Environmental Impact of Waste Treatment and Synchronous Hydrogen Production: Based on Life Cycle Assessment Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Principles of the Life Cycle Methods
2.2. The Fundamental Analytical Framework of the Life Cycle Approach
- (1)
- Definition of the goal and the scope
- (2)
- Life cycle inventory analysis
- (3)
- Impact evaluation
- (4)
- The result interpretation
2.3. Data Sources
3. Results and Discussion
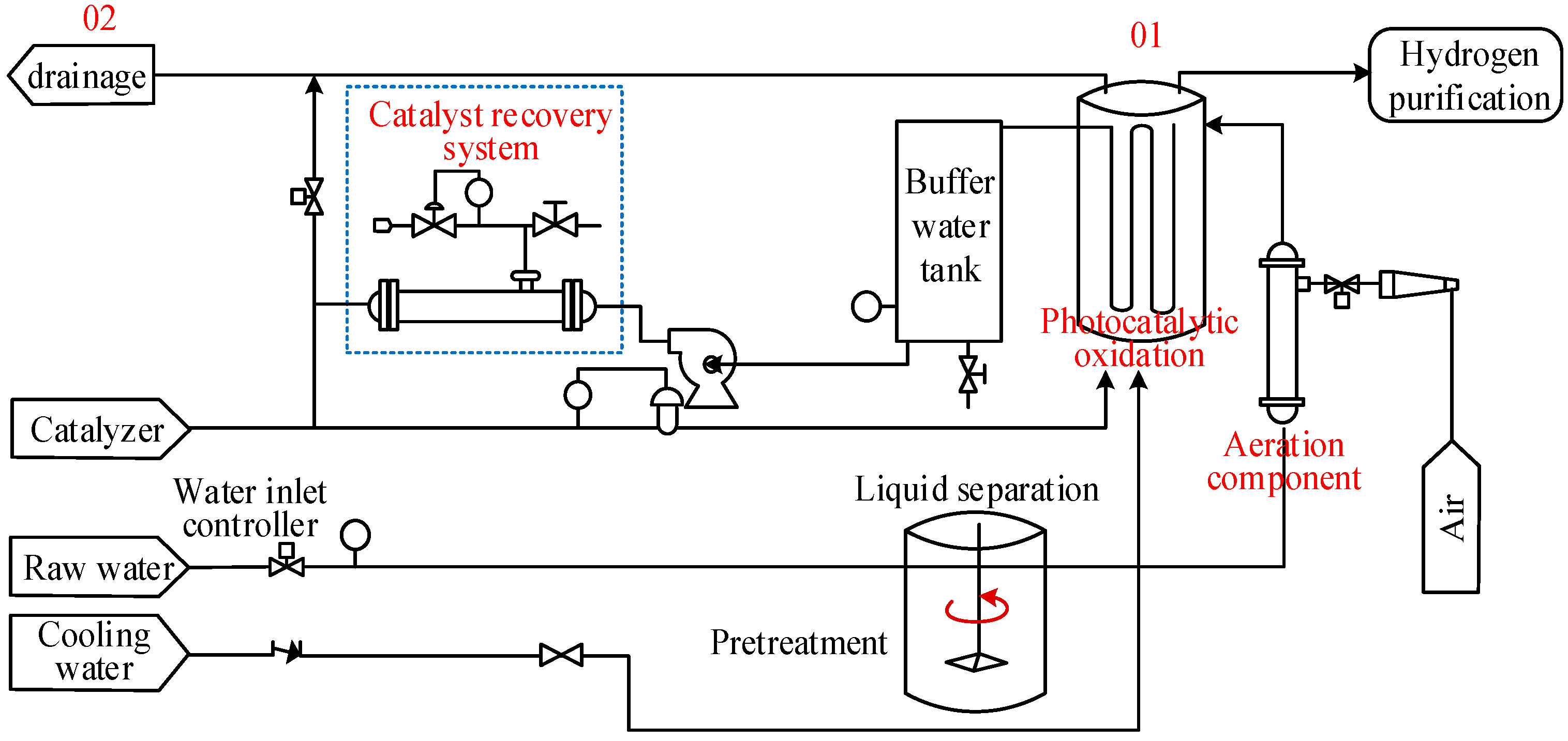
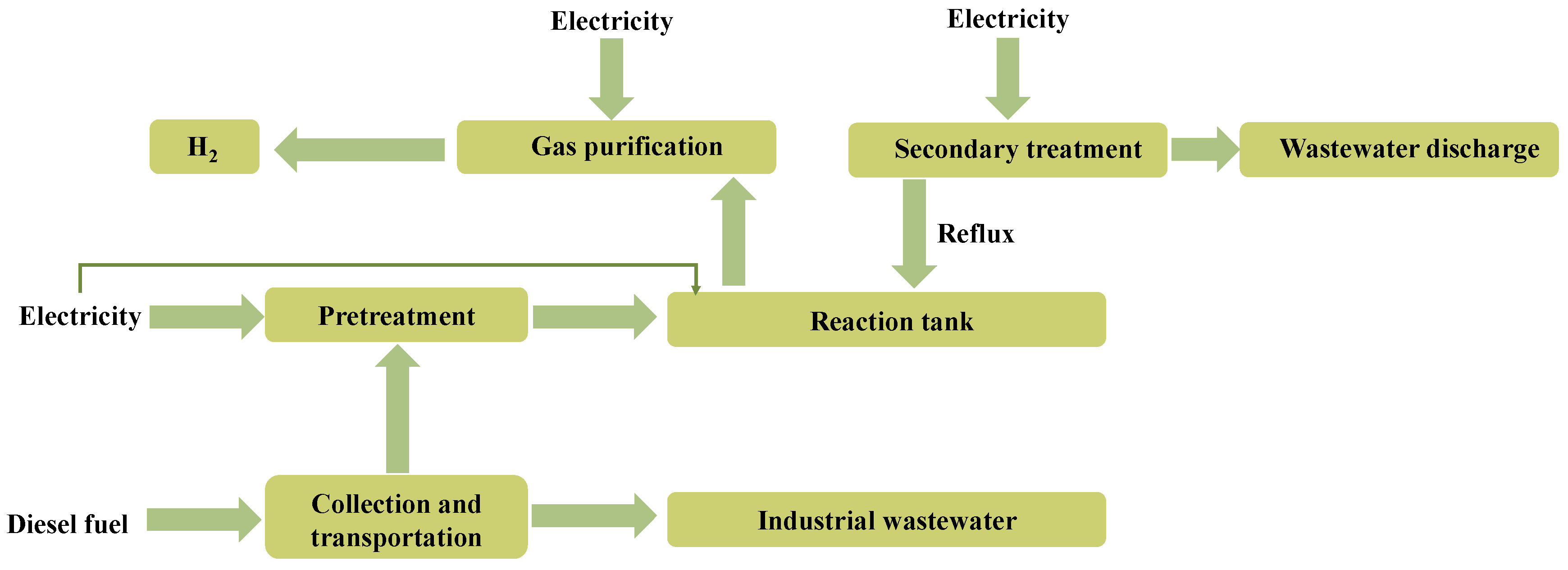
3.1. The Basic Framework of the LCA Model for Synchronous Hydrogen Production and Environmental Waste Treatment
- (1)
- Definition of the goal and the scope
- (2)
- Inventory analysis
| Material Input | Material Output | ||||
|---|---|---|---|---|---|
| Wastewater Collection and Transportation Stage | Unit | Magnitude | Photocatalytic Hydrogen Production Stage | Unit | Magnitude |
| Wastewater [51] | t | 10,000 | Annual hydrogen production [40,41] | m3 | 5264 |
| Wastewater transportation [52] | km | 50 | Gas emission in photocatalytic process (for CDs/CdS/treatment of 4-NP wastewater with CNU) [53,54] | Unit | Magnitude |
| Wastewater transportation fuel consumption [52] | L·a−1 | 13,800 | |||
| Wastewater pretreatment stage | Unit | Magnitude | CO2 | g·m−3 | 9450 |
| Fan power consumption (4 units) [51] | kWh·a−1 | 3160 | SO2 | g·m−3 | 50 |
| Solid–liquid separator power consumption [55] | kWh·a−1 | 3800 | N2O | g·m−3 | 0 |
| Photocatalyst preparation stage | Unit | Magnitude | CO | g·m−3 | 5.3 |
| Catalyst dosage | kg | 2000 | NOx | g·m−3 | 0.3 |
| Power consumption for CDs/CdS/CNU preparation [40] | kWh·a−1 | 8.0 × 104 | VOC | g·m−3 | 0.02 |
| Wastewater treatment stage | Unit | Magnitude | Content of contaminated elements [56] | Unit | Magnitude |
| CDs/CdS/CNU treatment containing 4-NP wastewater [40] | kWh·a−1 | 6.0 × 104 | TN | g/kg | 348.2 |
| Power consumption purification by voltage washing in hydrogen purification stage [57] | kWh·a−1 | 2.0 × 105 | TP | g/kg | 0 |
| Waste Disposal and Synchronous Hydrogen Production | Wastewater Transportation (L·a−1) | Pretreatment (kWh·a−1) | Photocatalytic Preparation (kWh·a−1) | Reaction Pool Processing (kWh·a−1) | Hydrogen Purification (kWh·a−1) |
|---|---|---|---|---|---|
| Energy consumption | 13,800 | 6.96 × 103 | 8.0 × 104 | 4.0 × 105 | 2.0 × 105 |
| Coal conversion coefficient | 6.12 × 10−4 | 3.09 × 10−3 | 1.23 × 10−4 | 2.35 × 10−4 | 1.23 × 10−4 |
| Coal conversion | 16.89 | 8.53 | 9.84 | 46.87 | 24.60 |
3.2. Analysis of the Potential Environmental Impact
- (1)
- Determining the types of impact:
- (2)
- Quantifying Standardized Environmental Impact Metrics:
3.3. Result Analysis and Improvement Measures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Octavianthy, D.; Syauqi, A.; Reyseliani, N.; Purwanto, W.W. Multi-period enviro-economic optimization of municipal solid waste to electricity. Waste Biomass Valorization 2022, 13, 3707–3722. [Google Scholar] [CrossRef]
- Den Boer, E.; Banaszkiewicz, K.; den Boer, J.; Pasiecznik, I. Energy recovery from waste-closing the municipal loop. Energies 2022, 15, 1246. [Google Scholar] [CrossRef]
- Issock, P.B.; Mpinganjira, M.; Roberts-Lombard, M. Trying to recycle domestic waste and feelings of guilt: A moderated mediation model applied to South African households. Sustain. Prod. Consum. 2021, 27, 1286–1296. [Google Scholar] [CrossRef]
- Zheng, Y.P.; Zhang, Q.G.; Zhang, Z.P.; Jing, Y.Y.; Hu, J.J.; He, C.; Lu, C.Y. A review on biological recycling in agricultural waste-based biohydrogen production: Recent developments. Bioresour. Technol. 2022, 347, 126595. [Google Scholar] [CrossRef]
- Su, R.; Li, Z.; Ma, X.; Li, Y.; Zeng, Z.; Li, L.; Sheng, P.; Wang, H.; Wang, S. Machine learning, experiments and molecular simulation demonstrate the adsorption mechanism of acetone on porous carbon at different pressures. Sep. Purif. Technol. 2023, 323, 124480. [Google Scholar] [CrossRef]
- Chen, T.; Duan, L.; Cheng, S.; Jiang, S.; Yan, B. The preparation of paddy soil amendment using granite and marble waste: Performance and mechanisms. J. Environ. Sci. 2023, 127, 564–576. [Google Scholar] [CrossRef]
- Su, R.; Xue, R.; Ma, X.; Zeng, Z.; Li, L.; Wang, S. Targeted improvement of narrow micropores in porous carbon for enhancing trace benzene vapor removal: Revealing the adsorption mechanism via experimental and molecular simulation. J. Colloid Interface Sci. 2024, 671, 770–778. [Google Scholar] [CrossRef]
- Zhang, J.L.; Fu, S.Y.; Lan, X.Y.; Yang, X.D. Agricultural residue-based bioplastics: Potential options for high-value agricultural residue utilization. Bioresources 2023, 18, 4383–4385. [Google Scholar] [CrossRef]
- Naqvi, M.; Dahlquist, E.; Yan, J.; Raza Naqvi, S.; Nizami, D.A.-S.; Salman, C.A.; Muhammad, D.; Farooq, U.; Rehan, M.; Khan, Z.; et al. Polygeneration system integrated with small non-wood pulp mills for substitute natural gas production. Appl. Energ. 2018, 224, 636–646. [Google Scholar] [CrossRef]
- Wang, S.; Tarroja, B.; Schell, L.S.; Shaffer, B.; Samuelsen, S. Prioritizing among the end uses of excess renewable energy for cost-effective greenhouse gas emission reductions. Appl. Energ. 2019, 235, 284–298. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. A technical review of bioenergy and resource recovery from municipal solid waste. J. Hazard. Mater. 2021, 403, 123970. [Google Scholar] [CrossRef] [PubMed]
- Arpia, A.A.; Chen, W.H.; Lam, S.S.; Rousset, P.; de Luna, M.D.G. Sustainable biofuel and bioenergy production from biomass waste residues using microwave-assisted heating: A comprehensive review. Chem. Eng. J. 2021, 403, 126233. [Google Scholar] [CrossRef]
- IEA International Energy Agency Database. Available online: http://data.iea.org/ (accessed on 15 January 2024).
- Yu, B.; Liu, X.Q.; Ji, C.; Sun, H. Greenhouse gas mitigation strategies and decision support for the utilization of agricultural waste systems: A case study of Jiangxi Province, China. Energy 2023, 265, 126380. [Google Scholar] [CrossRef]
- Su, R.; Li, Z.; Cheng, F.; Dai, X.; Wang, H.; Luo, Y.; Huang, L. Advances in the Degradation of Emerging Contaminants by Persulfate Oxidation Technology. Water Air Soil Pollut. 2023, 234, 754. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. Assessment of energy recovery potential and analysis of environmental impacts of waste to energy options using life cycle assessment. J. Clean. Prod. 2022, 365, 132854. [Google Scholar] [CrossRef]
- Su, R.; Chai, L.; Tang, C.; Li, B.; Yang, Z. Comparison of the degradation of molecular and ionic ibuprofen in a UV/H2O2 system. Water Sci. Technol. 2018, 77, 2174–2183. [Google Scholar] [CrossRef]
- Liu, B.C.; Han, Z.Y.; Li, J.; Yan, B. Comprehensive evaluation of municipal solid waste power generation and carbon emission potential in Tianjin based on Grey Relation Analysis and Long Short Term Memory. Process Saf. Environ. Environ. 2022, 168, 918–927. [Google Scholar] [CrossRef]
- Vyas, S.; Prajapati, P.; Shah, A.V.; Varjani, S. Municipal solid waste management: Dynamics, risk assessment, ecological influence, advancements, constraints and perspectives. Sci. Total Environ. 2022, 814, 152802. [Google Scholar] [CrossRef]
- Pheakdey, D.V.; Quan, N.V.; Xuan, T.D. Economic and environmental benefits of energy recovery from municipal solid waste in phnom penh municipality, Cambodia. Energies 2023, 16, 3234. [Google Scholar] [CrossRef]
- Su, R.; Xie, C.; Alhassan, S.I.; Huang, S.; Chen, R.; Xiang, S.; Wang, Z.; Huang, L. Oxygen reduction reaction in the field of water environment for application of nanomaterials. Nanomaterials 2020, 10, 1719. [Google Scholar] [CrossRef]
- Negri, C.; Ricci, M.; Zilio, M.; D’Imporzano, G.; Qiao, W.; Dong, R.; Adani, F. Anaerobic digestion of food waste for bio-energy production in China and Southeast Asia: A review. Renew. Sustain. Energy Rev. 2020, 133, 110138. [Google Scholar] [CrossRef]
- Li, Z.Y.; Kuo, Y.K.; Mahmud, A.R.; Nassani, A.A.; Haffar, M.; Muda, I. Integration of renewable energy, environmental policy stringency, and climate technologies in realizing environmental sustainability: Evidence from OECD countries. Renew. Energy 2022, 196, 1376–1384. [Google Scholar] [CrossRef]
- Selvey, L.A.; Carpenter, M.; Lazarou, M.; Cullerton, K. Communicating about energy policy in a resource-rich jurisdiction during the climate crisis: Lessons from the people of brisbane, queensland, australia. Int. J. Environ. Res. Public Health 2022, 19, 4635. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.H.; Yang, X.M.; Yu, H. Cleaner production and total factor productivity of polluting enterprises. J. Clean. Prod. 2023, 423, 138827. [Google Scholar]
- Luo, Y.; Su, R.; Yao, H.; Zhang, A.; Xiang, S.; Huang, L. Degradation of trimethoprim by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Environ. Environ. Sci. Pollut. Res. 2021, 28, 62572–62582. [Google Scholar] [CrossRef]
- Su, R.; Dai, X.; Wang, H.; Wang, Z.; Li, Z.; Chen, Y.; Luo, Y.; Ouyang, D. Metronidazole degradation by UV and UV/H2O2 advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Int. J. Environ. Res. Public Health 2022, 19, 12354. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting–a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Yang, L.; Hong, Y.Z.; Liu, E.L.; Zhang, X.; Wang, L.Y.; Lin, X.; Shi, J.Y. Significant enhancement of photocatalytic H2 production simultaneous with dye degradation over Ni2P modified In2O3 nanocomposites. Sep. Purif. Technol. 2021, 263, 118366. [Google Scholar] [CrossRef]
- Li, X.B.; Han, T.; Zhou, Y.T.; Xie, Y.; Luo, Y.D.; Huang, J.T.; Chen, Z.; Deng, F. Photoelectrocatalytic hydrogen evolution and synchronous degradation of organic pollutants by pg-C3N4/β-FeOOH S-scheme heterojunction. Sci. China-Technol. Sci. 2024, 67, 1238–1252. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, J.; Shangguan, W. A review on photocatalysis in antibiotic wastewater: Pollutant degradation and hydrogen production. Chin. J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Ablat, H.; Nurmamat, X.; Tian, J.R.; Zhao, Z.X. Progress of photocatalytic oxidation-adsorption synergistic removal of organic arsenic in water. Water Environ. Res. 2024, 96, e11057. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yang, D.L.; Xiang, W.Y.; Guo, Y.; Yu, Z.W.; Wang, J. An in-depth study of integrating cascaded photocatalytic H2O2 generation and activation with solar-driven interfacial evaporation for in-situ organic contaminant remediation. J. Hazard. Mater. 2024, 476, 13963. [Google Scholar] [CrossRef]
- Yu, W.; Yan, X.; Kai, L.; Jing, Q. New Two-Layer power control scheme in islanded Cyber-physical microgrids. J. Energy Eng. 2019, 145, 04019017. [Google Scholar]
- Musa, E.N.; Kaur, S.; Gallagher, T.C.; Anthony, T.M.; Stickle, W.F.; Arnadottir, L.; Stylianou, K.C. Two birds, one stone: Coupling hydrogen production with herbicide degradation over metal-organic framework-derived titanium dioxide. ACS Catal. 2023, 13, 3710–3722. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.H.; Liu, M.Z.; Li, X.W.; Han, C.H.; Niu, L.Y.; Zhang, F.; Wu, X.; Sun, J.M.; Shao, C.L.; et al. Hollow bi-Janus hetero-nanofibers as a bi-functional photoreforming catalyst for prominently boosting hydrogen evolution from water-pollutant system. Nano Energy 2023, 108, 108226. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Z.Y.; Zhang, D.P.; Liu, X.Y.; Wang, P.F.; Li, Y.; Zhan, S.H. Superexchange-induced Pt-O-Ti3+ site on single photocatalyst for efficient H2 production with organics degradation in wastewater. Proc. Natl. Acad. Sci. USA 2023, 120, e2302873120. [Google Scholar] [CrossRef]
- Goodarzi, N.; Ashrafi-Peyman, Z.; Khani, E.; Moshfegh, A.Z. Recent progress on semiconductor heterogeneous photocatalysts in clean energy production and environmental remediation. Catalysts 2023, 13, 1102. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Dhodamani, A.G.; Kang, S.W. Hydrothermally synthesized Ag@Mos2 composite for enhanced photocatalytic hydrogen production. Catalysts 2023, 13, 716. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, L.; Yu, F.; Nie, Y.; Xing, Q.; Liu, X.; Pei, Y.; Zou, J.; Dai, W. Photodegradation of organic pollutants coupled with simultaneous photocatalytic evolution of hydrogen using quantum-dot-modified g-C3N4 catalysts under visible-light irradiation. ACS Sustain. Chem. Eng. 2018, 6, 12695–12705. [Google Scholar] [CrossRef]
- Nie, Y.C.; Yu, F.; Wang, L.C.; Xing, Q.J.; Liu, X.; Pei, Y.; Zou, J.P.; Dai, W.L.; Li, Y.; Suib, S.L. Photocatalytic degradation of organic pollutants coupled with simultaneous photocatalytic H2 evolution over graphene quantum dots/Mn-N-TiO2/g-C3N4 composite catalysts: Performance and mechanism. Appl. Catal. B Environ. 2018, 227, 312–321. [Google Scholar] [CrossRef]
- Ma, Y.; Li, F.Y.; Wang, L.M.; Wang, G. Multidimensional evaluation method and application based on life cycle carbon efficiency considering carbon emission, cost, and function. Environ. Sci. Pollut. Res. 2023, 30, 70918–70936. [Google Scholar] [CrossRef]
- Ro, J.W.; Zhang, Y.Z.; Kendall, A. Developing guidelines for waste designation of biofuel feedstocks in carbon footprints and life cycle assessment. Sustain. Prod. Consum. 2023, 37, 320–330. [Google Scholar] [CrossRef]
- Mulya, K.S.; Zhou, J.Q.; Phuang, Z.X.; Laner, D.; Woon, K.S. A systematic review of life cycle assessment of solid waste management: Methodological trends and prospects. Sci. Total Environ. 2022, 831, 154903. [Google Scholar] [CrossRef]
- Li, H.M.; Tian, Y.; Li, Z.L.; Wu, C.; Liu, Y.L. Life-cycle economic and environmental impacts of municipal solid waste reverse logistics in residential areas. Waste Manag. 2023, 164, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Xie, J.L.; Xu, M.; Wang, Y.T.; Liu, Y.B. An infinite life cycle assessment model to re-evaluate resource efficiency and environmental impacts of circular economy systems. Waste Manag. 2022, 145, 72–82. [Google Scholar] [CrossRef]
- Zhao, X.; You, F.Q. Life cycle assessment of microplastics reveals their greater environmental hazards than mismanaged polymer waste losses. Environ. Sci. Technol. 2022, 56, 11780–11797. [Google Scholar] [CrossRef] [PubMed]
- Weidema, B.P.; Pizzol, M.; Schmidt, J.; Thoma, G. Attributional or consequential Life Cycle Assessment: A matter of social responsibility. J. Clean. Prod. 2018, 174, 305–314. [Google Scholar] [CrossRef]
- Jing, F. Study on Environmental Cost Control Based on Product Lifecycle Method. Master’s Thesis, Traffic University Of East China, Shanghai, China, 2019. [Google Scholar]
- Bianco, I.; Panepinto, D.; Zanetti, M. Environmental impacts of electricity from incineration and gasification: How the lca approach can affect the results. Sustainability 2022, 14, 92. [Google Scholar] [CrossRef]
- Lim, S.R.; Park, J.M. Environmental impact minimization of a total wastewater treatment network system from a life cycle perspective. J. Environ. Manag. 2009, 90, 1454–1462. [Google Scholar] [CrossRef]
- He, J.; Chen, D.; Li, Y.; Shao, J.; Jiao, X.; Yuejuan, S.; Zhiying, Y.; Jiaqiang, W. Diatoms templated TiO2 with enhanced photocatalytic activity: Biomimetics of photonic crystals. Appl. Phys. A 2014, 113, 327–332. [Google Scholar] [CrossRef]
- XD, H.; XY, W.; H, J.; S, L. Comprehensive benefit evaluation method and case application of sewage treatment environment. Chin. Water Supply Drain. 2019, 35, 6–15. [Google Scholar]
- Sabeen, A.H.; Noor, Z.Z.; Ngadi, N.; Almuraisy, S.; Raheem, A.B. Quantification of environmental impacts of domestic wastewater treatment using life cycle assessment: A review. J. Clean. Prod. 2018, 190, 221–233. [Google Scholar] [CrossRef]
- P, L.; HL, H.; GG, L.; QT, L.; K, Y.; WY, L. Engineering experiment on advanced treatment of electroplating organic wastewater by photocatalytic oxidation. Chin. J. Environ. Eng. 2013, 7, 3885–3889. [Google Scholar]
- J, Y.; D, Z.; L, W.; MT, H.; YP, J. Microwave photocatalytic reaction enhances the process of organic wastewater treatment and recycling. Chem. Ind. Eng. Prog. 2009, 28, 416. [Google Scholar]
- Li, H.; Liao, Z.; Sun, J.; Jiang, B.; Wang, J.; Yang, Y. Modelling and simulation of two-bed PSA process for separating H2 from methane steam reforming. Chin. J. Chem. Eng. 2019, 27, 1870–1878. [Google Scholar] [CrossRef]
- Huijbregts, M.A.; Steinmann, Z.J.; Elshout, P.M.; Stam, G.; Verones, F.; Vieira, M.D.; Hollander, A.; Zijp, M.; van Zelm, R. ReCiPe 2016: A harmonized life cycle impact assessment method at midpoint and endpoint level Report I: Characterization. Int. J. Life Cycle Assess. 2016, 22, 1–10. [Google Scholar]
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: A harmonised life cycle impact assessment method at midpoint and endpoint level. Int. J. Life Cycle Assess. 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Borda, F.; La Rosa, A.D.; Filice, L.; Gagliardi, F. Environmental impact of process constrained topology optimization design on automotive component’ life. Int. J. Mater. Form. 2023, 16, 48. [Google Scholar] [CrossRef]
- Zhao, L.X.; Meng, H.B.; Shen, Y.J.; Ding, J.T.; Zhang, X. Investigation and analysis on the current situation and development of planting and breeding circular agriculture in the plain area of northern China. Trans. Chin. Soc. Agric. Eng. 2017, 33, 1–10. [Google Scholar]
- Wang, L.; Gao, C.; Bi, Y.; Wang, Y.; Wang, H.; Sun, N.; Yu, J. Estimation of greenhouse gas emission reduction in large-scale straw biogas centralized gas supply project. Trans. Chin. Soc. Agric. Eng. 2017, 33, 223–228. [Google Scholar]
- L, Z.; YW, L.; HX, R.; H, L. Life cycle assessment of large straw biogas centralized gas supply project. Anhui Agric. Sci. 2010, 38, 19462–19464+19495. [Google Scholar]
- Saini, S.; Dhania, G. Cadmium as an environmental pollutant: Ecotoxicological effects, health hazards, and bioremediation approaches for its detoxification from contaminated sites. In Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 357–387. [Google Scholar]
- Xue, W.; Liu, H.; Li, J.; Chen, X.; Wen, S.; Guo, J.; Shi, X.; Cao, S.; Gao, Y.; Wang, R.; et al. Immobilization of cadmium in river sediments by different modified nanoscale zero-valent iron: Performance, mechanisms, and Fe dissolution. Environ. Sci. Pollut. Res. 2023, 30, 117892–117908. [Google Scholar] [CrossRef]
- Jameel, M.; Rauf, M.A.; Khan, M.T.; Farooqi, M.K.; Alam, M.A.; Mashkoor, F.; Shoeb, M.; Jeong, C. Ingestion and effects of green synthesized cadmium sulphide nanoparticle on Spodoptera Litura as an insecticidal and their antimicrobial and anticancer activities. Pestic. Biochem. Phys. 2023, 190, 105332. [Google Scholar] [CrossRef]
- Marmiroli, M.; Birarda, G.; Gallo, V.; Villani, M.; Zappettini, A.; Vaccari, L.; Marmiroli, N.; Pagano, L. Cadmium sulfide quantum dots, mitochondrial function and environmental stress: A mechanistic reconstruction through in vivo cellular approaches in saccharomyces cerevisiae. Nanomaterials 2023, 13, 1944. [Google Scholar] [CrossRef] [PubMed]
- Verma, Y.; Rani, V.; Rana, S.V.S. Assessment of cadmium sulphide nanoparticles toxicity in the gills of a fresh water fish. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100280. [Google Scholar] [CrossRef]
- Ning, X.M.; Wu, Y.L.; Ma, X.F.; Zhang, Z.; Gao, R.Q.; Chen, J.; Shan, D.L.; Lu, X.Q. A novel charge transfer channel to simultaneously enhance photocatalytic water splitting activity and stability of CdS. Adv. Funct. Mater. 2019, 29, 1902992. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Lian, J.H.; Ma, Q.; Zhang, S.L.; He, J.J.; Zhong, J.B.; Li, J.Z.; Huang, H.H.; Su, B. Preparation of carbon spheres supported CdS photocatalyst for enhancement its photocatalytic H2 evolution. Catal. Today 2017, 281, 662–668. [Google Scholar] [CrossRef]
- Jing, D.W.; Guo, L.J. A novel method for the preparation of a highly stable and active CdS photocatalyst with a special surface nanostructure. J. Phys. Chem. B 2006, 110, 11139–11145. [Google Scholar] [CrossRef]
- Kudo, A.; Sekizawa, M. Photocatalytic H2 evolution under visible light irradiation on Ni-doped ZnS photocatalyst. Chem. Commun. 2000, 15, 1371–1372. [Google Scholar] [CrossRef]
- Peng, K.; Wang, H.J.; Li, X.Y.; Wang, J.W.; Xu, L.; Gao, H.F.; Niu, M.; Ma, M.B.; Yang, J. One-step hydrothermal growth of MoS2 nanosheets/CdS nanoparticles heterostructures on montmorillonite for enhanced visible light photocatalytic activity. Appl. Clay Sci. 2019, 175, 86–93. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Ghosh, I.; Khamrai, J.; Savateev, A.; Shlapakov, N.; Antonietti, M.; König, B. Organic semiconductor photocatalyst can bifunctionalize arenes and heteroarenes. Sci. Justice 2019, 365, 360–366. [Google Scholar] [CrossRef]
- Chen, S.; Qi, Y.; Hisatomi, T.; Ding, Q.; Asai, T.; Li, Z.; Ma, S.S.K.; Zhang, F.; Domen, K.; Li, C. Efficient visible-light-driven Z-scheme overall water splitting using a MgTa2O6− xNy/TaON heterostructure photocatalyst for H2 evolution. Angew. Chem. 2015, 127, 8618–8621. [Google Scholar] [CrossRef]
- Hara, M.; Nunoshige, J.; Takata, T.; Kondo, J.N.; Domen, K. Unusual enhancement of H2 evolution by Ru on TaON photocatalyst under visible light irradiation. Chem. Commun. 2003, 24, 3000–3001. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Zhang, H.W.; Fan, J.J.; Xiang, Q.J. Design and application of active sites in g-C3N4-based photocatalysts. J. Mater. Sci. Technol. 2020, 56, 69–88. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Bai, X.J.; Wang, L.; Wang, Y.J.; Yao, W.Q.; Zhu, Y.F. Enhanced oxidation ability of g-C3N4 photocatalyst via C60 modification. Appl. Catal. B Environ. 2014, 152, 262–270. [Google Scholar] [CrossRef]
- Sapountzi, F.M.; Gracia, J.M.; Weststrate, C.J.; Fredriksson, H.O.A.; Niemantsverdriet, J.W. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. 2017, 58, 1–35. [Google Scholar] [CrossRef]
- Huang, M.; Liu, C.; Cui, P.; Dang, F.; Zhou, J.; Liu, M.; Wang, Y. Facet-specific cation exchange and heterogeneous transformation of cadmium sulfide nanoparticles induced by Cu (ii). Environ. Sci. Nano 2023, 10, 463–475. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Al Harby, N.; El Batouti, M.; Abd El-Monaem, E.M. Engineering a sustainable cadmium sulfide/polyethyleneimine-functionalized biochar/chitosan composite for effective chromium adsorption: Optimization, co-interfering anions, and mechanisms. RSC Adv. 2024, 14, 22266–22279. [Google Scholar] [CrossRef]
- Wang, C.; Bi, L.; Liu, J.; Huang, B.; Wang, F.; Zhang, Y.; Yao, C.; Pan, G.; Song, M. Microalgae-derived carbon quantum dots mediated formation of metal sulfide nano-adsorbents with exceptional cadmium removal performance. J. Colloid Interface Sci. 2023, 629, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Lacave, J.M.; Bilbao, E.; Gilliland, D.; Mura, F.; Dini, L.; Cajaraville, M.P.; Orbea, A. Bioaccumulation, cellular and molecular effects in adult zebrafish after exposure to cadmium sulphide nanoparticles and to ionic cadmium. Chemosphere 2020, 238, 124588. [Google Scholar] [CrossRef] [PubMed]
- Ghasempour, A.; Dehghan, H.; Ataee, M.; Chen, B.; Zhao, Z.; Sedighi, M.; Guo, X.; Shahbazi, M.-A. Cadmium sulfide nanoparticles: Preparation, characterization, and biomedical applications. Molecules 2023, 28, 3857. [Google Scholar] [CrossRef] [PubMed]

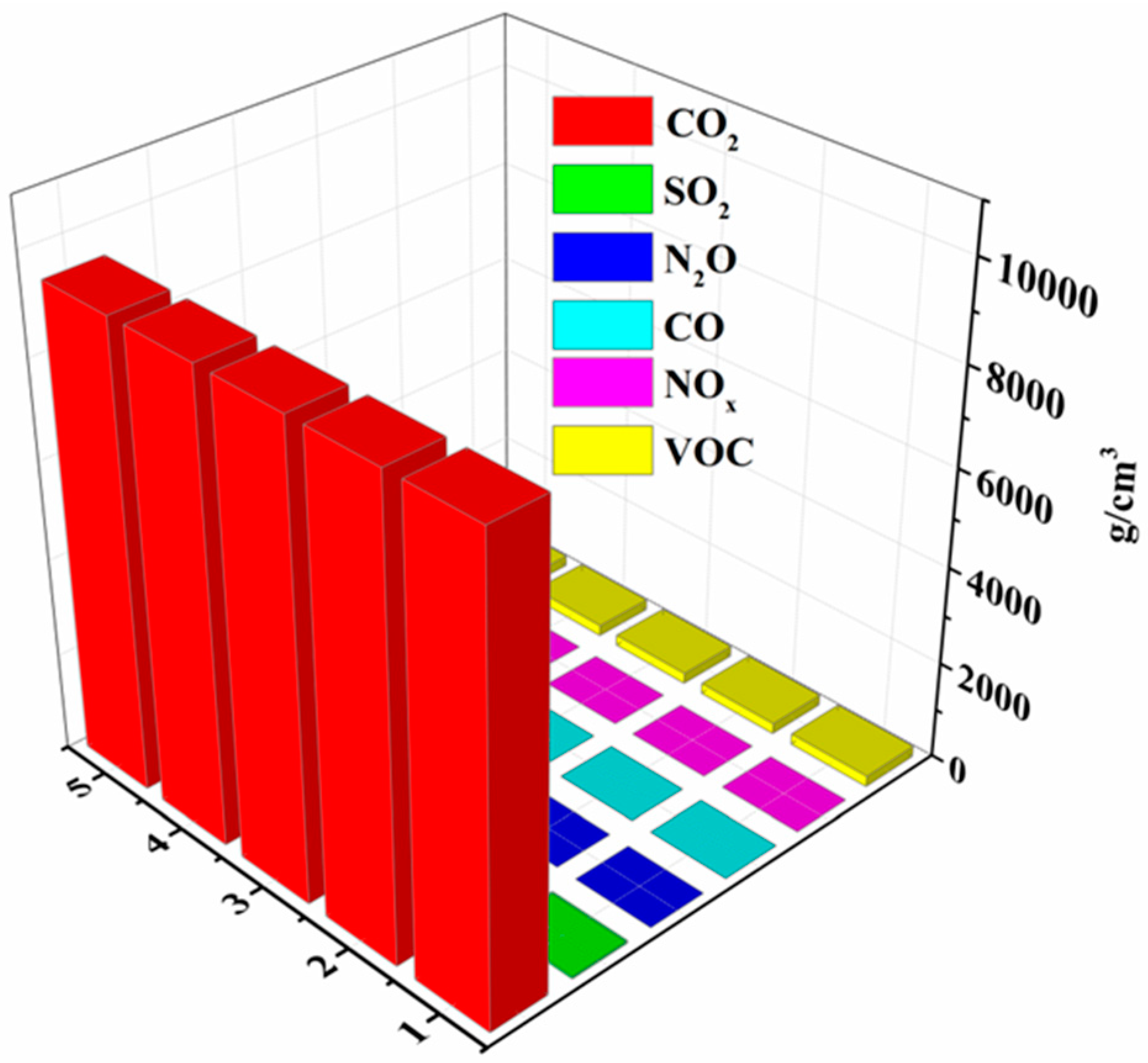
| Project | Detection 1 | Detection 2 | Detection 3 | Detection 4 | Detection 5 | Average Value |
|---|---|---|---|---|---|---|
| Carbon dioxide (CO2) g·m−3 | 9450 | 9448 | 9449 | 9452 | 9448 | 9449.4 |
| Sulfur dioxide (SO2) g·m−3 | 49 | 51 | 47 | 50 | 51 | 49.6 |
| nitrous oxide (N2O) g·m−3 | 0 | 0 | 0 | 0 | 0 | 0 |
| carbon monoxide (CO)g·m−3 | 5 | 5.5 | 5 | 5.8 | 5.6 | 5.38 |
| nitrogen oxide (NOx)g·m−3 | 0.3 | 0.4 | 0.3 | 0.38 | 0.35 | 0.346 |
| VOC g·m−3 | 225 | 220 | 223 | 228 | 221 | 223.4 |
| Impact Category | Unit | Altogether | Cadmium Sulfide | Phenylethane | Urea | Muriate | Citric Acid | Ammonia | Alcohol | Deionized Water | Power Consumption |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Global warming | kg CO2 eq | 23,340.50 | 0.60 | 45.20 | 82.74 | 0.43 | 5.74 | 1.72 | 329.9 | 0.024 | 22,871.2 |
| Stratospheric ozone depletion | kg CFC11 eq | 0.006 | 1.90 × 10−7 | 3.70 × 10−6 | 1.3 × 10−4 | 7.80 × 10−8 | 8.50 × 10−6 | 5.90 × 10−7 | 6.10 × 10−5 | 2.04 × 10−8 | 0.0062 |
| Ionizing radiation | kBq Co-60 eq | 326.34 | 0.06 | 0.67 | 4.67 | 0.02 | 0.13 | 0.02 | 10.96 | 0.001 | 309.82 |
| Human health | kg NOx eq | 53.98 | 0.0018 | 0.071 | 0.16 | 0.0009 | 0.01 | 0.002 | 0.65 | 4.81 × 10−5 | 53.09 |
| Fine particulate matter formation | kg PM2.5 eq | 9.71 | 0.0004 | 0.023 | 0.052 | 0.0005 | 0.0029 | 0.001 | 0.11 | 1.60 × 10−5 | 9.52 |
| Land | kg NOx eq | 54.15 | 0.002 | 0.08 | 0.16 | 0.0009 | 0.014 | 0.002 | 0.73 | 4.87 × 10−5 | 53.09 |
| Ground acidification | kg SO2 eq | 0.14 | 0.014 | 0.10 | 0.47 | 0.001 | 0.03 | 0.01 | 0.88 | 0.0001 | 136.24 |
| Eutrophication of freshwater. | kg P eq | 252.00 | 0.0003 | 0.009 | 0.03 | 9.60 × 10−5 | 0.002 | 0.0001 | 0.07 | 7.68 × 10−6 | 2.42 |
| Eutrophication of the sea | kg N eq | 0.21 | 1.70 × 10−5 | 0.0006 | 0.03 | 7.50 × 10−6 | 0.001 | 7.10 × 10−6 | 0.02 | 8.51 × 10−7 | 0.16 |
| Land ecological toxicity | kg 1,4-DCB eq | 6451.90 | 1.68 | 20.12 | 123.58 | 0.31 | 6.77 | 6.97 | 297.04 | 0.042 | 5995.37 |
| Freshwater ecological toxicity | kg 1,4-DCB eq | 417.53 | 0.06 | 0.50 | 4.65 | 0.01 | 0.26 | 0.03 | 6.19 | 0.017 | 405.83 |
| Seawater ecological toxicity | kg 1,4-DCB eq | 108.96 | 0.0167 | 0.17 | 1.27 | 0.003 | 0.07 | 0.01 | 2.07 | 0.0004 | 105.35 |
| Human carcinogenic toxicity | kg 1,4-DCB eq | 2.72 | 0.0002 | 0.049 | 0.082 | 7.50 × 10−5 | 0.0020 | 0.0013 | 0.12 | 1.15 × 10−5 | 2.46 |
| Non-carcinogenic toxicity in humans | kg 1,4-DCB eq | 125.67 | 0.025 | 0.38 | 2.17 | 0.005 | 0.14 | 0.023 | 4.86 | 0.0007 | 118.06 |
| Land use | m2a crop eq | 253.95 | 0.18 | 0.32 | 11.18 | 1.43 | 65.37 | 0.05 | 13.91 | 0.0033 | 161.49 |
| Scarcity of mineral resources. | kg Cu eq | 9.61 | 0.05 | 0.03 | 0.49 | 0.003 | 0.05 | 0.002 | 0.54 | 0.00045 | 8.45 |
| Scarcity of fossil resources | kg oil eq | 3277.05 | 0.18 | 20.67 | 22.47 | 0.071 | 0.99 | 0.55 | 152.46 | 0.0052 | 3079.64 |
| Water consumption | m3 | 83.84 | 0.0065 | 0.48 | 1.51 | 0.0027 | 0.15 | 0.033 | 3.94 | 0.043 | 77.68 |
| Impact Category | Altogether | Cadmium Sulfide | Phenylethane | Urea | Muriate | Citric Acid | Aqueous | Alcohol | Deionized Water | Power Consumption |
|---|---|---|---|---|---|---|---|---|---|---|
| Global warming | 2.17 | 5.61 × 10−5 | 0.0042 | 0.00769 | 4.02 × 10−5 | 0.0005 | 0.0001 | 0.0306 | 2.3 × 10−6 | 2.13 |
| Ozone layer consumption | 0.098 | 2.88 × 10−6 | 5.71 × 10−5 | 0.00192 | 1.12 × 10−6 | 0.0001 | 8.99 × 10−6 | 0.0009 | 3.12 × 10−7 | 0.095 |
| Ionizing radiation | 0.695 | 0.0001 | 0.0014 | 0.0099 | 3.963 × 10−5 | 0.0002 | 4.42 × 10−5 | 0.0233 | 2.85 × 10−6 | 0.066 |
| Ozone formation, and human health | 2.621 | 8.63 × 10−5 | 0.003 | 0.0077 | 4.35 × 10−5 | 0.0006 | 9.44 × 10−5 | 0.0315 | 2.34 × 10−6 | 2.58 |
| Particulate matter formation | 0.606 | 2.65 × 10−5 | 0.001 | 0.0032 | 3.136 × 10−5 | 0.0001 | 5.98 × 10−5 | 0.0071 | 9.97 × 10−7 | 0.59 |
| Land ecology | 3.041 | 0.0001 | 0.0045 | 0.0092 | 5.102 × 10−5 | 0.0007 | 0.0001 | 0.0410 | 2.75 × 10−6 | 2.99 |
| Ground acidification | 3.360 | 0.0003 | 0.0024 | 0.0113 | 2.858 × 10−5 | 0.0007 | 0.0002 | 0.0215 | 2.89 × 10−6 | 3.32 |
| Water eutrophication | 3.880 | 0.0003 | 0.0138 | 0.0405 | 0.0001 | 0.0025 | 0.0001 | 0.1028 | 1.18 × 10−5 | 3.72 |
| Ocean eutrophication | 0.0457 | 3.77 × 10−6 | 0.0001 | 0.0057 | 1.62 × 10−6 | 0.0002 | 1.54 × 10−6 | 0.0048 | 1.85 × 10−7 | 0.034 |
| Land ecological toxicity | 11.613 | 0.0030 | 0.0362 | 0.2224 | 0.0005 | 0.0121 | 0.0125 | 0.5346 | 7.63 × 10−5 | 10.79 |
| Freshwater ecological toxicity | 410.8 | 0.05864254 | 0.4954 | 4.5725 | 0.0097 | 0.2529 | 0.0288 | 6.0905 | 0.0016 | 399.33 |
| Marine ecological toxicity | 274.5 | 93452 | 0.4316 | 3.19 | 0.0074 | 0.1811 | 0.0283 | 5.2045 | 0.0011 | 265.48 |
| Human carcinogenic toxicity | 1.167 | 0.0001 | 0.0210 | 0.0353 | 3.19 × 10−5 | 0.0008 | 0.0005 | 0.0527 | 4.93 × 10−6 | 1.06 |
| Non-carcinogenic toxicity in humans | 4.046 | 0.0007 | 0.0122 | 0.0698 | 0.0001 | 0.0045 | 0.0007 | 0.1563 | 2.28 × 10−5 | 3.80 |
| Land use | 0.041 | 2.94 × 10−5 | 5.16 × 10−5 | 0.0018 | 0.0002 | 0.0105 | 8.79 | 0.0022 | 5.29 × 10−7 | 0.03 |
| Scarcity of mineral resources. | 4.97 × 10−5 | 2.48 × 10−7 | 1.61 × 10−7 | 2.55E-06 | 1.54 × 10−8 | 2.46 × 10−7 | 1.13 × 10−8 | 2.78 × 10−6 | 2.33 × 10−9 | 4.38 × 10−9 |
| Scarcity of fossil resources | 3.34 | 0.0001 | 0.0210 | 0.0229 | 7.232 × 10−5 | 0.0010 | 0.0005 | 0.1555 | 5.35 × 10−6 | 3.14 |
| Water consumption | 0.31 | 2.45 × 10−5 | 0.002 | 0.0056 | 1.00 × 10−5 | 0.00055 | 0.00012 | 0.015 | 0.00016 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Su, R. Environmental Impact of Waste Treatment and Synchronous Hydrogen Production: Based on Life Cycle Assessment Method. Toxics 2024, 12, 652. https://doi.org/10.3390/toxics12090652
Luo Y, Su R. Environmental Impact of Waste Treatment and Synchronous Hydrogen Production: Based on Life Cycle Assessment Method. Toxics. 2024; 12(9):652. https://doi.org/10.3390/toxics12090652
Chicago/Turabian StyleLuo, Yiting, and Rongkui Su. 2024. "Environmental Impact of Waste Treatment and Synchronous Hydrogen Production: Based on Life Cycle Assessment Method" Toxics 12, no. 9: 652. https://doi.org/10.3390/toxics12090652









