Green Tea Polyphenol Nanoparticles Reduce Anxiety Caused by Tobacco Smoking Withdrawal in Rats by Suppressing Neuroinflammation †
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
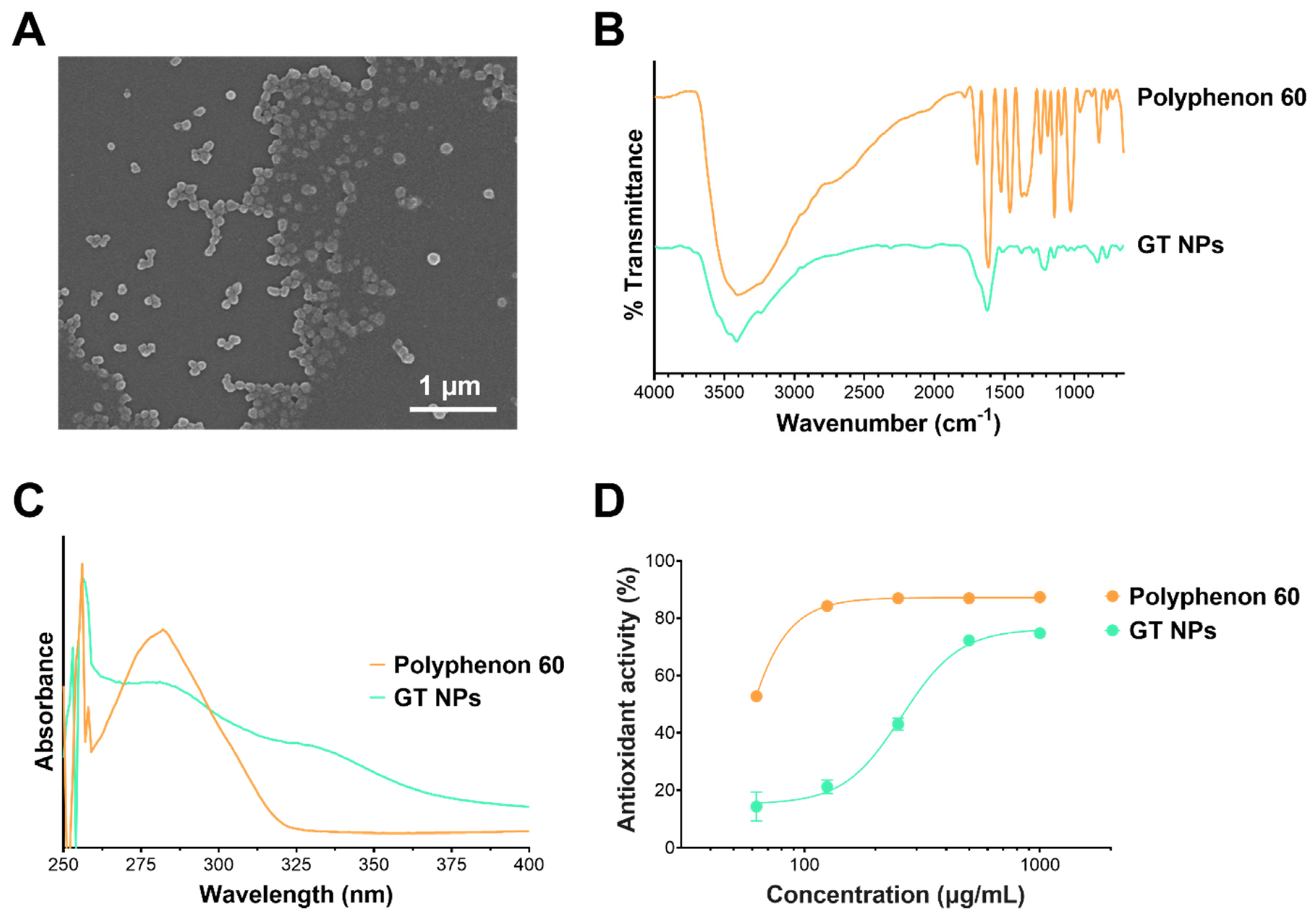
2.2. Preparation and Characterization of Green Tea Nanoparticles (GT NPs)
2.3. Characterization of GT NPs
2.4. Antioxidant Activity of GT NPs
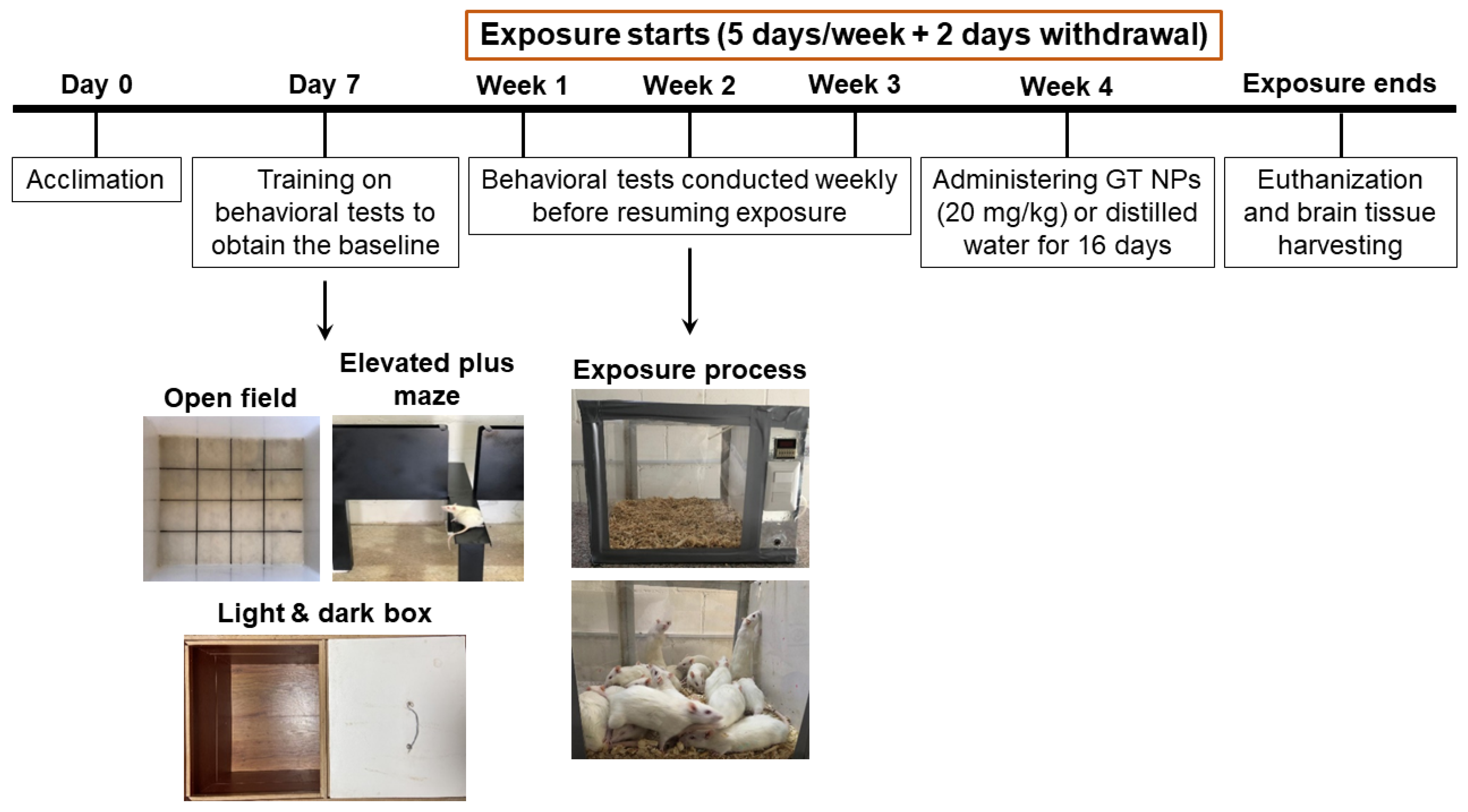
3. Animals
Tobacco Smoke Exposure Animal Model
4. Behavioral Tests
4.1. Open Field (OF) Test
4.2. Light and Dark Box (LDB) Test
4.3. Elevated Plus Maze (EPM) Test
4.4. Brain Tissue Harvesting
4.5. Real-Time Quantitative PCR (RT-PCR)
4.6. Western Blot
4.7. Statistical Analysis
5. Results
5.1. Preparation of NPs from Green Tea Polyphenols via Oxidative Coupling Assembly
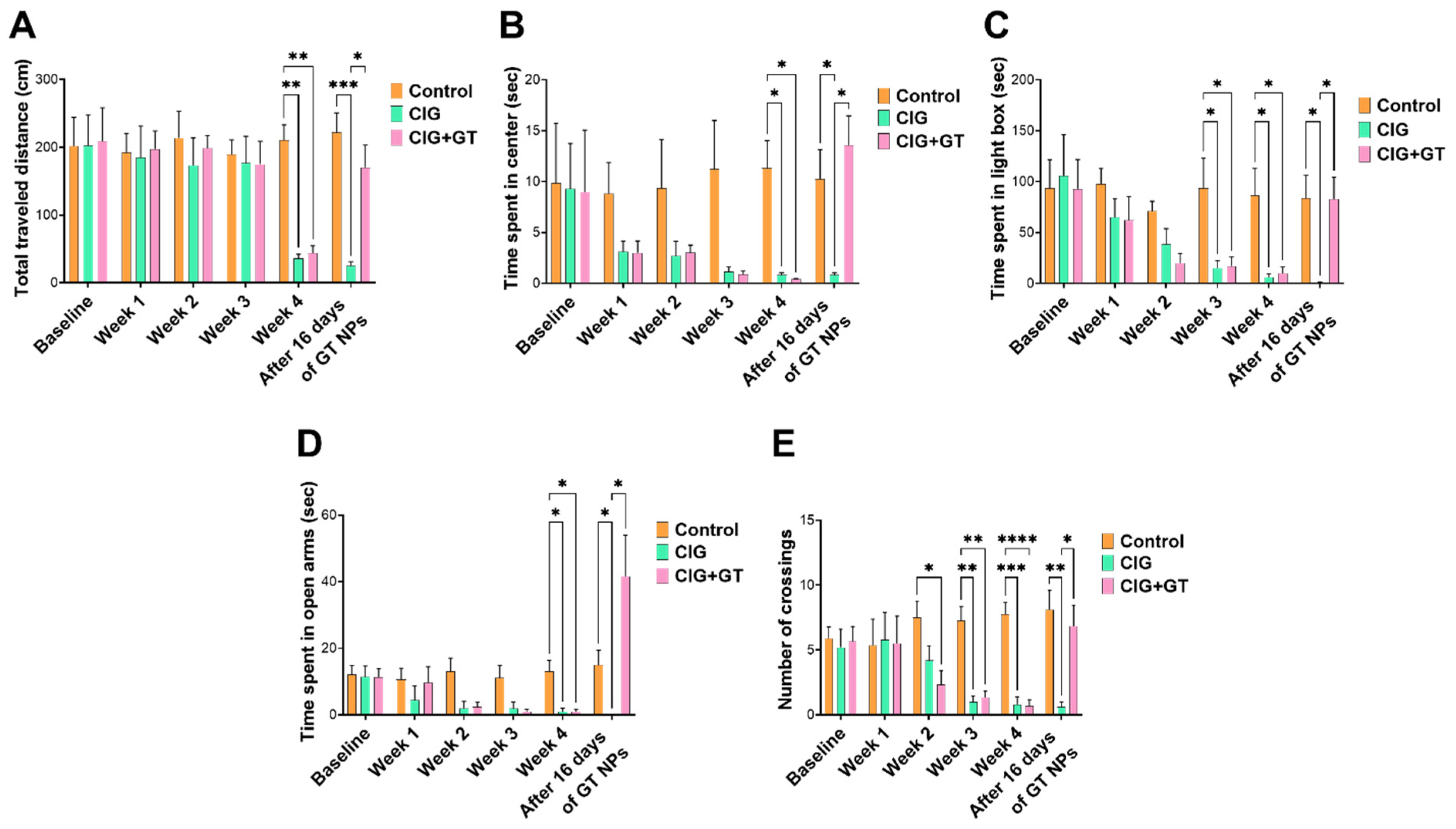
5.2. Impact of Tobacco Smoke Exposure and GT NP Intervention on Anxiety-like Behavior
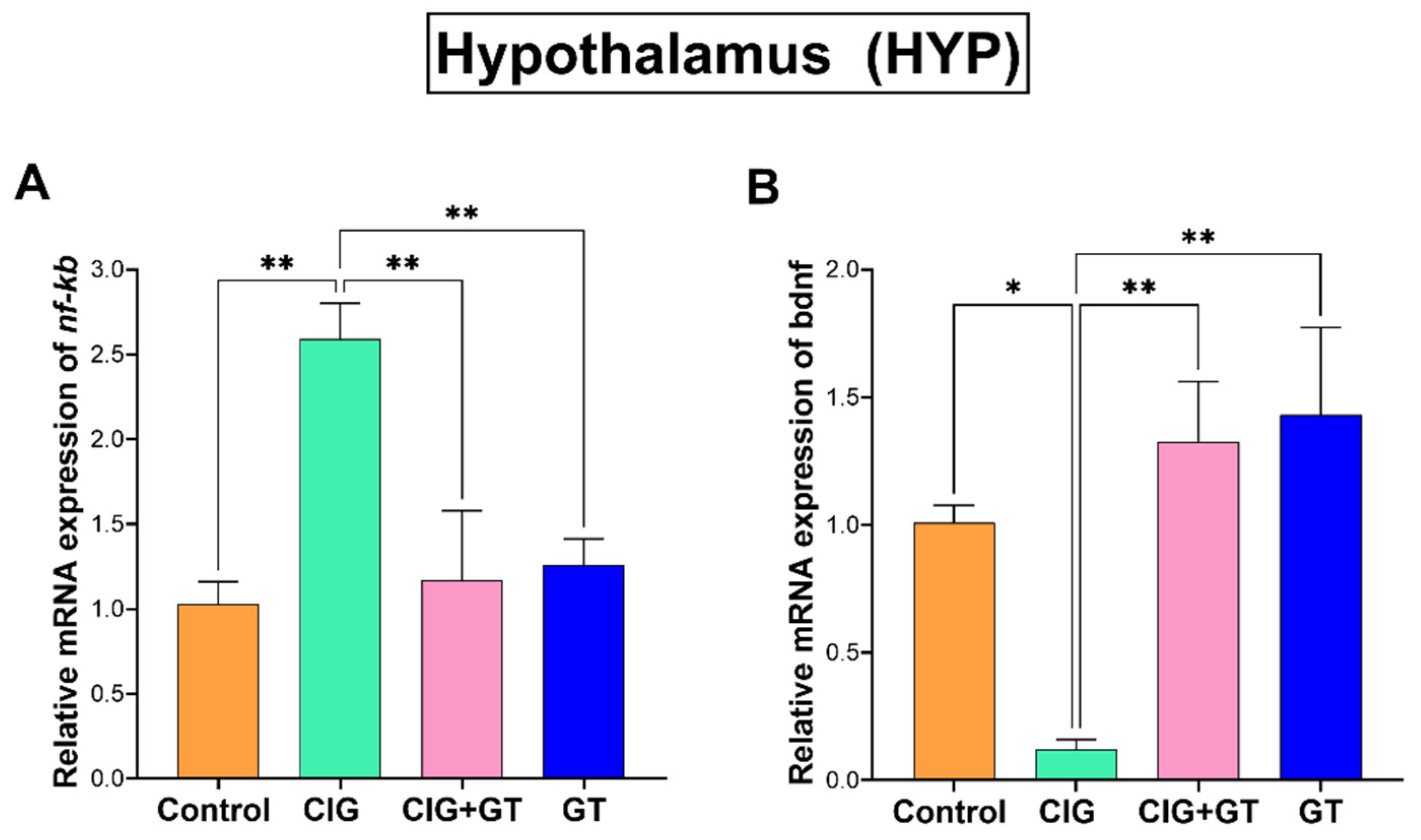
5.3. Impact of Tobacco Smoke Exposure and GT NP Intervention on nf-κb and bdnf Relative mRNA Expression in the HIP and HYP Brain Subregions
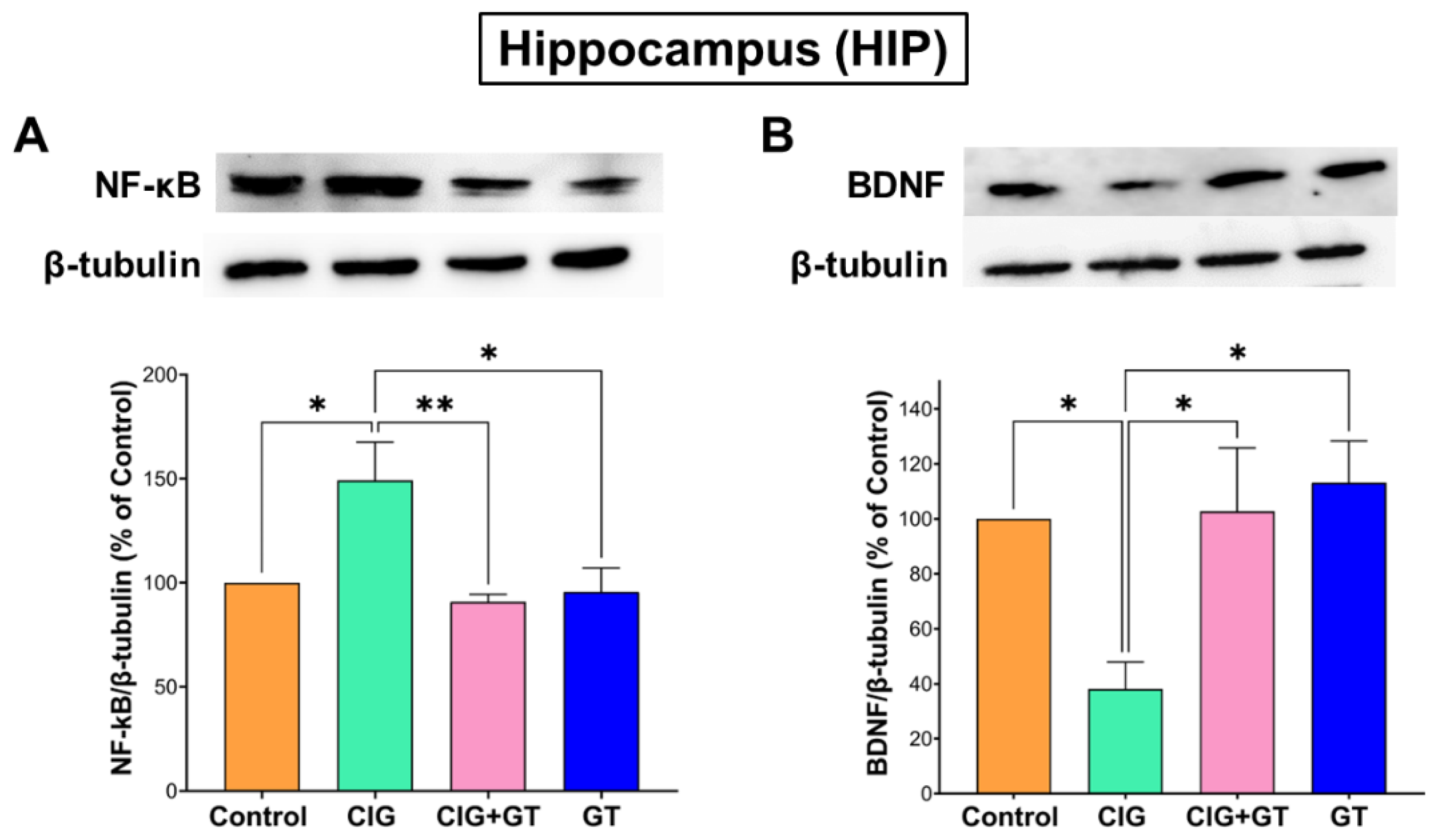
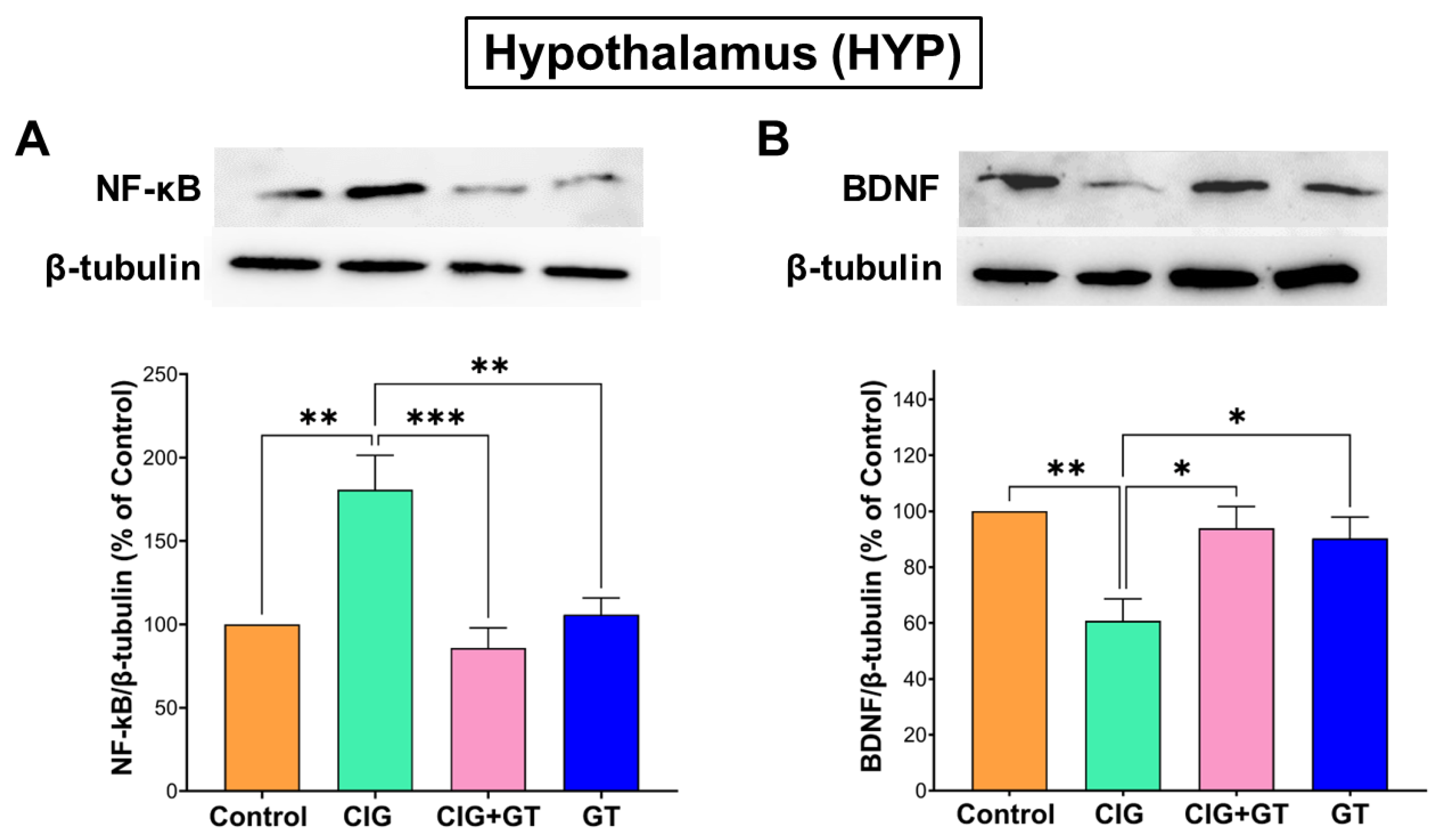
5.4. Impact of Tobacco Smoke Exposure and GT Intervention on Protein Expression Levels of NF-κB and BDNF in the HIP and HYP Brain Subregions
6. Discussion
7. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauer, U.E.; Briss, P.A.; Goodman, R.A.; Bowman, B.A. Prevention of chronic disease in the 21st century: Elimination of the leading preventable causes of premature death and disability in the USA. Lancet 2014, 384, 45–52. [Google Scholar] [CrossRef]
- Gebreslassie, M.; Feleke, A.; Melese, T. Psychoactive substances use and associated factors among Axum University students, Axum Town, North Ethiopia. BMC Public Health 2013, 13, 693. [Google Scholar] [CrossRef]
- Fluharty, M.; Taylor, A.E.; Grabski, M.; Munafò, M.R. The association of cigarette smoking with depression and anxiety: A systematic review. Nicotine Tob. Res. 2016, 19, 3–13. [Google Scholar] [CrossRef]
- Manhaes, A.C.; Guthierrez, M.C.; Filgueiras, C.C.; Abreu-Villaca, Y. Anxiety-like behavior during nicotine withdrawal predict subsequent nicotine consumption in adolescent C57BL/6 mice. Behav. Brain Res. 2008, 193, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R.; Ray, R.; Lee, B.; Everett, L.; Xiang, J.; Jepson, C.; Kaestner, K.H.; Lerman, C.; Blendy, J.A. Evidence from mouse and man for a role of neuregulin 3 in nicotine dependence. Mol. Psychiatry 2014, 19, 801–810. [Google Scholar] [CrossRef]
- Fisher, M.L.; LeMalefant, R.M.; Zhou, L.; Huang, G.; Turner, J.R. Distinct roles of CREB within the ventral and dorsal hippocampus in mediating nicotine withdrawal phenotypes. Neuropsychopharmacology 2017, 42, 1599–1609. [Google Scholar] [CrossRef]
- Levita, L.; Hoskin, R.; Champi, S. Avoidance of harm and anxiety: A role for the nucleus accumbens. Neuroimage 2012, 62, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.J.; Nestler, E.J. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 2013, 14, 609–625. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Sarraj, N.; Taneja, M.; Saha, K.; Tejada-Simon, M.V.; Chugh, G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav. Brain Res. 2010, 208, 545–552. [Google Scholar] [CrossRef]
- Adeluyi, A.; Guerin, L.; Fisher, M.L.; Galloway, A.; Cole, R.D.; Chan, S.S.; Wyatt, M.D.; Davis, S.W.; Freeman, L.R.; Ortinski, P.I. Microglia morphology and proinflammatory signaling in the nucleus accumbens during nicotine withdrawal. Sci. Adv. 2019, 5, eaax7031. [Google Scholar] [CrossRef]
- Rahman, I.; Marwick, J.; Kirkham, P. Redox modulation of chromatin remodeling: Impact on histone acetylation and deacetylation, NF-κB and pro-inflammatory gene expression. Biochem. Pharmacol. 2004, 68, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kang, J.; Ferguson, M.E.; Nagarajan, S.; Badger, T.M.; Wu, X. Blueberries reduce pro-inflammatory cytokine TNF-α and IL-6 production in mouse macrophages by inhibiting NF-κB activation and the MAPK pathway. Mol. Nutr. Food Res. 2011, 55, 1587–1591. [Google Scholar] [CrossRef]
- De Luca, C.; Colangelo, A.M.; Alberghina, L.; Papa, M. Neuro-immune hemostasis: Homeostasis and diseases in the central nervous system. Front. Cell. Neurosci. 2018, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Adachi, N.; Numakawa, T.; Richards, M.; Nakajima, S.; Kunugi, H. New insight in expression, transport, and secretion of brain-derived neurotrophic factor: Implications in brain-related diseases. World J. Biol. Chem. 2014, 5, 409. [Google Scholar] [CrossRef] [PubMed]
- Cirulli, F.; Berry, A.; Chiarotti, F.; Alleva, E. Intrahippocampal administration of BDNF in adult rats affects short-term behavioral plasticity in the Morris water maze and performance in the elevated plus-maze. Hippocampus 2004, 14, 802–807. [Google Scholar] [CrossRef]
- Abu Khalaf, R. Exploring natural products as a source for antidiabetic lead compounds and possible lead optimization. Curr. Top. Med. Chem. 2016, 16, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.B.; Bolling, B.W.; Chen, C.; Xiao, H. Review and perspective on the composition and safety of green tea extracts. Eur. J. Nutr. Food Saf. 2014, 5, 1–31. [Google Scholar] [CrossRef]
- Senanayake, S.N. Green tea extract: Chemistry, antioxidant properties and food applications–A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Park, C.-S.; Kim, D.-J.; Cho, M.-H.; Jin, B.-K.; Pie, J.-E.; Chung, W.-G. Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced Parkinson’s disease in mice by tea phenolic epigallocatechin 3-gallate. Neurotoxicology 2002, 23, 367–374. [Google Scholar] [CrossRef]
- Levites, Y.; Youdim, M.B.; Maor, G.; Mandel, S. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-κB) activation and cell death by tea extracts in neuronal cultures. Biochem. Pharmacol. 2002, 63, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Levites, Y.; Weinreb, O.; Maor, G.; Youdim, M.B.; Mandel, S. Green tea polyphenol (–)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 2001, 78, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Yan, J.; Yang, T.; Yang, X.; Bezard, E.; Zhao, B. Protective effects of green tea polyphenols in the 6-OHDA rat model of Parkinson’s disease through inhibition of ROS-NO pathway. Biol. Psychiatry 2007, 62, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yi, Z.; Sun, Z.; Ma, X.; Li, X. Functional nanoparticles of tea polyphenols for doxorubicin delivery in cancer treatment. J. Mater. Chem. B 2017, 5, 7622–7631. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Yang, P.; Guo, H.; Zhang, S.; Zhang, X.; Zhu, F.; Li, Y. Green Tea Makes Polyphenol Nanoparticles with Radical-Scavenging Activities. Macromol. Rapid Commun. 2017, 38, 1700446. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, C.; Chen, J.; Li, X. Biocompatible, functional spheres based on oxidative coupling assembly of green tea polyphenols. J. Am. Chem. Soc. 2013, 135, 4179–4182. [Google Scholar] [CrossRef] [PubMed]
- Sunoqrot, S.; Al-Shalabi, E.; Al-Bakri, A.G.; Zalloum, H.; Abu-Irmaileh, B.; Ibrahim, L.H.; Zeno, H. Coffee bean polyphenols can form biocompatible template-free antioxidant nanoparticles with various sizes and distinct colors. ACS Omega 2021, 6, 2767–2776. [Google Scholar] [CrossRef]
- Wang, T.; Fan, Q.; Hong, J.; Chen, Z.; Zhou, X.; Zhang, J.; Dai, Y.; Jiang, H.; Gu, Z.; Cheng, Y.; et al. Therapeutic nanoparticles from grape seed for modulating oxidative stress. Small 2021, 17, 2102485. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Al-Bakri, A.G.; Ibrahim, L.H.; Aldaken, N.a. Amphotericin B-loaded plant-inspired polyphenol nanoparticles enhance its antifungal activity and biocompatibility. ACS Appl. Bio Mater. 2022, 5, 5156–5164. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Al-Debsi, T.; Al-Shalabi, E.; Hasan Ibrahim, L.; Faruqu, F.N.; Walters, A.; Palgrave, R.; Al-Jamal, K.T. Bioinspired polymerization of quercetin to produce a curcumin-loaded nanomedicine with potent cytotoxicity and cancer-targeting potential in vivo. ACS Biomater. Sci. Eng. 2019, 5, 6036–6045. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Al-Shalabi, E.; Hasan Ibrahim, L.; Zalloum, H. Nature-inspired polymerization of quercetin to produce antioxidant nanoparticles with controlled size and skin tone-matching colors. Molecules 2019, 24, 3815. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Al-Shalabi, E.; Messersmith, P.B. Facile synthesis and surface modification of bioinspired nanoparticles from quercetin for drug delivery. Biomater. Sci. 2018, 6, 2656–2666. [Google Scholar] [CrossRef]
- Hogg, S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol. Biochem. Behav. 1996, 54, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Seibenhener, M.L.; Wooten, M.C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. JoVE (J. Vis. Exp.) 2015, 96, e52434. [Google Scholar]
- Arrant, A.E.; Schramm-Sapyta, N.L.; Kuhn, C.M. Use of the light/dark test for anxiety in adult and adolescent male rats. Behav. Brain Res. 2013, 256, 119–127. [Google Scholar] [CrossRef]
- Hammad, A.M.; Alzaghari, L.F.; Alfaraj, M.; Al-Shawaf, L.; Sunoqrot, S. Nanoassemblies from the aqueous extract of roasted coffee beans modulate the behavioral and molecular effects of smoking withdrawal-induced anxiety in female rats. Drug Deliv. Transl. Res. 2023, 13, 1967–1982. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Alkurdi, M.; Al Bawab, A.Q.; Hammad, A.M.; Tayyem, R.; Abu Obeed, A.; Abufara, M. Encapsulation of morin in lipid core/PLGA shell nanoparticles significantly enhances its anti-inflammatory activity and oral bioavailability. Saudi Pharm. J. 2023, 31, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, R.E. The declaration of Helsinki. In The Oxford Textbook of Clinical Research Ethics; Oxford University Press: Oxford, UK, 2008; pp. 141–148. [Google Scholar]
- Shao, X.M.; Lopez, B.; Nathan, D.; Wilson, J.; Bankole, E.; Tumoyan, H.; Munoz, A.; Espinoza-Derout, J.; Hasan, K.M.; Chang, S. A mouse model for chronic intermittent electronic cigarette exposure exhibits nicotine pharmacokinetics resembling human vapers. J. Neurosci. Methods 2019, 326, 108376. [Google Scholar] [CrossRef]
- Hammad, A.M.; Alzaghari, L.F.; Alfaraj, M.; Al-Qerem, W.; Talib, W.H.; Alasmari, F.; Amawi, H.; Hall, F.S. Acetylsalicylic acid reduces cigarette smoke withdrawal-induced anxiety in rats via modulating the expression of NFĸB, GLT-1, and xCT. Front. Pharmacol. 2023, 13, 1047236. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2006; Volume 170, p. 10.1016. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Obata, K.; Noguchi, K. BDNF in sensory neurons and chronic pain. Neurosci. Res. 2006, 55, 1–10. [Google Scholar] [CrossRef]
- Elshazly, S.M.; Abd El Motteleb, D.M.; Nassar, N.N. The selective 5-LOX inhibitor 11-keto-β-boswellic acid protects against myocardial ischemia reperfusion injury in rats: Involvement of redox and inflammatory cascades. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2013, 386, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Chen, K.; Zhou, Q.; Wei, W.; Wang, Y.; Wang, Y. The role of oxidized low-density lipoprotein in breaking peripheral Th17/Treg balance in patients with acute coronary syndrome. Biochem. Biophys. Res. Commun. 2010, 394, 836–842. [Google Scholar] [CrossRef]
- Hammad, A.M.; Alasmari, F.; Sari, Y. Effect of Modulation of the Astrocytic Glutamate Transporters’ Expression on Cocaine-Induced Reinstatement in Male P Rats Exposed to Ethanol. Alcohol. Alcohol. 2020, 56, 210–219. [Google Scholar] [CrossRef]
- Hammad, A.M.; Swiss, G.M.S.; Hall, F.S.; Hikmat, S.; Sari, Y.; Al-Qirim, T.M.; Amawi, H.A. Ceftriaxone Reduces Waterpipe Tobacco Smoke Withdrawal-induced Anxiety in rats via Modulating the Expression of TNF-α/NFĸB, Nrf2, and GLT-1. Neuroscience 2021, 463, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Hakami, A.Y.; Hammad, A.M.; Sari, Y. Effects of amoxicillin and augmentin on cystine-glutamate exchanger and glutamate transporter 1 isoforms as well as ethanol intake in alcohol-preferring rats. Front. Neurosci. 2016, 10, 171. [Google Scholar] [CrossRef]
- Zang, Y.; Tai, Y.; Wan, B.; Jia, X. miR-200a-3p promotes the proliferation of human esophageal cancer cells by post-transcriptionally regulating cytoplasmic collapsin response mediator protein-1. Int. J. Mol. Med. 2016, 38, 1558–1564. [Google Scholar] [CrossRef]
- Yongvongsoontorn, N.; Chung, J.E.; Gao, S.J.; Bae, K.H.; Yamashita, A.; Tan, M.-H.; Ying, J.Y.; Kurisawa, M. Carrier-Enhanced Anticancer Efficacy of Sunitinib-Loaded Green Tea-Based Micellar Nanocomplex beyond Tumor-Targeted Delivery. ACS Nano 2019, 13, 7591–7602. [Google Scholar] [CrossRef]
- Chen, X.; Yi, Z.; Chen, G.; Ma, X.; Su, W.; Cui, X.; Li, X. DOX-assisted functionalization of green tea polyphenol nanoparticles for effective chemo-photothermal cancer therapy. J. Mater. Chem. B 2019, 7, 4066–4078. [Google Scholar] [CrossRef]
- Chen, X.; Ren, Q.; Chen, G.; Yi, Z.; Tong, Q.; Ran, Y.; Ma, L.; Fu, P.; Ma, L.; Li, X. Self-Assembled Phytochemical Nanomedicines with Enhanced Bioactivities for Effective Acute Kidney Injury Therapy. ACS Sustain. Chem. Eng. 2023, 11, 7288–7300. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, R.; Li, J.; Wang, S.; Ding, J.; Zhao, K.; Lu, B.; Zhou, W. Intrinsic Radical Species Scavenging Activities of Tea Polyphenols Nanoparticles Block Pyroptosis in Endotoxin-Induced Sepsis. ACS Nano 2022, 16, 2429–2441. [Google Scholar] [CrossRef]
- de la Peña, J.B.; Ahsan, H.M.; Botanas, C.J.; Dela Peña, I.J.; Woo, T.; Kim, H.J.; Cheong, J.H. Cigarette smoke exposure during adolescence but not adulthood induces anxiety-like behavior and locomotor stimulation in rats during withdrawal. Int. J. Dev. Neurosci. 2016, 55, 49–55. [Google Scholar] [CrossRef] [PubMed]
- De Biasi, M.; Dani, J.A. Reward, addiction, withdrawal to nicotine. Annu. Rev. Neurosci. 2011, 34, 105–130. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.-W.; Hsiao, Y.-M.; Chen, C.-J.; Wang, J.-P.; Chen, W.-C.; Ko, J.-L. Benzo [a] pyrene diol epoxide up-regulates COX-2 expression through NF-κB in rat astrocytes. Toxicol. Lett. 2004, 151, 345–355. [Google Scholar] [CrossRef]
- Yang, S.; Xu, K.; Xu, X.; Zhu, J.; Jin, Y.; Liu, Q.; Xu, R.; Gu, X.; Liu, Y.; Huang, Y. S-Ketamine Pretreatment Alleviates Anxiety-Like Behaviors and Mechanical Allodynia and Blocks the Pro-inflammatory Response in Striatum and Periaqueductal Gray From a Post-traumatic Stress Disorder Model. Front. Behav. Neurosci. 2022, 16, 848232. [Google Scholar] [CrossRef] [PubMed]
- Morosini, C.; Vivarelli, F.; Rullo, L.; Volino, E.; Losapio, L.M.; Paolini, M.; Romualdi, P.; Canistro, D.; Candeletti, S. Unburned Tobacco Smoke Affects Neuroinflammation-Related Pathways in the Rat Mesolimbic System. Int. J. Mol. Sci. 2024, 25, 5259. [Google Scholar] [CrossRef]
- Vivarelli, F.; Morosini, C.; Rullo, L.; Losapio, L.M.; Lacorte, A.; Sangiorgi, S.; Ghini, S.; Fagiolino, I.; Franchi, P.; Lucarini, M.; et al. Effects of unburned tobacco smoke on inflammatory and oxidative mediators in the rat prefrontal cortex. Front. Pharmacol. 2024, 15, 1328917. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Podlutsky, A.; Wolin, M.S.; Losonczy, G.; Pacher, P.; Ungvari, Z. Oxidative stress and accelerated vascular aging: Implications for cigarette smoking. Front. Biosci. A J. Virtual Libr. 2009, 14, 3128. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.-H. Effects of epigallocatechin gallate on behavioral and cognitive impairments, hypothalamic–pituitary–adrenal Axis dysfunction, and alternations in hippocampal BDNF expression under single prolonged stress. J. Med. Food 2018, 21, 979–989. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Wang, C.; Liu, Y.; You, J.; Wang, P.; Xu, G.; Shen, H.; Yao, H.; Lan, X. Chronic ethanol exposure induces neuroinflammation in h4 cells through tlr3/nf-κb pathway and anxiety-like behavior in male c57bl/6 mice. Toxicology 2020, 446, 152625. [Google Scholar] [CrossRef]
- Yang, X.; Guo, A.-L.; Pang, Y.-P.; Cheng, X.-J.; Xu, T.; Li, X.-R.; Liu, J.; Zhang, Y.-Y.; Liu, Y. Astaxanthin attenuates environmental tobacco smoke-induced cognitive deficits: A critical role of p38 MAPK. Mar. Drugs 2019, 17, 24. [Google Scholar] [CrossRef]
- Cheng, L.; Li, F.; Ma, R.; Hu, X. Forsythiaside inhibits cigarette smoke-induced lung inflammation by activation of Nrf2 and inhibition of NF-κB. Int. Immunopharmacol. 2015, 28, 494–499. [Google Scholar] [CrossRef]
- Ghosh, D.; Mishra, M.K.; Das, S.; Kaushik, D.K.; Basu, A. Tobacco carcinogen induces microglial activation and subsequent neuronal damage. J. Neurochem. 2009, 110, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.; Bromberg, E.; de Vries, E.F. Brain-derived neurotrophic factor in brain disorders: Focus on neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef]
- Gold, P.W. The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatry 2015, 20, 32–47. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J. Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef] [PubMed]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef] [PubMed]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Chugh, G.; Asghar, M. Inflammation in anxiety. Adv. Protein Chem. Struct. Biol. 2012, 88, 1–25. [Google Scholar] [PubMed]
- Xiao, L.; Kish, V.L.; Benders, K.M.; Wu, Z.-X. Prenatal and early postnatal exposure to cigarette smoke decreases BDNF/TrkB signaling and increases abnormal behaviors later in life. Int. J. Neuropsychopharmacol. 2016, 19, pyv117. [Google Scholar] [CrossRef]
- Naha, N.; Gandhi, D.; Gautam, A.; Prakash, J.R. Nicotine and cigarette smoke modulate Nrf2-BDNF-dopaminergic signal and neurobehavioral disorders in adult rat cerebral cortex#. Hum. Exp. Toxicol. 2018, 37, 540–556. [Google Scholar]
- Williams, J.; Sergi, D.; McKune, A.J.; Georgousopoulou, E.N.; Mellor, D.D.; Naumovski, N. The beneficial health effects of green tea amino acid l-theanine in animal models: Promises and prospects for human trials. Phytother. Res. 2019, 33, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Vignes, M.; Maurice, T.; Lanté, F.; Nedjar, M.; Thethi, K.; Guiramand, J.; Récasens, M. Anxiolytic properties of green tea polyphenol (−)-epigallocatechin gallate (EGCG). Brain Res. 2006, 1110, 102–115. [Google Scholar] [CrossRef]
- Adachi, N.; Tomonaga, S.; Tachibana, T.; Denbow, D.M.; Furuse, M. (−)-Epigallocatechin gallate attenuates acute stress responses through GABAergic system in the brain. Eur. J. Pharmacol. 2006, 531, 171–175. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Qin, T.; Sun, D.; Zhao, X.; Zhang, B. Protective effect of epigallocatechin-3-gallate against neuroinflammation and anxiety-like behavior in a rat model of myocardial infarction. Brain Behav. 2020, 10, e01633. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, L.; Wang, X.; Liu, J.; Wang, Y.; Zhou, Y.; Ho, W. (-)-Epigallocatechin gallate inhibits endotoxin-induced expression of inflammatory cytokines in human cerebral microvascular endothelial cells. J. Neuroinflamm. 2012, 9, 161. [Google Scholar] [CrossRef]
- Mandrekar, P.; Catalano, D.; Szabo, G. Inhibition of lipopolysaccharide-mediated NFκB activation by ethanol in human monocytes. Int. Immunol. 1999, 11, 1781–1790. [Google Scholar] [CrossRef]
- Wahyudi, S.; Sargowo, D. Green tea polyphenols inhibit oxidized LDL-induced NF-KB activation in human umbilical vein endothelial cells. Acta Med. Indones. 2007, 39, 66–70. [Google Scholar] [PubMed]
- Yang, F.; Oz, H.S.; Barve, S.; De Villiers, W.J.; McClain, C.J.; Varilek, G.W. The green tea polyphenol (−)-epigallocatechin-3-gallate blocks nuclear factor-κB activation by inhibiting IκB kinase activity in the intestinal epithelial cell line IEC-6. Mol. Pharmacol. 2001, 60, 528–533. [Google Scholar] [PubMed]
- Wakabayashi, C.; Numakawa, T.; Ninomiya, M.; Chiba, S.; Kunugi, H. Behavioral and molecular evidence for psychotropic effects in L-theanine. Psychopharmacology 2012, 219, 1099–1109. [Google Scholar] [CrossRef]
- Yamada, T.; Terashima, T.; Honma, H.; Nagata, S.; Okubo, T.; Juneja, L.R.; Yokogoshi, H. Effects of theanine, a unique amino acid in tea leaves, on memory in a rat behavioral test. Biosci. Biotechnol. Biochem. 2008, 72, 1356–1359. [Google Scholar] [CrossRef]
- Yang, J.R.; Ren, T.T.; Lan, R.; Qin, X.Y. Tea polyphenols attenuate staurosporine-induced cytotoxicity and apoptosis by modulating BDNF-TrkB/Akt and Erk1/2 signaling axis in hippocampal neurons. IBRO Rep. 2020, 8, 115–121. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer | Sequence | Reference |
|---|---|---|---|
| bdnf | Forward | 5′-GGCAGGTTCGAGAGGTCTGA-3′ | [42] |
| Reverse | 5′-CGCTGTGACCCACTCGCTAA-3′ | ||
| nf-kb | Forward | 5′-TGGCAGACGACGATCCTTTC-3′ | [43] |
| Reverse | 5′-GAAGGTATGGGCCATCTGTTGA-3′ | ||
| b-actin | Forward | 5′-ATCTGGCACCACACCTTC-3′ | [44] |
| Reverse | 5′-AGCCAGGTCCAGACGCA-3′ |
| Antibody | Company (cat#) | Host Species | Dilution |
|---|---|---|---|
| Recombinant anti-BDNF antibody [EPR1292] [47] | Abcam, (ab108319) | Rabbit | 1/1000 |
| Recombinant anti-NF-kB p65 antibody [EP2161Y] | Abcam, (ab76311) | Rabbit | 1/1000 |
| Anti-beta tubulin antibody | Abcam, (ab6046) | Rabbit | 1/2000 |
| Goat anti-rabbit IgG H&L (HRP) [48] | Abcam, (ab6721) | Goat | 1/1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammad, A.M.; Alzaghari, L.F.; Alfaraj, M.; Lux, V.; Sunoqrot, S. Green Tea Polyphenol Nanoparticles Reduce Anxiety Caused by Tobacco Smoking Withdrawal in Rats by Suppressing Neuroinflammation. Toxics 2024, 12, 598. https://doi.org/10.3390/toxics12080598
Hammad AM, Alzaghari LF, Alfaraj M, Lux V, Sunoqrot S. Green Tea Polyphenol Nanoparticles Reduce Anxiety Caused by Tobacco Smoking Withdrawal in Rats by Suppressing Neuroinflammation. Toxics. 2024; 12(8):598. https://doi.org/10.3390/toxics12080598
Chicago/Turabian StyleHammad, Alaa M., Lujain F. Alzaghari, Malek Alfaraj, Vanessa Lux, and Suhair Sunoqrot. 2024. "Green Tea Polyphenol Nanoparticles Reduce Anxiety Caused by Tobacco Smoking Withdrawal in Rats by Suppressing Neuroinflammation" Toxics 12, no. 8: 598. https://doi.org/10.3390/toxics12080598





