Per- and Polyfluoroalkyl Substances (PFAS) Accumulation, Reproductive Impairment, and Associations with Nestling Body Condition in Great (Parus major)- and Blue Tits (Cyanistes caeruleus) Living near a Hotspot in Belgium
Abstract
1. Introduction
2. Materials and Methods
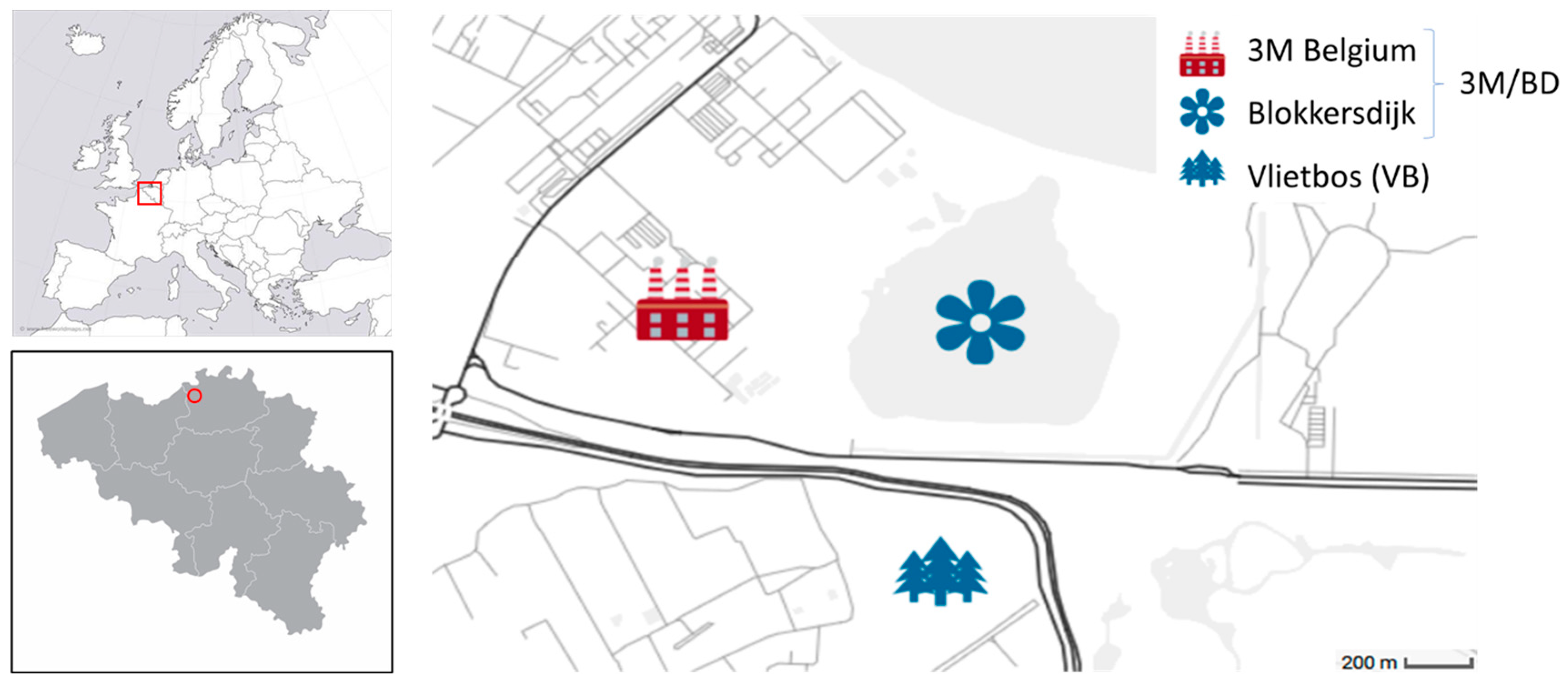
2.1. Study Species and Sample Collection
2.2. PFAS Extraction and Analysis
2.3. Quality Control and Assurance
2.4. Statistical Analysis
3. Results
3.1. PFAS Concentrations and Accumulation Profiles
3.2. Correlations between Egg and Plasma Concentrations
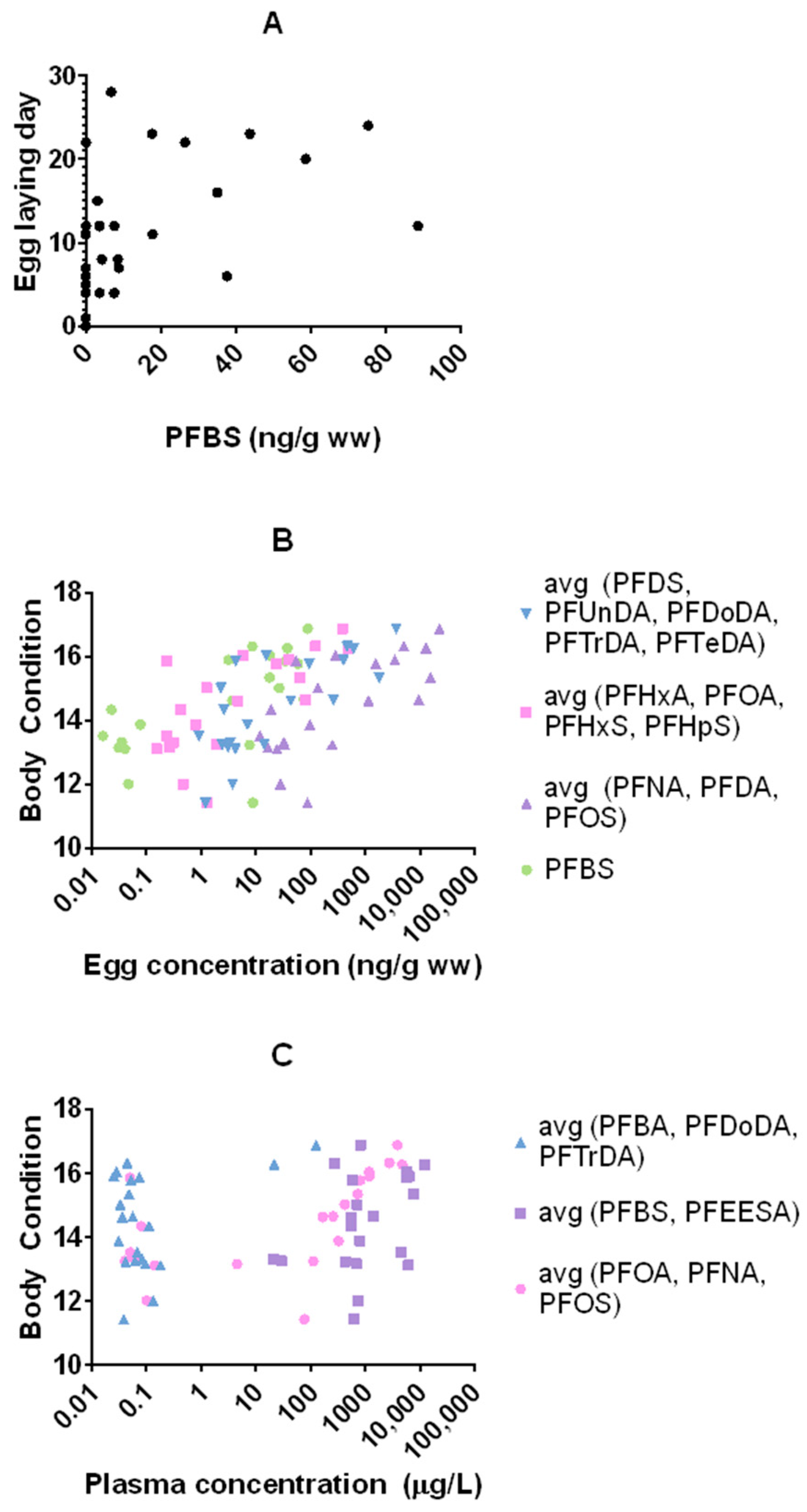
3.3. Reproductive Toxicity and Associations with Nestling Body Condition
4. Discussion
4.1. PFAS Accumulation: Tissue- and Species-Specific Differences
4.2. Temporal Trends
4.3. Reproductive Toxicity and Associations with Nestling Body Condition
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Buser, A.M.; Cousins, I.T.; Demattio, S.; Drost, W.; Johansson, O.; Ohno, K.; Patlewicz, G.; Richard, A.M.; Walker, G.W.; et al. A new OECD definition for per- and polyfluoroalkyl substances. Environ. Sci. Technol. 2021, 55, 15575–15578. [Google Scholar] [CrossRef] [PubMed]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2022, 22, 2345–2373. [Google Scholar] [CrossRef]
- Muir, D.; Bossi, R.; Carlsson, P.; Evans, M.; De Silva, A.; Halsall, C.; Rauert, C.; Herzke, D.; Hung, H.; Letcher, R.; et al. Levels and trends of poly- and perfluoroalkyl substances in the Arctic environment–an update. Emerg. Contam. 2019, 5, 240–271. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef]
- Sims, J.L.; Stroski, K.M.; Kim, S.; Killeen, G.; Ehalt, R.; Simcik, M.F.; Brooks, B.W. Global occurrence and probabilistic environmental health hazard assessment of per- and polyfluoroalkyl substances (PFASs) in groundwater and surface waters. Sci. Total Environ. 2022, 816, 151535. [Google Scholar] [CrossRef]
- Habib, Z.; Song, M.; Ikram, S.; Zahra, Z. Overview of per- and polyfluoroalkyl substances (PFAS), their applications, sources, and potential impacts on human health. Pollutants 2024, 4, 136–152. [Google Scholar] [CrossRef]
- Miranda, D.A.; Peaslee, G.F.; Zachritz, A.M.; Lamberti, G.A. A worldwide evaluation of trophic magnification of per- and polyfluoroalkyl substances in aquatic ecosystems. Integr. Environ. Assess. Manag. 2022, 18, 1500–1512. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; De Felice, B.; Rusconi, M.; Morganti, M.; Polesello, S.; Valsecchi, S. A review of the bioaccumulation and adverse effects of PFAS in free-living organisms from contaminated sites nearby fluorochemical production plants. Water Emerg. Contam. Nanoplastics 2022, 1, 18. [Google Scholar] [CrossRef]
- Brunn, H.; Arnold, G.; Körner, W.; Rippen, G.; Steinhäuser, K.G.; Valentin, I. PFAS: Forever chemicals–persistent, bioaccumulative and mobile. Reviewing the status and the need for their phase out and remediation of contaminated sites. Environ. Sci. Eur. 2023, 35, 20. [Google Scholar] [CrossRef]
- Kuo, D.T.F.; Rattner, B.A.; Marteinson, S.C.; Letcher, R.; Fernie, K.J.; Treu, G.; Deutsch, M.; Johnson, M.S.; Deglin, S.; Embry, M. A critical review of bioaccumulation and biotransformation of organic chemicals in birds. Rev. Environ. Contam. Toxicol. 2022, 260, 6. [Google Scholar] [CrossRef]
- Ankley, G.T.; Cureton, P.; Hoke, R.A.; Houde, M.; Kumar, A.; Kurias, J.; Lanno, R.; McCarthy, C.; Newsted, J.; Salice, C.J.; et al. Assessing the ecological risks of per- and polyfluoroalkyl substances: Current state-of-the science and a proposed path forward. Environ. Toxicol. Chem. 2021, 40, 564–605. [Google Scholar] [CrossRef] [PubMed]
- Custer, C.M. Linking field and laboratory studies: Reproductive effects of perfluorinated substances on avian populations. Integr. Environ. Assess. Manag. 2021, 17, 690–696. [Google Scholar] [CrossRef]
- Tartu, S.; Gabrielsen, G.W.; Blévin, P.; Ellis, H.; Bustnes, J.O.; Herzke, D.; Chastel, O. Endocrine and fitness correlates of long-chain perfluorinated carboxylates exposure in Arctic breeding black-legged kittiwakes. Environ. Sci. Technol. 2014, 48, 13504–13510. [Google Scholar] [CrossRef] [PubMed]
- Groffen, T.; Lasters, R.; Lopez-Antia, A.; Prinsen, E.; Bervoets, L.; Eens, M. Limited reproductive impairment in a passerine bird species exposed along a perfluoroalkyl acid (PFAA) pollution gradient. Sci. Total Environ. 2019, 652, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Custer, C.M.; Custer, T.W.; Schoenfuss, H.L.; Poganski, B.H.; Solem, L. Exposure and effects of perfluoroalkyl compounds on tree swallows nesting at Lake Johanna in east central Minnesota, USA. Reprod. Toxicol. 2012, 33, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Custer, C.M.; Custer, T.W.; Dummer, P.M.; Etterson, M.A.; Thogmartin, W.E.; Wu, Q.; Kannan, K.; Trowbridge, A.; McKann, P.C. Exposure and effects of perfluoroalkyl substances in tree swallows nesting in Minnesota and Wisconsin, USA. Arch. Environ. Contam. Toxicol. 2014, 66, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Custer, C.M.; Custer, T.W.; Delaney, R.; Dummer, P.M.; Schultz, S.; Karouna-Renier, N. Perfluoroalkyl contaminant exposure and effects in tree swallows nesting at Clarks Marsh, Oscoda, Michigan, USA. Arch. Environ. Contam. Toxicol. 2019, 77, 1–13. [Google Scholar] [CrossRef]
- Morganti, M.; Polesello, S.; Pascariello, S.; Ferrario, C.; Rubolini, D.; Valsecchi, S.; Parolini, M. Exposure assessment of PFAS-contaminated sites using avian eggs as a biomonitoring tool: A frame of reference and a case study in the Po River valley (Northern Italy). Integr. Environ. Assess. Manag. 2021, 17, 733–745. [Google Scholar] [CrossRef]
- Groffen, T.; Lopez-Antia, A.; D’Hollander, W.; Prinsen, E.; Eens, M.; Bervoets, L. Perfluoroalkylated acids in the eggs of great tits (Parus major) near a fluorochemical plant in Flanders, Belgium. Environ. Pollut. 2017, 228, 140–148. [Google Scholar] [CrossRef]
- Buytaert, J.; Eens, M.; Abd Elgawad, H.; Bervoets, L.; Beemster, G.; Groffen, T. Associations between PFAS concentrations and the oxidative status in a free-living songbird (Parus major) near a fluorochemical facility. Environ. Pollut. 2023, 335, 122304. [Google Scholar] [CrossRef]
- Lopez-Antia, A.; Groffen, T.; Lasters, R.; AbdElgawad, H.; Sun, J.; Asard, H.; Bervoets, L.; Eens, M. Perfluoroalkyl acids (PFAAs) concentrations and oxidative status in two generations of great tits inhabiting a contamination hotspot. Environ. Sci. Technol. 2019, 53, 1617–1626. [Google Scholar] [CrossRef]
- Lasters, R.; Groffen, T.; Bervoets, L.; Eens, M. Perfluoroalkyl acid (PFAA) profile and concentrations in two co-occurring tit species: Distinct differences indicate non-generalizable results across passerines. Sci. Total Environ. 2021, 761, 143301. [Google Scholar] [CrossRef] [PubMed]
- Hoff, P.T.; Van de Vijver, K.; Dauwe, T.; Covaci, A.; Maervoet, J.; Eens, M.; Blust, R.; De Coen, W. Evaluation of biochemical effects related to perfluorooctane sulfonic acid exposure in organohalogen-contaminated great tit (Parus major) and blue tit (Parus caeruleus) nestlings. Chemosphere 2005, 61, 1558–1569. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 2009, 118, 1883–1891. [Google Scholar] [CrossRef]
- Lopez-Antia, A.; Ortiz-Santaliestra, M.E.; Mougeot, F.; Mateo, R. Experimental exposure of red-legged partridges (Alectoris rufa) to seeds coated with imidacloprid, thiram and difenoconazole. Ecotoxicology 2013, 22, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Powley, C.R.; George, S.W.; Ryan, T.W.; Buck, R.C. Matrix effect-free analytical methods for determination of perfluorinated carboxylic acids in environmental matrices. Anal. Chem. 2005, 77, 6353–6358. [Google Scholar] [CrossRef]
- Groffen, T.; Lasters, R.; Lemière, F.; Willems, T.; Eens, M.; Bervoets, L.; Prinsen, E. Development and validation of an extraction method for the analysis of perfluoroalkyl substances (PFASs) in environmental and biotic matrices. J. Chromatogr. B 2019, 1116, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Groffen, T.; Bervoets, L.; Jeong, Y.; Willems, T.; Eens, M.; Prinsen, E. A rapid method for the detection and quantification of legacy and emerging per- and polyfluoroalkyl substances (PFAS) in bird feathers using UPLC-MS/MS. J. Chromatogr. B 2021, 1172, 122653. [Google Scholar] [CrossRef]
- Villanueva, P. MLE-Based Procedure for Left-Censored Data Excel Spreadsheet; 2005 Office of Pesticide Programs; U.S. Environmental protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- Robuck, A.R.; McCord, J.P.; Strynar, M.J.; Cantwell, M.G.; Wiley, D.N.; Lohmann, R. Tissue-specific distribution of legacy and novel per- and polyfluoroalkyl substances in juvenile seabirds. Environ. Sci. Technol. Lett. 2021, 8, 457–462. [Google Scholar] [CrossRef]
- Rupp, J.; Guckert, M.; Berger, U.; Drost, W.; Mader, A.; Nödler, K.; Nürenberg, G.; Schulze, J.; Söhlmann, R.; Reemtsma, T. Comprehensive target analysis and TOP assay of per- and polyfluoroalkyl substances (PFAS) in wild boar livers indicate contamination hot-spots in the environment. Sci. Total Environ. 2023, 871, 162028. [Google Scholar] [CrossRef]
- Verreault, J.; Houde, M.; Gabrielsen, G.W.; Berger, U.; Haukås, M.; Letcher, R.J.; Muir, D.C.G. Perfluorinated alkyl substances in plasma, liver, brain, and eggs of glaucous gulls (Larus hyperboreus) from the Norwegian Arctica. Environ. Sci. Technol. 2005, 39, 7439–7445. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.E.; Berger, U. Tissue distribution of perfluorinated surfactants in common Guillemot (Uria aalge) from the Baltic Sea. Environ. Sci. Technol. 2008, 42, 5879–5884. [Google Scholar] [CrossRef] [PubMed]
- Gebbink, W.A.; Letcher, R.J. Comparative tissue and body compartment accumulation and maternal transfer to eggs of perfluoroalkyl sulfonates and carboxylates in Great Lakes herring gulls. Environ. Pollut. 2012, 162, 40–47. [Google Scholar] [CrossRef]
- Witt, C.C.; Gadek, C.R.; Cartron, J.E.; Andersen, M.J.; Campbell, M.L.; Castro-Farías, M.; Gyllenhaal, E.F.; Johnson, A.B.; Malaney, J.L.; Montoya, K.N.; et al. Extraordinary levels of per- and polyfluoroalkyl substances (PFAS) in vertebrate animals at a New Mexico desert oasis: Multiple pathways for wildlife and human exposure. Environ. Res. 2024, 249, 118229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Teng, M.; Zhao, X.; Li, Y.; Sun, J.; Zhao, W.; Ruan, Y.; Leung, K.M.Y.; Wu, F. Insight into the binding model of per- and polyfluoroalkyl substances to proteins and membranes. Environ. Int. 2023, 175, 107951. [Google Scholar] [CrossRef]
- Bertolero, A.; Vicente, J.; Meyer, J.; Lacorte, S. Accumulation and maternal transfer of perfluorooctane sulphonic acid in yellow-legged (Larus michahellis) and Audouin’s gull (Larus audouinii) from the Ebro Delta Natural Park. Environ. Res. 2015, 137, 208–214. [Google Scholar] [CrossRef]
- Ricolfi, L.; Taylor, M.D.; Yang, Y.; Lagisz, M.; Nakagawa, S. Maternal transfer of per- and polyfluoroalkyl substances (PFAS) in wild birds: A systematic review and meta-analysis. Chemosphere 2024, 361, 142346. [Google Scholar] [CrossRef]
- Lasters, R.; Groffen, T.; Lopez-Antia, A.; Bervoets, L.; Eens, M. Variation in PFAA concentrations and egg parameters throughout the egg-laying sequence in a free-living songbird (the great tit, Parus major): Implications for biomonitoring studies. Environ. Pollut. 2019, 246, 237–248. [Google Scholar] [CrossRef] [PubMed]
- 3M Company. Phase-Out Plan for POSF-Based Products. USEPA Administrative Record AR 226-0600. 2000. Available online: https://www.regulations.gov/document/EPA-HQ-OPPT-2012-0268-0008 (accessed on 17 May 2024).
- Hekster, F.M.; de Voogt, P.; Pijnenburg, A.M.C.M.; Laane, R.W.P.M. Perfluoroalkylated Substances: Aquatic Environmental Assessment; Report RIKZ/2002.043; National Institute for Coastal and Marine Management (RIKZ): The Hague, The Netherlands, 2002. [Google Scholar]
- Brendel, S.; Fetter, É.; Staude, C.; Vierke, L.; Biegel-Engler, A. Short-chain perfluoroalkyl acids: Environmental concerns and a regulatory strategy under REACH. Environ. Sci. Eur. 2018, 30, 9. [Google Scholar] [CrossRef]
- Lasters, R.; Groffen, T.; Eens, M.; Bervoets, L. Dynamic spatiotemporal changes of per- and polyfluoroalkyl substances (PFAS) in soil and eggs of private gardens at different distances from a fluorochemical plant. Environ. Pollut. 2024, 346, 123613. [Google Scholar] [CrossRef]
- Eriksson, U.; Roos, A.; Lind, Y.; Hope, K.; Ekblad, A.; Kärrman, A. Comparison of PFASs contamination in the freshwater and terrestrial environments by analysis of eggs from osprey (Pandion haliaetus), tawny owl (Strix aluco), and common kestrel (Falco tinnunculus). Environ. Res. 2016, 149, 40–47. [Google Scholar] [CrossRef]
- Land, M.; de Wit, C.A.; Bignert, A.; Cousins, I.T.; Herzke, D.; Johansson, J.H.; Martin, J.W. What is the effect of phasing out long-chain per- and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review. Environ. Evid. 2018, 7, 4. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Zhao, H.; Li, J. Spatiotemporal distribution of perfluoroalkyl acid in Chinese eggs. Food Addit. Contam. B 2022, 15, 2. [Google Scholar] [CrossRef]
- Groffen, T.; Bervoets, L.; Eens, M. Temporal trends in PFAS concentrations in livers of a terrestrial raptor (common buzzard; Buteo buteo) collected in Belgium during the period 2000–2005 and in 2021. Environ. Res. 2023, 216, 114644. [Google Scholar] [CrossRef]
- Barba, E.; Gil-Delgado, J.A.; Monrós, J.S. The costs of being late: Consequences of delaying great tit Parus major first clutches. J. Anim. Ecol. 1995, 64, 642–651. [Google Scholar] [CrossRef]
- Bańbura, J.; Bańbura, M.; Glądalski, M.; Kaliński, A.; Markowski, M.; Michalski, M.; Nadolski, J.; Skwarska, J.; Zieliński, P. Body condition parameters of nestling great tits Parus major in relation to experimental food supplementation. Acta Ornithol. 2011, 46, 207–212. [Google Scholar] [CrossRef]
- Oddie, K.R. Size matters: Competition between male and female great tit offspring. J. Anim. Ecol. 2001, 69, 903–912. [Google Scholar] [CrossRef] [PubMed]


| 2011 Groffen et al. [19] | 2016 Groffen et al. [14] | 2022 (The Present Study) | ||||
|---|---|---|---|---|---|---|
| 3M | Vlietbos | 3M | Vlietbos | 3M/BD | Vlietbos | |
| PFBS | ND | ND | ND | ND | 24.9 (<0.762–88.5) | 4.45 (<0.762–35.1) |
| PFHxS | 162 (36.9–355) | 1.6 (<0.45–5.6) | ND | ND | 166 (<2.79–1005) | <2.79 |
| PFOS | 20,122 (3237–69,218) | 254 (55.1–782) | 48,056 (5111–187,032) | 830 (<2.55–4035) | 15,545 (261–67,916) | 101 (34.5–253) |
| PFDS | ND | ND | 315 (9.4–1489) | <5.92 | 1939 (1.61–13,765) | <0.587 |
| PFBA | ND | ND | 1.7 (<0.261–11) | <0.261 (<0.261–1.7) | ND | ND |
| PFPeA | ND | ND | ND | ND | ND | ND |
| PFHxA | ND | ND | ND | ND | 0.431 (<0.375–1.25) | <0.375 (<0.375–0.440) |
| PFHpA | ND | ND | ND | ND | <0.485 (<0.485–4.03) | <0.485 |
| PFOA | 26.9 (2.7–56.3) | 1.0 (0.3–1.9) | 39 (3.4–359) | 1.8 (<0.045–3.5) | 33.3 (0.836–243) | 1.12 (0.503–2.22) |
| PFNA | 4.2 (<1.8–20.5) | <1.8 | 9.1 (2.1–28) | 1.8 (<0.586–5.7) | 7.13 (<0.198–26.4) | 0.565 (<0.198–1.48) |
| PFDA | 12.3 (<1.4–37.2) | <1.4 | 25 (1.6–102) | 0.7 (<0.425–4.1) | 7.71 (<0.334–26.9) | 1.47 (0.641–2.67) |
| PFUnDA | ND | ND | ND | ND | 8.60 (<0.230–53.7) | 0.995 (0.344–2.17) |
| PFDoDA | 22.0 (2.0–104) | 0.7 (<0.32–1.50) | 29 (1.1–133) | 1.8 (<0.444–7.8) | 355 (3.04–2953) | 8.94 (2.61–21.5) |
| PFTrDA | 7.9 (<0.38–32.3) | 0.4 (<0.38–0.9) | 25 (<0.256–156) | 6.0 (<0.256–22) | 139 (1.19–1034) | 5.07 (1.46–13.9) |
| PFTeDA | ND | ND | 3.4 (<0.355–22) | 1.2 (<0.355–4.1) | 50.1 (<0.535–450) | 1.77 (<0.535–5.10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groffen, T.; Buytaert, J.; Prinsen, E.; Bervoets, L.; Eens, M. Per- and Polyfluoroalkyl Substances (PFAS) Accumulation, Reproductive Impairment, and Associations with Nestling Body Condition in Great (Parus major)- and Blue Tits (Cyanistes caeruleus) Living near a Hotspot in Belgium. Toxics 2024, 12, 636. https://doi.org/10.3390/toxics12090636
Groffen T, Buytaert J, Prinsen E, Bervoets L, Eens M. Per- and Polyfluoroalkyl Substances (PFAS) Accumulation, Reproductive Impairment, and Associations with Nestling Body Condition in Great (Parus major)- and Blue Tits (Cyanistes caeruleus) Living near a Hotspot in Belgium. Toxics. 2024; 12(9):636. https://doi.org/10.3390/toxics12090636
Chicago/Turabian StyleGroffen, Thimo, Jodie Buytaert, Els Prinsen, Lieven Bervoets, and Marcel Eens. 2024. "Per- and Polyfluoroalkyl Substances (PFAS) Accumulation, Reproductive Impairment, and Associations with Nestling Body Condition in Great (Parus major)- and Blue Tits (Cyanistes caeruleus) Living near a Hotspot in Belgium" Toxics 12, no. 9: 636. https://doi.org/10.3390/toxics12090636
APA StyleGroffen, T., Buytaert, J., Prinsen, E., Bervoets, L., & Eens, M. (2024). Per- and Polyfluoroalkyl Substances (PFAS) Accumulation, Reproductive Impairment, and Associations with Nestling Body Condition in Great (Parus major)- and Blue Tits (Cyanistes caeruleus) Living near a Hotspot in Belgium. Toxics, 12(9), 636. https://doi.org/10.3390/toxics12090636







