Development and Application of a Slot-Blot Assay Using the Damage Sensing Protein Atl1 to Detect and Quantify O6-Alkylated Guanine Bases in DNA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DNA Samples Containing a Variety of O6-AlkG Adducts
2.3. Production of CT DNA Standards of Known O6-MeG Levels
2.4. ASB Assay
2.4.1. Analysis of Membrane-Bound DNA by Propidium Iodide Staining
2.4.2. MGMT-Treated DNA Sample Preparation
2.5. Culture and Treatment of MCF-10A Cells
3. Results
3.1. ASB Assay Detects a Range of Different O6-AlkGs in Alkylated CT DNA
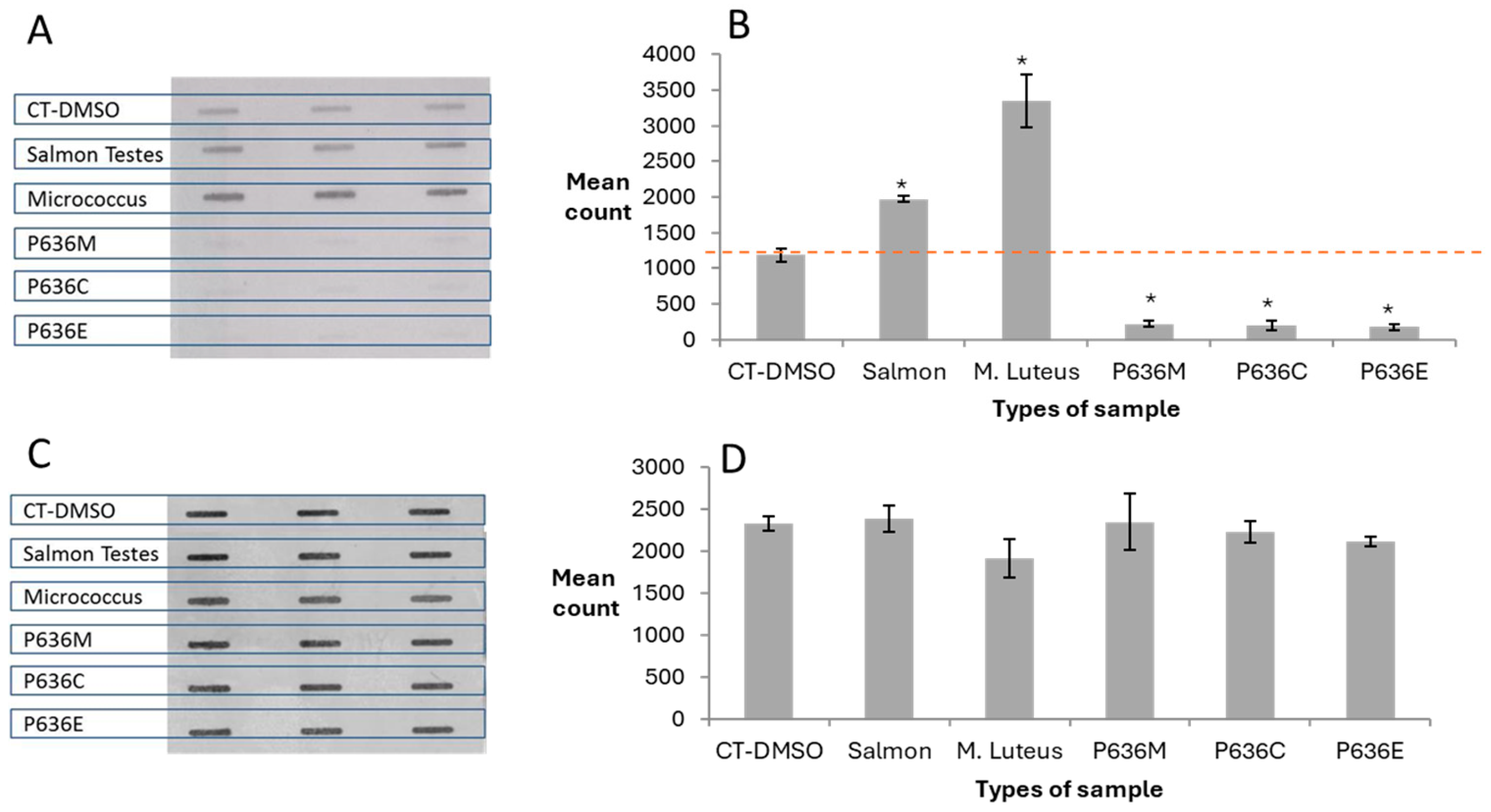
3.2. Investigation of ISB Background Levels
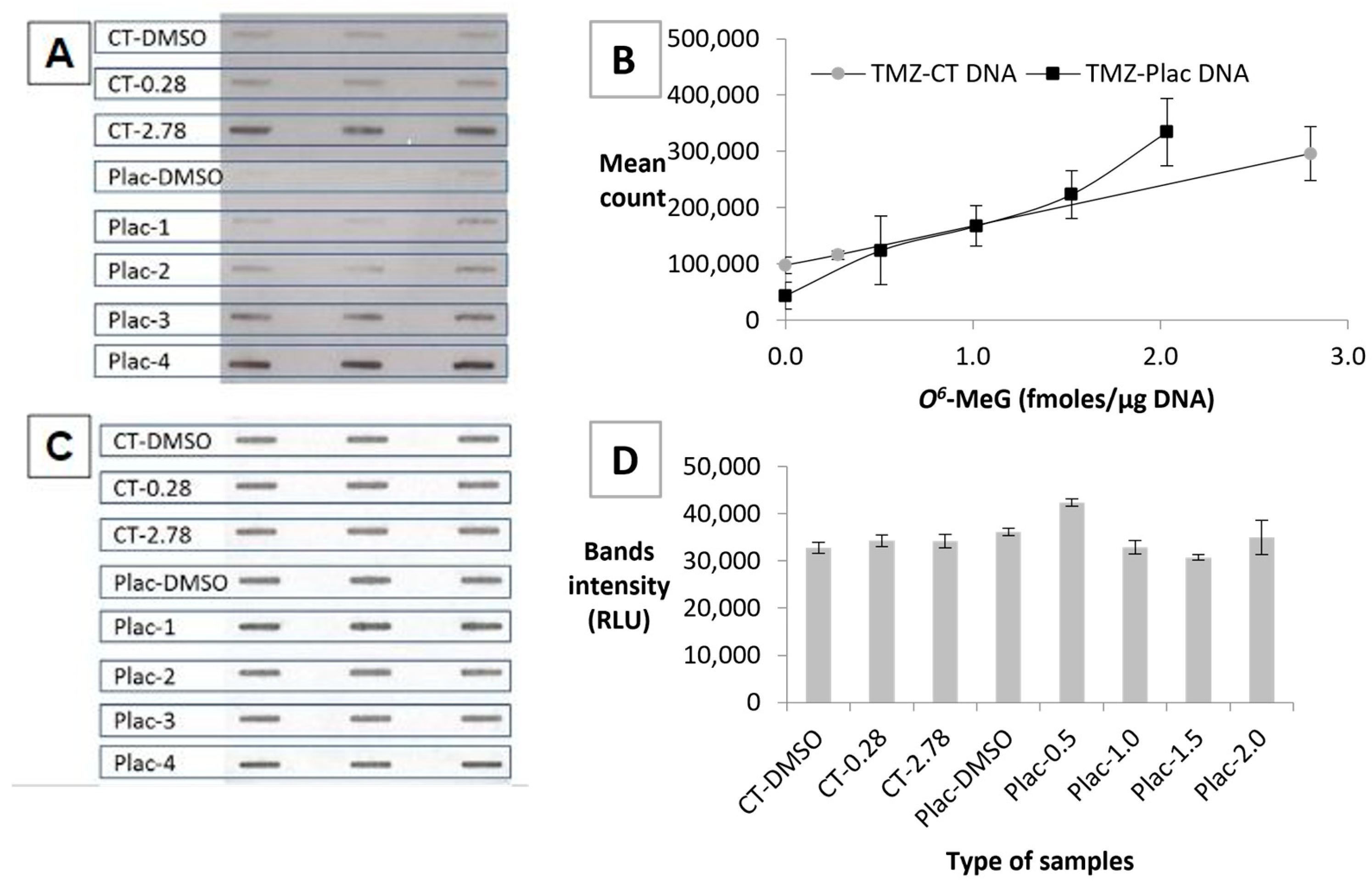
3.3. HRP-MBP-Atl1 Binding to TMZ-CT DNA Is Reduced by Pretreatment with MGMT
3.4. Establishing a Standard Curve Using TMZ-Treated DNA
3.5. Analysis of Human Breast Tumour Cell Line DNA Samples
3.6. Analysis of Human DNA Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magee, P.N.; Barnes, J.M. The production of malignant primary hepatic tumours in the rat by feeding dimethylnitrosamine. Br. J. Cancer 1956, 10, 114–122. [Google Scholar] [CrossRef]
- Druckrey, H.; Preussmann, R.; Ivankovic, S.; Schmähl, D. Organotropic carcinogenic effects of 65 various N-nitroso- compounds on BD rats. Z. Krebsforsch. 1967, 69, 103–201. [Google Scholar] [CrossRef]
- NTP (National Toxicology Program). Report on Carcinogens, 15th ed.; Department of Health and Human Services, Public Health Service: Research Triangle Park, NC, USA, 2021. [Google Scholar]
- Kobets, T.; Smith, B.P.C.; Williams, G.M. Food-Borne Chemical Carcinogens and the Evidence for Human Cancer Risk. Foods 2022, 11, 2828. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (EFSA CONTAM Panel); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Hogstrand, C.; Ron Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; et al. Risk assessment of N-nitrosamines in food. EFSA J. 2023, 21, e07884. [Google Scholar]
- Gushgari, A.J.; Halden, R.U. Critical review of major sources of human exposure to N-nitrosamines. Chemosphere 2018, 210, 1124–1136. [Google Scholar] [CrossRef]
- Tricker, A.R. N-nitroso compounds and man: Sources of exposure, endogenous formation and occurrence in body fluids. Eur. J. Cancer Prev. 1997, 6, 226–268. [Google Scholar] [CrossRef]
- Taverna, P.; Sedgwick, B. Generation of an Endogenous DNA-Methylating Agent by Nitrosation in Escherichia coli. J. Bacteriol. 1996, 178, 5105–5111. [Google Scholar] [CrossRef]
- Hu, C.W.; Shih, Y.M.; Liu, H.H.; Chiang, Y.C.; Chen, C.M.; Chao, M.R. Elevated urinary levels of carcinogenic N-nitrosamines in patients with urinary tract infections measured by isotope dilution online SPE LC-MS/MS. J. Hazard Mater. 2016, 310, 207–216. [Google Scholar] [CrossRef]
- Rietjens, I.M.C.M.; Michael, A.; Bolt, H.M.; Siméon, B.; Andrea, H.; Nils, H.; Christine, K.; Angela, M.; Gloria, P.; Daniel, R.; et al. The role of endogenous versus exogenous sources in the exposome of putative genotoxins and consequences for risk assessment. Arch. Toxicol. 2022, 96, 1297–1352. [Google Scholar] [CrossRef]
- Li, Y.; Hecht, S.S. Metabolic Activation and DNA Interactions of Carcinogenic N-Nitrosamines to Which Humans Are Commonly Exposed. Int. J. Mol. Sci. 2022, 23, 4559. [Google Scholar] [CrossRef]
- Drabløs, F.; Feyzi, E.; Aas, P.A.; Vaagbø, C.B.; Kavli, B.; Bratlie, M.S.; Peña-Diaz, J.; Otterlei, M.; Slupphaug, G.; Krokan, H.E. Alkylation damage in DNA and RNA--repair mechanisms and medical significance. DNA Repair 2004, 3, 1389–1407. [Google Scholar] [CrossRef]
- Fahrer, J.; Christmann, M. DNA Alkylation Damage by Nitrosamines and Relevant DNA Repair Pathways. Int. J. Mol. Sci. 2023, 24, 4684. [Google Scholar] [CrossRef] [PubMed]
- Beranek, D.T. Distribution of methyl and ethyl adducts following alkylation with mono- functional alkylating agents. Mutat. Res. 1990, 231, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, N.; Li, D.; Essigmann, J.M. Chemical biology of mutagenesis and DNA repair: Cellular responses to DNA alkylation. Carcinogenesis 2010, 31, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.D. Biological Basis for Threshold Responses to Methylating Agents. Chem. Res. Toxicol. 2020, 33, 2219–2224. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y. Cytotoxic and mutagenic properties of O6-alkyl-2′-deoxyguanosine lesions in Escherichia coli cells. J. Biol. Chem. 2018, 293, 15033–15042. [Google Scholar] [CrossRef]
- Knijnenburg, T.A.; Wang, L.; Zimmermann, M.T.; Chambwe, N.; Gao, G.F.; Cherniack, A.D.; Fan, H.; Shen, H.; Way, G.P.; Greene, C.S.; et al. Genomic and Molecular Landscape of DNA Damage Repair Deficiency across the Cancer Genome Atlas. Cell Rep. 2018, 23, 239–254. [Google Scholar] [CrossRef]
- Gurjao, C.; Zhong, R.; Haruki, K.; Li, Y.Y.; Spurr, L.F.; Lee-Six, H.; Reardon, B.; Ugai, T.; Zhang, X.; Cherniack, A.D.; et al. Discovery and Features of an Alkylating Signature in Colorectal Cancer. Cancer Discov. 2021, 11, 2446–2455. [Google Scholar] [CrossRef]
- Hall, C.N.; Badawi, A.F.; O’Connor, P.J.; Saffhill, R. The detection of alkylation damage in the DNA of human gastrointestinal tissues. Br. J. Cancer 1991, 64, 59–63. [Google Scholar] [CrossRef]
- Wilson, V.L.; Weston, A.; Manchester, D.K.; Trivers, G.E.; Roberts, D.W.; Kadlubar, F.F. Alkyl and aryl carcinogen adducts detected in human peripheral lung. Carcinogenesis 1989, 10, 2149–2153. [Google Scholar] [CrossRef]
- Hemeryck, L.Y.; Decloedt, A.I.; Vanden Bussche, J.; Geboes, K.P.; Vanhaecke, L. High resolution mass spectrometry-based profiling of diet-related deoxyribonucleic acid adducts. Anal. Chim. Acta 2015, 892, 123–131. [Google Scholar] [CrossRef]
- Tessmer, I.; Margison, G.P. The DNA Alkyltransferase Family of DNA Repair Proteins: Common Mechanisms, Diverse Functions. Int. J. Mol. Sci. 2024, 25, 463. [Google Scholar] [CrossRef]
- Fang, Q. The Versatile Attributes of MGMT: Its Repair Mechanism, Crosstalk with Other DNA Repair Pathways, and Its Role in Cancer. Cancers 2024, 16, 331. [Google Scholar] [CrossRef]
- Kaina, B.; Christmann, M.; Naumann, S.; Roos, W.P. MGMT: Key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair 2007, 6, 1079–1099. [Google Scholar]
- Abdelhady, R.; Senthong, P.; Eyers, C.E.; Reamtong, O.; Cowley, E.; Cannizzaro, L.; Stimpson, J.; Cain, K.; Wilkinson, O.J.; Williams, N.H.; et al. Mass Spectrometric Analysis of the Active Site Tryptic Peptide of Recombinant O6-Methylguanine-DNA Methyltransferase Following Incubation with Human Colorectal DNA Reveals the Presence of an O6-Alkylguanine Adductome. Chem. Res. Toxicol. 2023, 36, 1921–1929. [Google Scholar] [CrossRef]
- Pearson, S.J.; Wharton, S.; Watson, A.J.; Begum, G.; Butt, A.; Glynn, N.; Williams, D.M.; Shibata, T.; Santibáñez-Koref, M.F.; Margison, G.P. A novel DNA damage recognition protein in Schizosaccharomyces pombe. Nucleic Acids Res. 2006, 34, 2347–2354. [Google Scholar] [CrossRef] [PubMed]
- Latypov, V.F.; Tubbs, J.L.; Watson, A.J.; Marriott, A.S.; McGown, G.; Thorncroft, M.; Wilkinson, O.J.; Senthong, P.; Butt, A.; Arvai, A.S.; et al. Atl1 regulates choice between global genome and transcription-coupled repair of O6-alkylguanines. Mol. Cell. 2012, 47, 50–60. [Google Scholar] [CrossRef]
- Watson, A.J.; Middleton, M.R.; McGown, G.; Thorncroft, M.; Ranson, M.; Hersey, P.; McArthur, G.; Davis, I.D.; Thomson, D.; Beith, J.; et al. O6-methylguanine-DNA methyltransferase depletion and DNA damage in patients with melanoma treated with temozolomide alone or with lomeguatrib. Br. J. Cancer 2009, 100, 1250–1256. [Google Scholar] [CrossRef]
- Pearson, S.J.; Ferguson, J.; Santibanez-Koref, M.; Margison, G.P. Inhibition of O6-methylguanine-DNA methyltransferase by an alkyltransferase-like protein from Escherichia coli. Nucleic Acids Res. 2005, 33, 3837–3844. [Google Scholar] [CrossRef]
- Sobol, R.W. Temozolomide. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Göder, A.; Nagel, G.; Kraus, A.; Dörsam, B.; Seiwert, N.; Kaina, B.; Fahrer, J. Lipoic acid inhibits the DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) and triggers its depletion in colorectal cancer cells with concomitant autophagy induction. Carcinogenesis 2015, 36, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Cupid, B.C.; Zeng, Z.; Singh, R.; Shuker, D.E.G. Detection of O6-carboxymethyl-2′-deoxyguanosine in DNA following reaction of nitric oxide with glycine and in human blood DNA using a quantitative immunoslot blot assay. Chem. Res. Toxicol. 2004, 17, 294–300. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaakub, H.; Howell, A.; Margison, G.P.; Povey, A.C. Development and Application of a Slot-Blot Assay Using the Damage Sensing Protein Atl1 to Detect and Quantify O6-Alkylated Guanine Bases in DNA. Toxics 2024, 12, 649. https://doi.org/10.3390/toxics12090649
Yaakub H, Howell A, Margison GP, Povey AC. Development and Application of a Slot-Blot Assay Using the Damage Sensing Protein Atl1 to Detect and Quantify O6-Alkylated Guanine Bases in DNA. Toxics. 2024; 12(9):649. https://doi.org/10.3390/toxics12090649
Chicago/Turabian StyleYaakub, Hanum, Anthony Howell, Geoffrey P. Margison, and Andrew C. Povey. 2024. "Development and Application of a Slot-Blot Assay Using the Damage Sensing Protein Atl1 to Detect and Quantify O6-Alkylated Guanine Bases in DNA" Toxics 12, no. 9: 649. https://doi.org/10.3390/toxics12090649
APA StyleYaakub, H., Howell, A., Margison, G. P., & Povey, A. C. (2024). Development and Application of a Slot-Blot Assay Using the Damage Sensing Protein Atl1 to Detect and Quantify O6-Alkylated Guanine Bases in DNA. Toxics, 12(9), 649. https://doi.org/10.3390/toxics12090649





