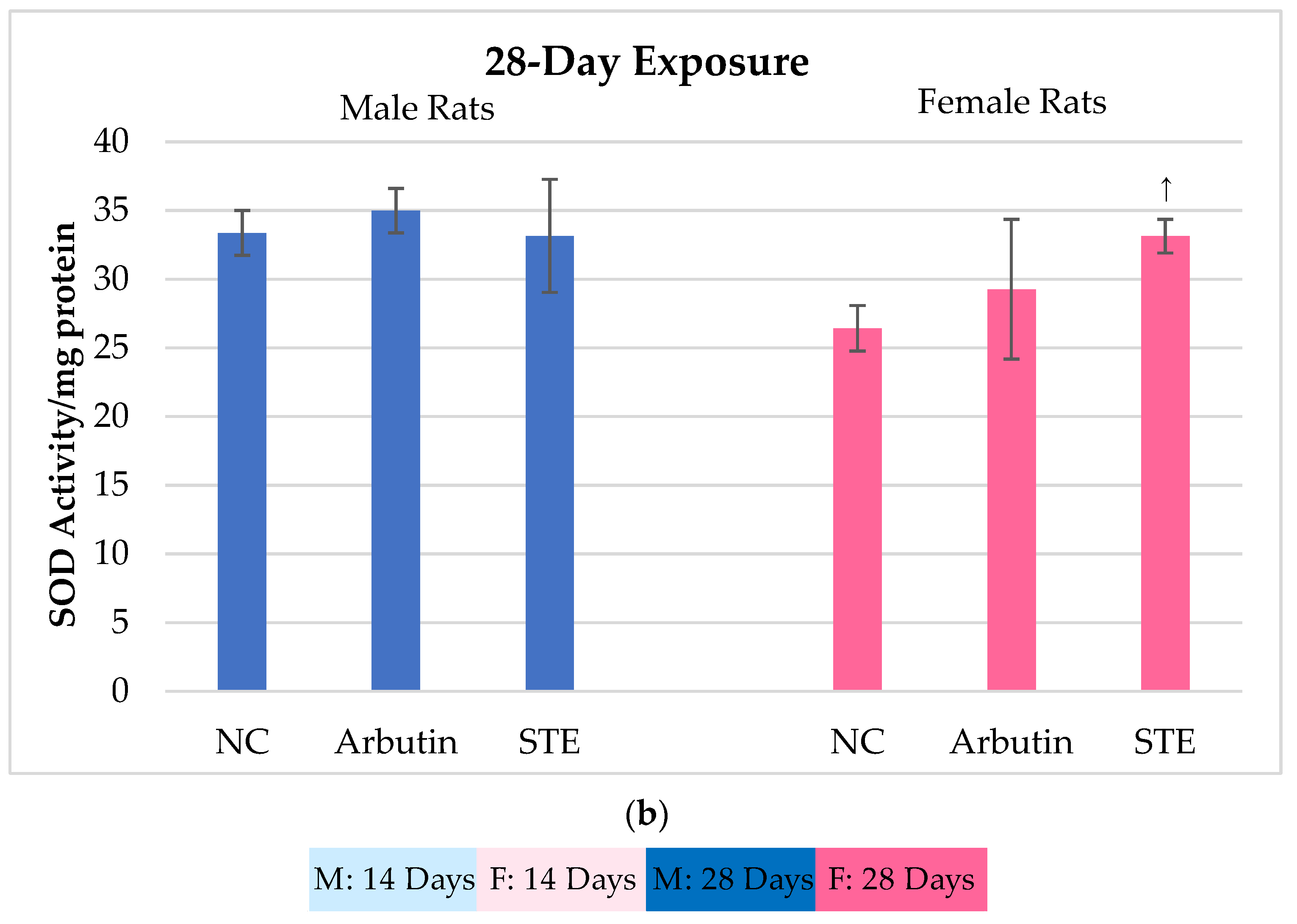
3.3. Superoxide Dismutase (SOD) Activity
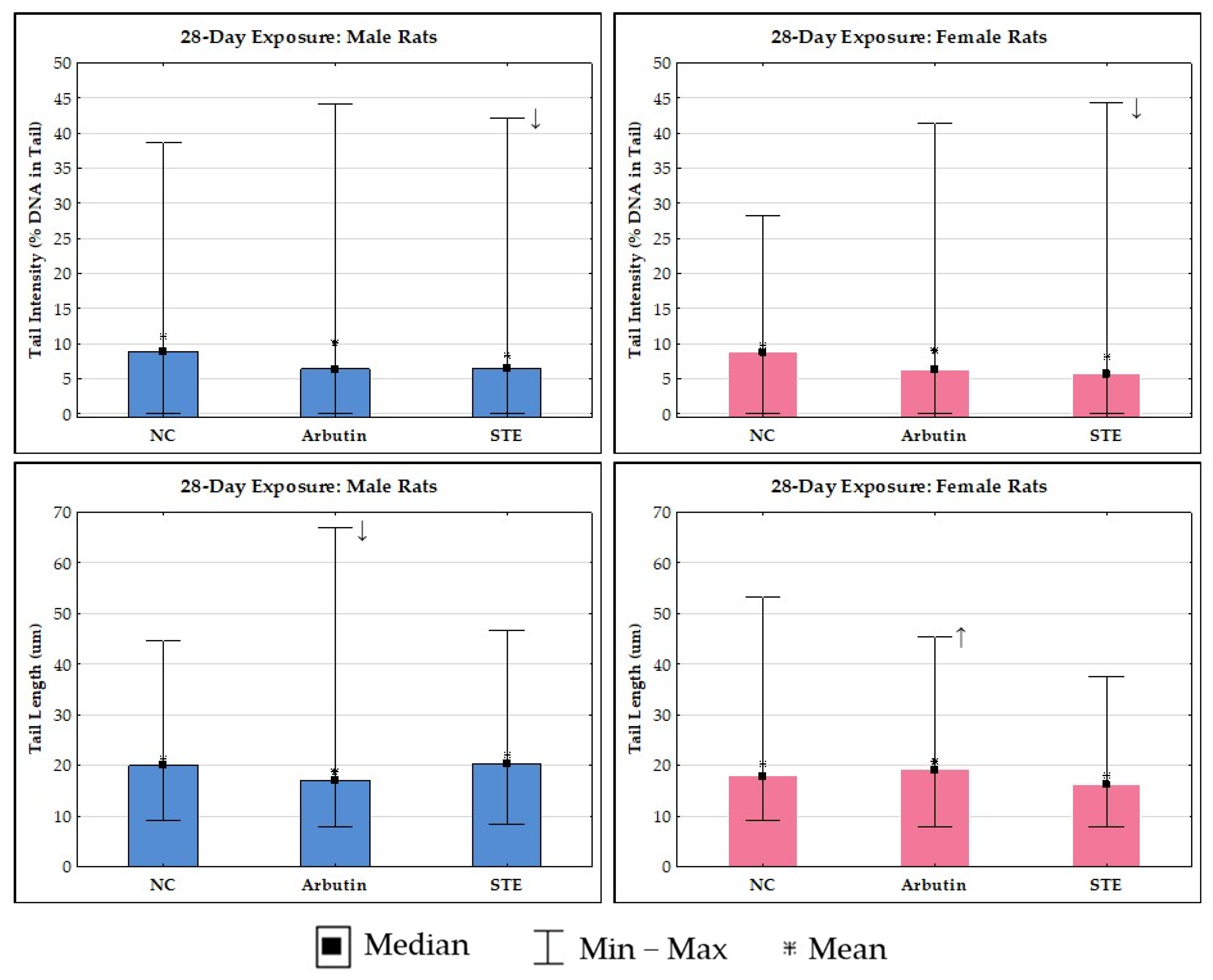
3.4. Alkaline Comet Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Migas, P.; Krauze-Baranowska, M. The Significance of Arbutin and Its Derivatives in Therapy and Cosmetics. Phytochem. Lett. 2015, 13, 35–40. [Google Scholar] [CrossRef]
- Jurica, K.; Gobin, I.; Kremer, D.; Vitali Čepo, D.; Jurišić Grubešić, R.; Brčić Karačonji, I.; Kosalec, I. Arbutin and Its Metabolite Hydroquinone as the Main Factors in the Antimicrobial Effect of Strawberry Tree (Arbutus unedo L.) Leaves. J. Herb. Med. 2017, 8, 17–23. [Google Scholar] [CrossRef]
- Afshar, K.; Fleischmann, N.; Schmiemann, G.; Bleidorn, J.; Hummers-Pradier, E.; Friede, T.; Wegscheider, K.; Moore, M.; Gágyor, I. Reducing Antibiotic Use for Uncomplicated Urinary Tract Infection in General Practice by Treatment with Uva-Ursi (REGATTA)—A Double-Blind, Randomized, Controlled Comparative Effectiveness Trial. BMC Complement Altern. Med. 2018, 18, 203. [Google Scholar] [CrossRef] [PubMed]
- Sadeghinezhad, S.; Khodamoradi, E.; Diojan, L.; Taeb, S.; Najafi, M. Radioprotective Mechanisms of Arbutin: A Systematic Review. Curr. Drug Res. Rev. 2022, 14, 132–138. [Google Scholar] [CrossRef]
- Hu, Z.M.; Zhou, Q.; Lei, T.C.; Ding, S.F.; Xu, S.Z. Effects of Hydroquinone and Its Glucoside Derivatives on Melanogenesis and Antioxidation: Biosafety as Skin Whitening Agents. J. Dermatol. Sci. 2009, 55, 179–184. [Google Scholar] [CrossRef]
- Ahmadian, S.R.; Ghasemi-Kasman, M.; Pouramir, M.; Sadeghi, F. Arbutin Attenuates Cognitive Impairment and Inflammatory Response in Pentylenetetrazol-Induced Kindling Model of Epilepsy. Neuropharmacology 2019, 146, 117–127. [Google Scholar] [CrossRef]
- Akkoyun, H.T.; Uyar, A.; Bayramoglu Akkoyun, M.; Bengü, A.Ş.; Melek, Ş.; Karagözoğlu, F.; Aydın, S.; Ekin, S.; Erdem, S.A. The Protective Effect of Arbutin against Potassium Bromate-Induced Oxidative Damage in the Rat Brain. J. Biochem. Mol. Toxicol. 2023, 37, e23248. [Google Scholar] [CrossRef]
- Dadgar, M.; Pouramir, M.; Dastan, Z.; Ghasemi-Kasman, M.; Ashrafpour, M.; Moghadamnia, A.A.; Khafri, S.; Pourghasem, M. Arbutin Attenuates Behavioral Impairment and Oxidative Stress in an Animal Model of Parkinson’s Disease. Avicenna J. Phytomed. 2018, 8, 533–542. [Google Scholar]
- Ebrahim-Tabar, F.; Nazari, A.; Pouramir, M.; Ashrafpour, M.; Pourabdolhossein, F. Arbutin Improves Functional Recovery and Attenuates Glial Activation in Lysolecethin-Induced Demyelination Model in Rat Optic Chiasm. Mol. Neurobiol. 2020, 57, 3228–3242. [Google Scholar] [CrossRef]
- Tan, J.; Yadav, M.K.; Devi, S.; Kumar, M. Neuroprotective Effects of Arbutin against Oxygen and Glucose Deprivation-Induced Oxidative Stress and Neuroinflammation in Rat Cortical Neurons. Acta Pharm. 2022, 72, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Zha, Z.; Liu, S.; Liu, Y.; Li, C.; Wang, L. Potential Utility of Natural Products against Oxidative Stress in Animal Models of Multiple Sclerosis. Antioxidants 2022, 11, 1495. [Google Scholar] [CrossRef]
- Zhao, J.; Kumar, M.; Sharma, J.; Yuan, Z. Arbutin Effectively Ameliorates the Symptoms of Parkinson’s Disease: The Role of Adenosine Receptors and Cyclic Adenosine Monophosphate. Neural Regen. Res. 2021, 16, 2030–2040. [Google Scholar] [CrossRef]
- Jurica, K.; Brčić Karačonji, I.; Mikolić, A.; Milojković-Opsenica, D.; Benković, V.; Kopjar, N. In Vitro Safety Assessment of the Strawberry Tree (Arbutus Unedo L.) Water Leaf Extract and Arbutin in Human Peripheral Blood Lymphocytes. Cytotechnology 2018, 70, 1261–1278. [Google Scholar] [CrossRef]
- Jurica, K.; Brčić Karačonji, I.; Kopjar, N.; Shek-Vugrovečki, A.; Cikač, T.; Benković, V. The Effects of Strawberry Tree Water Leaf Extract, Arbutin and Hydroquinone on Haematological Parameters and Levels of Primary DNA Damage in White Blood Cells of Rats. J. Ethnopharmacol. 2018, 215, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Jurica, K.; Benković, V.; Sikirić, S.; Kopjar, N.; Brčić Karačonji, I. Liver Function and DNA Integrity in Hepatocytes of Rats Evaluated after Treatments with Strawberry Tree (Arbutus Unedo L.) Water Leaf Extract and Arbutin. Drug Chem. Toxicol. 2020, 43, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Jurica, K.; Benković, V.; Sikirić, S.; Brčić Karačonji, I.; Kopjar, N. The Effects of Strawberry Tree (Arbutus Unedo L.) Water Leaf Extract and Arbutin upon Kidney Function and Primary DNA Damage in Renal Cells of Rats. Nat. Prod. Res. 2020, 34, 2354–2357. [Google Scholar] [CrossRef]
- NTP. Chemical Information Review Document for Arbutin [CAS No. 497-76-7] and Extracts from Arctostaphylos Uva-Ursi; National Toxicology Program; National Institute of Environmental Health Sciences: Durham, NC, USA, 2006; pp. 1–38. [Google Scholar]
- Garcia de Arriba, S.; Naser, B.; Nolte, K.-U. Risk Assessment of Free Hydroquinone Derived from Arctostaphylos Uva-Ursi Folium Herbal Preparations. Int. J. Toxicol. 2013, 32, 442–453. [Google Scholar] [CrossRef]
- EU. Scientific Committee on Consumer Safety Opinion on β-Arbutin; European Commission: Luxembourg, 2015. [Google Scholar]
- Brčić Karačonji, I.; Jurica, K.; Gašić, U.; Dramićanin, A.; Tešić, Ž.; Milojković Opsenica, D. Comparative Study on the Phenolic Fingerprint and Antioxidant Activity of Strawberry Tree (Arbutus Unedo L.) Leaves and Fruits. Plants 2022, 11, 25. [Google Scholar] [CrossRef]
- Jurica, K.; Brčić Karačonji, I.; Šegan, S.; Milojković Opsenica, D.; Kremer, D. Quantitative Analysis of Arbutin and Hydroquinone in Strawberry Tree (Arbutus Unedo L., Ericaceae) Leaves by Gas Chromatography-Mass Spectrometry. Arh. Hig. Rada Toksikol. 2015, 66, 197–202. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 407: Repeated Dose 90-Day Oral Toxicity Study in Rodents; OECD: Paris, France, 2008. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Jutrić, D.; Đikić, D.; Boroš, A.; Odeh, D.; Gračan, R.; Beletić, A.; Landeka Jurčević, I. Combined Effects of Valproate and Naringin on Kidney Antioxidative and Serum Parameters of Kidney Function in C57BL6 Mice. Arh. Hig. Rada Toksikol. 2023, 74, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Benković, V.; Milić, M.; Oršolić, N.; Knežević, A.H.; Brozović, G.; Borojević, N. Different Damaging Effects of Volatile Anaesthetics Alone or in Combination with 1 and 2 Gy Gamma-Irradiation in Vivo on Mouse Liver DNA: A Preliminary Study. Arh. Hig. Rada Toksikol. 2023, 74, 22–33. [Google Scholar] [CrossRef]
- Kašuba, V.; Micek, V.; Milić, M.; Želježić, D.; Katić, A. The Effect of Low Doses of Chlorpyrifos on Blood and Bone Marrow Cells in Wistar Rats. Arh. Hig. Rada Toksikol. 2022, 73, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Jomová, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3′,4′-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper(II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef]
- Maliar, T.; Maliarová, M.; Blažková, M.; Kunštek, M.; Uváčková, L.; Viskupičová, J.; Purdešová, A.; Benovič, P. Simultaneously Determined Antioxidant and Pro-Oxidant Activity of Randomly Selected Plant Secondary Metabolites and Plant Extracts. Molecules 2023, 28, 6890. [Google Scholar] [CrossRef]
- Rajashekar, C.B. Dual Role of Plant Phenolic Compounds as Antioxidants and Prooxidants. Am. J. Plant Sci. 2023, 14, 15–28. [Google Scholar] [CrossRef]
- McGregor, D. Hydroquinone: An Evaluation of the Human Risks from Its Carcinogenic and Mutagenic Properties. Crit. Rev. Toxicol. 2007, 37, 887–914. [Google Scholar] [CrossRef]
- Li, J.; Jiang, S.; Chen, Y.; Ma, R.; Chen, J.; Qian, S.; Shi, Y.; Han, Y.; Zhang, S.; Yu, K. Benzene Metabolite Hydroquinone Induces Apoptosis of Bone Marrow Mononuclear Cells through Inhibition of β-Catenin Signalling. Toxicol. Vitro 2018, 46, 361–369. [Google Scholar] [CrossRef]
- Scucuglia Heluany, C.; De Palma, A.; Day, N.J.; Poliselli Farsky, S.H.; Nalesso, G. Hydroquinone, an Environmental Pollutant, Affects Cartilage through the Activation of the Aryl Hydrocarbon Pathway. Cells 2023, 12, 690. [Google Scholar] [CrossRef]
- Sakihama, Y.; Cohen, M.F.; Grace, S.C.; Yamasaki, H. Plant Phenolic Antioxidant and Prooxidant Activities: Phenolics-Induced Oxidative Damage Mediated by Metals in Plants. Toxicology 2002, 177, 67–80. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Polyphenols as Antioxidant/Pro-Oxidant Compounds and Donors of Reducing Species: Relationship with Human Antioxidant Metabolism. Processes 2023, 11, 2771. [Google Scholar] [CrossRef]
- EMA Assessment Report on Arctostaphylos Uva-Ursi (L.) Spreng. Folium. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-arctostaphylos-uva-ursi-l-spreng-folium-revision-2_en.pdf (accessed on 10 June 2024).
- Tóth, B.; Jávorházy, A.; Nyirády, P.; Csupor-Löffler, B.; Birinyi, P.; Zhanel, G.; Naber, K.; Länger, R.; Vörhendi, N.; Gede, N.; et al. Bearberry in the Treatment of Acute Uncomplicated Cystitis (BRUMI): Protocol of a Multicentre, Randomised Double-Blind Clinical Trial. BMJ Open 2022, 12, 8–14. [Google Scholar] [CrossRef]
- Yang, Q.; Fang, Y.; Zhang, C.; Liu, X.; Wu, Y.; Zhang, Y.; Yang, J.; Yong, K. Exposure to Zinc Induces Lysosomal-Mitochondrial Axis-Mediated Apoptosis in PK-15 Cells. Ecotoxicol. Environ. Saf. 2022, 241, 113716. [Google Scholar] [CrossRef]
- Rasheed, Z. Therapeutic Potentials of Catalase: Mechanisms, Applications, and Future Perspectives. Int. J. Health Sci. 2024, 18, 1–6. [Google Scholar]
- Glorieux, C.; Calderon, P.B. Catalase, a Remarkable Enzyme: Targeting the Oldest Antioxidant Enzyme to Find a New Cancer Treatment Approach. Biol. Chem. 2017, 398, 1095–1108. [Google Scholar] [CrossRef]
- Lee, K.; Cha, M.; Lee, B. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Riley, D.P. Functional Mimics of Superoxide Dismutase Enzymes as Therapeutic Agents. Chem. Rev. 1999, 99, 2573–2587. [Google Scholar] [CrossRef]
- Trist, B.G.; Hilton, J.B.; Hare, D.J.; Crouch, P.J.; Double, K.L. Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic. Angew. Chem. Int. Ed. 2021, 60, 9215–9246. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Anand, N.; Varma, S.R.; Ramamurthy, S.; Vichitra, C.; Sharma, A.; Mahalakshmi, A.M.; Essa, M.M. Superoxide Dismutase and Neurological Disorders. IBRO Neurosci. Rep. 2024, 16, 373–394. [Google Scholar] [CrossRef]
- Azqueta, A.; Collins, A.R. The Essential Comet Assay: A Comprehensive Guide to Measuring DNA Damage and Repair. Arch. Toxicol. 2013, 87, 949–968. [Google Scholar] [CrossRef]
- Møller, P. The Comet Assay: Ready for 30 More Years. Mutagenesis 2018, 33, 1–7. [Google Scholar] [CrossRef]
- Collins, A.; Møller, P.; Gajski, G.; Vodenková, S.; Abdulwahed, A.; Anderson, D.; Bankoglu, E.E.; Bonassi, S.; Boutet-Robinet, E.; Brunborg, G.; et al. Measuring DNA Modifications with the Comet Assay: A Compendium of Protocols. Nat. Protoc. 2023, 18, 929–989. [Google Scholar] [CrossRef]
- OECD. Test No. 489: In Vivo Mammalian Alkaline Comet Assay; OECD: Paris, France, 2014. [Google Scholar]
- Collins, A.R. The Comet Assay for DNA Damage and Repair: Principles, Applications, and Limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Welch, G.; Tsai, L. Mechanisms of DNA Damage-mediated Neurotoxicity in Neurodegenerative Disease. EMBO Rep. 2022, 23, e54217. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Bahorun, T.; Jen, L.S. Neuroprotection by Bioactive Components in Medicinal and Food Plant Extracts. Mutat. Res. Rev. Mutat. Res. 2003, 544, 203–215. [Google Scholar] [CrossRef]
- Chang, C.L.; Lin, C.S.; Lai, G.H. Phytochemical Characteristics, Free Radical Scavenging Activities, and Neuroprotection of Five Medicinal Plant Extracts. Evid. Based Complement. Alternat. Med. 2012, 2012, 984295. [Google Scholar] [CrossRef]
- Akbarian, M.; Hosseini, M.; Mirzavi, F.; Amirahmadi, S.; Arab, F.L.; Rajabian, A. Punica Granatum Peel Supplementation Attenuates Cognitive Deficits and Brain Injury in Rat by Targeting the Nrf2-HO-1 Pathway. Food Sci. Nutr. 2023, 11, 168–180. [Google Scholar] [CrossRef]
- May, N.; de Sousa Alves Neri, J.L.; Clunas, H.; Shi, J.; Parkes, E.; Dongol, A.; Wang, Z.; Jimenez Naranjo, C.; Yu, Y.; Huang, X.F.; et al. Investigating the Therapeutic Potential of Plants and Plant-Based Medicines: Relevance to Antioxidant and Neuroprotective Effects. Nutrients 2023, 15, 3912. [Google Scholar] [CrossRef]
- Moura, M.V.N.; Mesquita da Conceição Bahia, G.; Gonçalves Correa, M.; Araujo Sarges, M.A.; Lobão, T.A.; Sanches, E.M.; Oliveira, K.R.H.M.; Herculano, A.M.; Bahia, C.P. Neuroprotective Effects of Crude Extracts, Compounds, and Isolated Molecules Obtained from Plants in the Central Nervous System Injuries: A Systematic Review. Front. Neurosci. 2023, 17, 1249685. [Google Scholar] [CrossRef]


| Experimental Groups (Total N = 48 Rats) | Treatment | |||
|---|---|---|---|---|
| 14-Day Exposure Per Os, Each Day | 28-Day Exposure Per Os, Each Day | |||
| Negative control | Double-distilled water | Double-distilled water | ||
| N = 4 male | N = 4 female | N = 4 male | N = 4 female | |
| Arbutin | 200 mg/kg b.w., dissolved in double-distilled water | 200 mg/kg b.w., dissolved in double-distilled water | ||
| N = 4 male | N = 4 female | N = 4 male | N = 4 female | |
| Strawberry tree water leaf extract (STE) | 200 mg/kg b.w., dissolved in double-distilled water | 200 mg/kg b.w., dissolved in double-distilled water | ||
| N = 4 male | N = 4 female | N = 4 male | N = 4 female | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benković, V.; Vuković, D.; Đelatić, I.; Popović, V.; Jurica, K.; Knežević, F.; Brčić Karačonji, I.; Lucić Vrdoljak, A.; Kopjar, N. Effects of Strawberry Tree Water Leaf Extract and Arbutin on Biochemical Markers and DNA Integrity in Brain Cells of Lewis Rats. Toxics 2024, 12, 595. https://doi.org/10.3390/toxics12080595
Benković V, Vuković D, Đelatić I, Popović V, Jurica K, Knežević F, Brčić Karačonji I, Lucić Vrdoljak A, Kopjar N. Effects of Strawberry Tree Water Leaf Extract and Arbutin on Biochemical Markers and DNA Integrity in Brain Cells of Lewis Rats. Toxics. 2024; 12(8):595. https://doi.org/10.3390/toxics12080595
Chicago/Turabian StyleBenković, Vesna, Dora Vuković, Iva Đelatić, Vanja Popović, Karlo Jurica, Fabijan Knežević, Irena Brčić Karačonji, Ana Lucić Vrdoljak, and Nevenka Kopjar. 2024. "Effects of Strawberry Tree Water Leaf Extract and Arbutin on Biochemical Markers and DNA Integrity in Brain Cells of Lewis Rats" Toxics 12, no. 8: 595. https://doi.org/10.3390/toxics12080595






