Plant Defense Mechanisms against Polycyclic Aromatic Hydrocarbon Contamination: Insights into the Role of Extracellular Vesicles
Abstract
:1. Introduction
2. Polycyclic Aromatic Hydrocarbons (PAHs)
3. Soil as a Critical Reservoir
4. Impact of PAHs on Animals, Humans and Environment
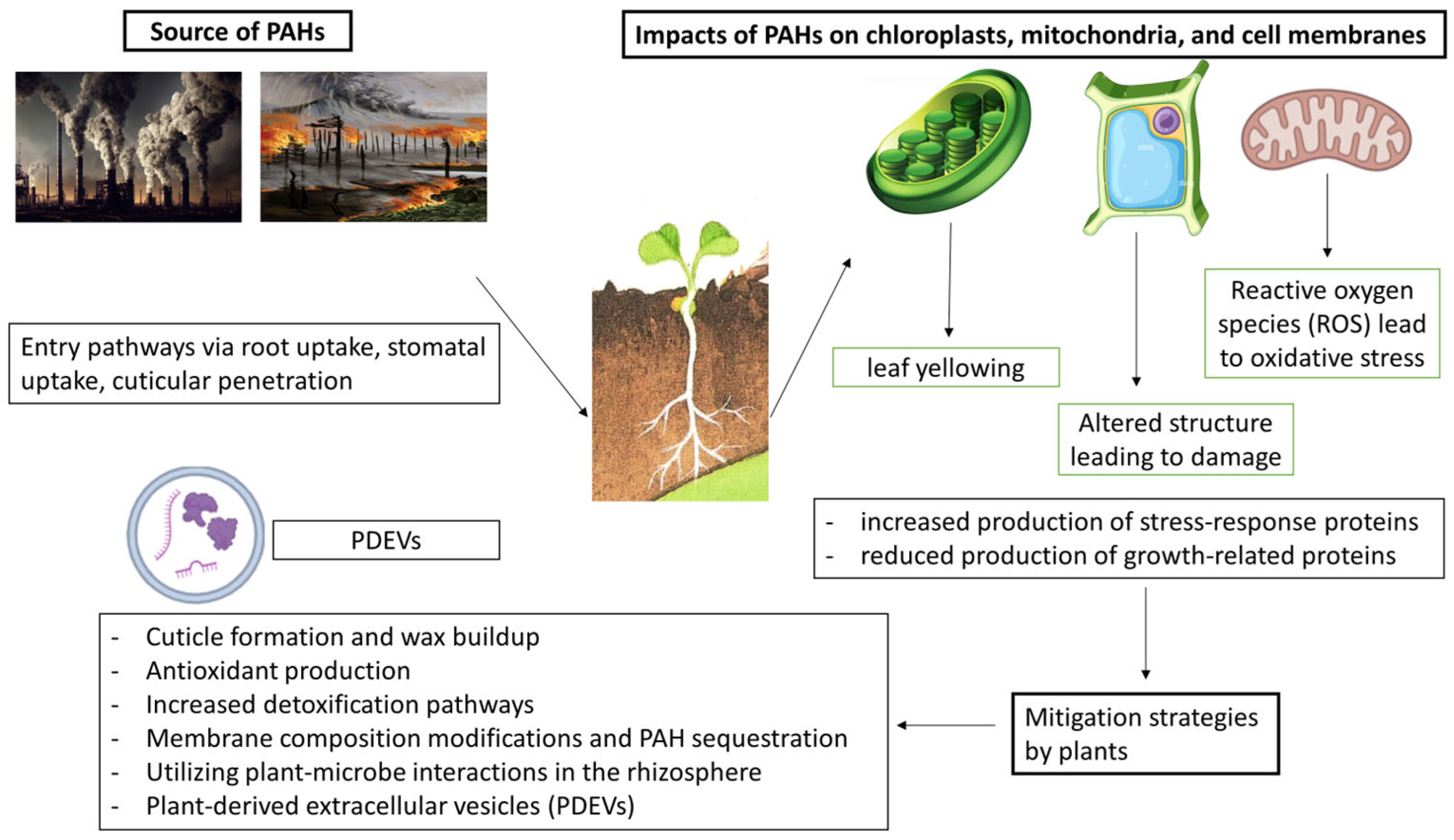
5. Pathways of PAH Entry into Plants
6. Impact of PAHs in Plants
7. Plant Derived Extracellular Vesicles (PDEVs)
8. Role of Plant EVs in PAH-Induced Stress Response
9. Mitigation Strategies and Practical Applications
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, G.; Giribabu, N.; Mohan, P.S.; Muttiah, B.; Govindarajan, V.K.; Alagiri, M.; Abdul Rahman, P.S.; Karsani, S.A. Environmental impact and human health effects of polycyclic aromatic hydrocarbons and remedial strategies: A detailed review. Chemosphere 2024, 351, 141227. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo[a]pyrene-Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef]
- Al-Nasir, F.; Hijazin, T.J.; Al-Alawi, M.M.; Jiries, A.; Mayyas, A.; Al-Dalain, S.A.; Al-Dmour, R.; Alahmad, A.; Al-Madanat, O.Y.; Batarseh, M.I. Accumulation, Source Identification, and Cancer Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Different Jordanian Vegetables. Toxics 2022, 10, 643. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Souza, R.H.Z.; Cardoso, M.D.G.; Machado, A.M.R.; Santiago, W.D.; Pedroso, M.P.; Brandão, R.M.; Oliveira, R.E.S.; Barbosa, R.B.; Alvarenga, G.F.; Caetano, A.R.S.; et al. Polycyclic aromatic hydrocarbons in cachaças packed in bottles of polyethylene terephthalate. J. Food Sci. 2022, 87, 1906–1915. [Google Scholar] [CrossRef]
- Samburova, V.; Zielinska, B.; Khlystov, A. Do 16 Polycyclic Aromatic Hydrocarbons Represent PAH Air Toxicity? Toxics 2017, 5, 17. [Google Scholar] [CrossRef]
- Starski, A.; Kukielska, A.; Postupolski, J. Occurrence of polycyclic aromatic hydrocarbons in human diet—Exposure and risk assessment to consumer health. Rocz. Panstw. Zakl. Hig. 2021, 72, 253–265. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Wang, Y.; Bai, P.; Hayakawa, K.; Zhang, L.; Tang, N. Characteristics and Influencing Factors of Polycyclic Aromatic Hydrocarbons Emitted from Open Burning and Stove Burning of Biomass: A Brief Review. Int. J. Environ. Res. Public Health 2022, 19, 3944. [Google Scholar] [CrossRef]
- Vistnes, H.; Sossalla, N.A.; Røsvik, A.; Gonzalez, S.V.; Zhang, J.; Meyn, T.; Asimakopoulos, A.G. The Determination of Polycyclic Aromatic Hydrocarbons (PAHs) with HPLC-DAD-FLD and GC-MS Techniques in the Dissolved and Particulate Phase of Road-Tunnel Wash Water: A Case Study for Cross-Array Comparisons and Applications. Toxics 2022, 10, 399. [Google Scholar] [CrossRef] [PubMed]
- Ailijiang, N.; Zhong, N.; Zhou, X.; Mamat, A.; Chang, J.; Cao, S.; Hua, Z.; Li, N. Levels, sources, and risk assessment of PAHs residues in soil and plants in urban parks of Northwest China. Sci. Rep. 2022, 12, 21448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, W.; Chuan, X.; Guo, X.; Shen, X.; Zhang, H.; Wu, F.; Hu, J.; Wu, Z.; Wang, X. Remediation of heavily PAHs-contaminated soil with high mineral content from a coking plant using surfactant-enhanced soil washing. Sci. Total Environ. 2024, 909, 168499. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jia, X.; Wu, Z.; Yu, P.; Zhang, L.; Wang, S.; Xia, T. Contamination and source-specific health risk assessment of polycyclic aromatic hydrocarbons in soil from a mega iron and steel site in China. Environ. Pollut. 2024, 340 Pt 2, 122851. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, A.G.; Chernikova, N.; Minkina, T.; Sushkova, S.; Dudnikova, T.; Antonenko, E.; Fedorenko, G.; Bauer, T.; Mandzhieva, S.; Barbashev, A. Effects of benzo[a]pyrene toxicity on morphology and ultrastructure of Hordeum sativum. Environ. Geochem. Health 2021, 43, 1551–1562. [Google Scholar] [CrossRef]
- Boente, C.; Baragaño, D.; Gallego, J.R. Benzo[a]pyrene sourcing and abundance in a coal region in transition reveals historical pollution, rendering soil screening levels impractical. Environ. Pollut. 2020, 266 Pt 1, 115341. [Google Scholar] [CrossRef]
- Bezberdaya, L.; Kosheleva, N.; Chernitsova, O.; Lychagin, M.; Kasimov, N. Pollution Level, Partition and Spatial Distribution of Benzo(a)pyrene in Urban Soils, Road Dust and Their PM10 Fraction of Health-Resorts (Alushta, Yalta) and Industrial (Sebastopol) Cities of Crimea. Water 2022, 14, 561. [Google Scholar] [CrossRef]
- Obrycki, J.F.; Basta, N.T.; Culman, S.W. Management Options for Contaminated Urban Soils to Reduce Public Exposure and Maintain Soil Health. J. Environ. Qual. 2017, 46, 420–430. [Google Scholar] [CrossRef]
- Twardowska, I.; Kolodziejczyk, A.M. Benzo[a]Pyrene in Soils and Ground Water: Occurrence, Sources, Distribution, Interrelation. Toxicol. Environ. Chem. 1998, 66, 127–144. [Google Scholar] [CrossRef]
- Olgun, B.; Doğan, G. Polycyclic aromatic hydrocarbon concentrations in soils of greenhouses located in Aksu Antalya, Turkey. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2020, 81, 283–292. [Google Scholar] [CrossRef]
- Li, R.; Cheng, M.; Cui, Y.; He, Q.; Guo, X.; Chen, L.; Wang, X. Distribution of the Soil PAHs and Health Risk Influenced by Coal Usage Processes in Taiyuan City, Northern China. Int. J. Environ. Res. Public Health 2020, 17, 6319. [Google Scholar] [CrossRef] [PubMed]
- Kasimov, N.S.; Kosheleva, N.E.; Nikiforova, E.M.; Vlasov, D.V. Benzo[a]pyrene in urban environments of eastern Moscow: Pollution levels and critical loads. Atmos. Chem. Phys. 2017, 17, 2217–2227. [Google Scholar] [CrossRef]
- Sanli, G.; Celik, S.; Joubi, V.; Tasdemir, Y. Concentrations, phase exchanges and source apportionment of polycyclic aromatic hydrocarbons (PAHs) In Bursa-Turkey. Environ. Res. 2023, 232, 116344. [Google Scholar] [CrossRef]
- Jiao, S.; Zhang, H.; Cai, Y.; Jin, C.; Shen, S. Polycyclic aromatic hydrocarbons (PAHs) evidence for frequent combustion events on land during the Permian–Triassic transition in Northwest China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2024, 642, 112152. [Google Scholar] [CrossRef]
- Ilić, P.; Nišić, T.; Farooqi, Z.U.R. Polycyclic aromatic hydrocarbons contamination of soil in an industrial zone and evaluation of pollution sources. Pol. J. Environ. Stud. 2021, 30, 155–162. [Google Scholar] [CrossRef]
- Brukstiene, D.; Ruzgyte, A. Contamination of soil by polycyclic aromatic hydrocarbons (PAHs) in some urban areas. Polycycl. Aromat. Compd. 2001, 2001, 1–9. [Google Scholar] [CrossRef]
- Cai, H.; Yao, S.; Huang, J.; Zheng, X.; Sun, J.; Tao, X.; Lu, G. Polycyclic Aromatic Hydrocarbons Pollution Characteristics in Agricultural Soils of the Pearl River Delta Region, China. Int. J. Environ. Res. Public Health 2022, 19, 16233. [Google Scholar] [CrossRef]
- Chai, C.; Cheng, Q.; Wu, J.; Zeng, L.; Chen, Q.; Zhu, X.; Ma, D.; Ge, W. Contamination, source identification, and risk assessment of polycyclic aromatic hydrocarbons in the soils of vegetable greenhouses in Shandong, China. Ecotoxicol. Environ. Saf. 2017, 142, 181–188. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Guo, D.; Dang, J. Characteristics and Risk Assessment of PAH Pollution in Soil of a Retired Coking Wastewater Treatment Plant in Taiyuan, Northern China. Toxics 2023, 11, 415. [Google Scholar] [CrossRef]
- Muntean, N.; Muntean, E.; Duda, M.M. Polycyclic aromatic hydrocarbons in soil. ProEnvironment 2015, 8, 285–289. [Google Scholar]
- Bandowe, B.A.M.; Shukurov, N.; Leimer, S.; Kersten, M.; Steinberger, Y.; Wilcke, W. Polycyclic aromatic hydrocarbons (PAHs) in soils of an industrial area in semi-arid Uzbekistan: Spatial distribution, relationship with trace metals and risk assessment. Environ. Geochem. Health 2021, 43, 4847–4861. [Google Scholar] [CrossRef] [PubMed]
- Rabieimesbah, A.; Sobhanardakani, S.; Cheraghi, M.; Lorestani, B. Concentrations, Source Identification and Potential Ecological and Human Health Risks Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Agricultural Soils of Hamedan County, West of Iran. Soil Sediment Contam. Int. J. 2023, 33, 482–506. [Google Scholar] [CrossRef]
- Wei, L.; Lv, J.; Zuo, P.; Li, Y.; Yang, R.; Zhang, Q.; Jiang, G. The occurrence and sources of PAHs, oxygenated PAHs (OPAHs), and nitrated PAHs (NPAHs) in soil and vegetation from the Antarctic, Arctic, and Tibetan Plateau. Sci. Total Environ. 2024, 912, 169394. [Google Scholar] [CrossRef] [PubMed]
- Alawi, M.A.; Azeez, A.L. Study of polycyclic aromatic hydrocarbons (PAHs) in soil samples from Al-Ahdab oil field in Waset Region, Iraq. Toxin Rev. 2016, 35, 69–76. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Kim, T.T.; Quynh, N.T.; Vi, P.T.; Viet, P.H.; Anh, D.H. Polycyclic Aromatic Hydrocarbons (PAHs) in paddy soil around Nam Son landfill area, Ha Noi, Viet Nam. Vietnam J. Sci. Technol. 2023, 61, 875–888. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, J.; Zhai, L.; Yu, X.; Xiao, Y. Recent Evidence on Polycyclic Aromatic Hydrocarbon Exposure. Healthcare 2023, 11, 1958. [Google Scholar] [CrossRef]
- Dai, C.; Han, Y.; Duan, Y.; Lai, X.; Fu, R.; Liu, S.; Leong, K.H.; Tu, Y.; Zhou, L. Review on the contamination and remediation of polycyclic aromatic hydrocarbons (PAHs) in coastal soil and sediments. Environ. Res. 2022, 205, 112423. [Google Scholar] [CrossRef]
- Gregoris, E.; Barbaro, E.; Morabito, E.; Toscano, G.; Donateo, A.; Cesari, D.; Contini, D.; Gambaro, A. Impact of maritime traffic on polycyclic aromatic hydrocarbons, metals and particulate matter in Venice air. Environ. Sci. Pollut. Res. Int. 2016, 23, 6951–6959. [Google Scholar] [CrossRef]
- Lin, X.; Lin, L.; Liao, Z.; Wu, P.; Yang, C. Occurrence and distribution of polycyclic aromatic hydrocarbons (PAHs) in marine organisms from Shenzhen coastal waters and human health risk assessment. Mar. Pollut. Bull. 2023, 195, 115498. [Google Scholar] [CrossRef]
- Chen, F.; Lin, Y.; Cai, M.; Zhang, J.; Zhang, Y.; Kuang, W.; Liu, L.; Huang, P.; Ke, H. Occurrence and Risk Assessment of PAHs in Surface Sediments from Western Arctic and Subarctic Oceans. Int. J. Environ. Res. Public Health 2018, 15, 734. [Google Scholar] [CrossRef]
- Shen, Q.; Fu, W.; Chen, B.; Zhang, X.; Xing, S.; Ji, C.; Zhang, X. Community response of soil microorganisms to combined contamination of polycyclic aromatic hydrocarbons and potentially toxic elements in a typical coking plant. Front. Microbiol. 2023, 14, 1143742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.; Wang, Y.; Duan, L.; Liu, X.; Zhang, X.; Dong, L. Deep relationships between bacterial community and polycyclic aromatic hydrocarbons in soil profiles near typical coking plants. Environ. Sci. Pollut. Res. Int. 2023, 30, 64486–64498. [Google Scholar] [CrossRef]
- Geng, S.; Xu, G.; Cao, W.; You, Y.; Zhu, Y.; Ding, A.; Fan, F.; Dou, J. Occurrence of polycyclic aromatic compounds and potentially toxic elements contamination and corresponding interdomain microbial community assembly in soil of an abandoned gas station. Environ. Res. 2022, 212 Pt E, 113618. [Google Scholar] [CrossRef]
- Ai, F.; Eisenhauer, N.; Xie, Y.; Zhu, J.; Jousset, A.; Du, W.; Yin, Y.; Zhang, X.; Ji, R.; Guo, H. Elevated CO2 accelerates polycyclic aromatic hydrocarbon accumulation in a paddy soil grown with rice. PLoS ONE 2018, 13, e0196439. [Google Scholar] [CrossRef] [PubMed]
- Janneh, M.; Qu, C.; Zhang, Y.; Xing, X.; Nkwazema, O.; Nyihirani, F.; Qi, S. Distribution, sources, and ecological risk assessment of polycyclic aromatic hydrocarbons in agricultural and dumpsite soils in Sierra Leone. RSC Adv. 2023, 13, 7102–7116. [Google Scholar] [CrossRef] [PubMed]
- Tarigholizadeh, S.; Sushkova, S.; Rajput, V.D.; Ranjan, A.; Arora, J.; Dudnikova, T.; Barbashev, A.; Mandzhieva, S.; Minkina, T.; Wong, M.H. Transfer and Degradation of PAHs in the Soil-Plant System: A Review. J. Agric. Food Chem. 2024, 72, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Ukalska-Jaruga, A.; Smreczak, B. The Impact of Organic Matter on Polycyclic Aromatic Hydrocarbon (PAH) Availability and Persistence in Soils. Molecules 2020, 25, 2470. [Google Scholar] [CrossRef]
- Petruzzelli, L.; Celi, L.; Cignetti, A.; Marsan, F.A. Influence of soil organic matter on the leaching of polycyclic aromatic hydrocarbons in soil. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2002, 37, 187–199. [Google Scholar] [CrossRef]
- Kuśmierz, M.; Oleszczuk, P.; Kraska, P.; Pałys, E.; Andruszczak, S. Persistence of polycyclic aromatic hydrocarbons (PAHs) in biochar-amended soil. Chemosphere 2016, 146, 272–279. [Google Scholar] [CrossRef]
- Wang, D.; Ma, J.; Li, H.; Zhang, X. Concentration and Potential Ecological Risk of PAHs in Different Layers of Soil in the Petroleum-Contaminated Areas of the Loess Plateau, China. Int. J. Environ. Res. Public Health 2018, 15, 1785. [Google Scholar] [CrossRef]
- Wang, J.; Odinga, E.S.; Zhang, W.; Zhou, X.; Yang, B.; Waigi, M.G.; Gao, Y. Polyaromatic hydrocarbons in biochars and human health risks of food crops grown in biochar-amended soils: A synthesis study. Environ. Int. 2019, 130, 104899. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.S.; Ambade, B.; Mohammad, F.; Al-Lohedan, H.A.; Soleiman, A.A. Accumulation and Toxicity of Polycyclic Aromatic Hydrocarbons in Long-Term Soil Irrigated with Treated Wastewater. Sustainability 2023, 15, 13581. [Google Scholar] [CrossRef]
- Sui, Q.; Yang, X.; Sun, X.; Zhu, L.; Zhao, X.; Feng, Z.; Xia, B.; Qu, K. Bioaccumulation of polycyclic aromatic hydrocarbons and their human health risks depend on the characteristics of microplastics in marine organisms of Sanggou Bay, China. J. Hazard. Mater. 2024, 473, 134622. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, X.; Lu, S.; Zhang, T.; Jin, B.; Wang, Q.; Tang, Z.; Liu, Y.; Guo, X.; Zhou, J.; et al. A review on occurrence and risk of polycyclic aromatic hydrocarbons (PAHs) in lakes of China. Sci. Total Environ. 2019, 651 Pt 2, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, R.A.; Taniguchi, S.; da Silva, J.; Gallotta, F.D.C.; Bícego, M.C. Polycyclic aromatic hydrocarbons in marine mammals: A review and synthesis. Mar. Pollut. Bull. 2021, 171, 112699. [Google Scholar] [CrossRef]
- Power, A.; White, P.; McHugh, B.; Berrow, S.; McKeown, A.; Crowley, D.; Newton, S.; McGovern, E.; Murphy, S.; O’Connor, I. Polycyclic aromatic hydrocarbons (PAHs) in seabird eggs in Ireland. Mar. Pollut. Bull. 2021, 170, 112636. [Google Scholar] [CrossRef]
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A review of human and animals exposure to polycyclic aromatic hydrocarbons: Health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 2021, 773, 145403. [Google Scholar] [CrossRef]
- Xie, W.; Wang, G.; Yu, E.; Xie, J.; Gong, W.; Li, Z.; Zhang, K.; Xia, Y.; Tian, J.; Li, H. Residue character of polycyclic aromatic hydrocarbons in river aquatic organisms coupled with geographic distribution, feeding behavior, and human edible risk. Sci. Total Environ. 2023, 895, 164814. [Google Scholar] [CrossRef]
- Gregoris, E.; Argiriadis, E.; Vecchiato, M.; Zambon, S.; De Pieri, S.; Donateo, A.; Contini, D.; Piazza, R.; Barbante, C.; Gambaro, A. Gas-particle distributions, sources and health effects of polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs) and polychlorinated naphthalenes (PCNs) in Venice aerosols. Sci. Total Environ. 2014, 476–477, 393–405. [Google Scholar] [CrossRef]
- Yost, E.E.; Galizia, A.; Kapraun, D.F.; Persad, A.S.; Vulimiri, S.V.; Angrish, M.; Lee, J.S.; Druwe, I.L. Health Effects of Naphthalene Exposure: A Systematic Evidence Map and Analysis of Potential Considerations for Dose-Response Evaluation. Environ. Health Perspect. 2021, 129, 76002. [Google Scholar] [CrossRef] [PubMed]
- Ewa, B.; Danuta, M.Š. Polycyclic aromatic hydrocarbons and PAH-related DNA adducts. J. Appl. Genet. 2017, 58, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.N.; Gaither, K.A.; Pande, P. Competitive Metabolism of Polycyclic Aromatic Hydrocarbons (PAHs): An Assessment Using In Vitro Metabolism and Physiologically Based Pharmacokinetic (PBPK) Modeling. Int. J. Environ. Res. Public Health 2022, 19, 8266. [Google Scholar] [CrossRef] [PubMed]
- Stoddard, E.G.; Nag, S.; Martin, J.; Tyrrell, K.J.; Gibbins, T.; Anderson, K.A.; Shukla, A.K.; Corley, R.; Wright, A.T.; Smith, J.N. Exposure to an Environmental Mixture of Polycyclic Aromatic Hydrocarbons Induces Hepatic Cytochrome P450 Enzymes in Mice. Chem. Res. Toxicol. 2021, 34, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Šulc, M.; Indra, R.; Moserová, M.; Schmeiser, H.H.; Frei, E.; Arlt, V.M.; Stiborová, M. The impact of individual cytochrome P450 enzymes on oxidative metabolism of benzo[a]pyrene in human livers. Environ. Mol. Mutagen. 2016, 57, 229–235. [Google Scholar] [CrossRef]
- Indra, R.; Moserova, M.; Sulc, M.; Frei, E.; Stiborova, M. Oxidation of carcinogenic benzo[a]pyrene by human and rat cytochrome P450 1A1 and its influencing by cytochrome b5—A comparative study. Neuro Endocrinol. Lett. 2013, 34 (Suppl. S2), 55–63. [Google Scholar]
- Pietrogrande, M.C.; Bacco, D.; Demaria, G.; Russo, M.; Scotto, F.; Trentini, A. Polycyclic aromatic hydrocarbons and their oxygenated derivatives in urban aerosol: Levels, chemical profiles, and contribution to PM2.5 oxidative potential. Environ. Sci. Pollut. Res. 2022, 29, 54391–54406. [Google Scholar] [CrossRef]
- Zuo, J.; Brewer, D.S.; Arlt, V.M.; Cooper, C.S.; Phillips, D.H. Benzo pyrene-induced DNA adducts and gene expression profiles in target and non-target organs for carcinogenesis in mice. BMC Genom. 2014, 15, 880. [Google Scholar] [CrossRef]
- Rafiee, A.; Hoseini, M.; Akbari, S.; Mahabee-Gittens, E.M. Exposure to Polycyclic Aromatic Hydrocarbons and adverse reproductive outcomes in women: Current status and future perspectives. Rev. Environ. Health 2023, 39, 305–311. [Google Scholar] [CrossRef]
- Jo, Y.S.; Ko, H.S.; Kim, A.Y.; Jo, H.G.; Kim, W.J.; Choi, S.K. Effects of polycyclic aromatic hydrocarbons on the proliferation and differentiation of placental cells. Reprod. Biol. Endocrinol. 2022, 20, 47. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Jin, H.; Lu, Q. Effect of polycyclic aromatic hydrocarbons on immunity. J. Transl. Autoimmun. 2022, 5, 100177. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, J.; Valdés Hernández, M.D.C. Impact of polycyclic aromatic hydrocarbon exposure on cognitive function and neurodegeneration in humans: A systematic review and meta-analysis. Front. Neurol. 2023, 13, 1052333. [Google Scholar] [CrossRef]
- Moubarz, G.; Saad-Hussein, A.; Shahy, E.M.; Mahdy-Abdallah, H.; Mohammed, A.M.F.; Saleh, I.A.; Abo-Zeid, M.A.M.; Abo-Elfadl, M.T. Lung cancer risk in workers occupationally exposed to polycyclic aromatic hydrocarbons with emphasis on the role of DNA repair gene. Int. Arch. Occup. Environ. Health 2023, 96, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Olayinka, O.O.; Adewusi, A.A.; Olujimi, O.O.; Aladesida, A.A. Polycyclic Aromatic Hydrocarbons in Sediment and Health Risk of Fish, Crab and Shrimp Around Atlas Cove, Nigeria. J. Health Pollut. 2019, 9, 191204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Zhang, Y. Analytical chemistry, formation, mitigation, and risk assessment of polycyclic aromatic hydrocarbons: From food processing to in vivo metabolic transformation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1422–1456. [Google Scholar] [CrossRef] [PubMed]
- Ashok, A.; Høj, L.; Brinkman, D.L.; Negri, A.P.; Agusti, S. Food-chain length determines the level of phenanthrene bioaccumulation in corals. Environ. Pollut. 2022, 297, 118789. [Google Scholar] [CrossRef]
- Pullagurala, V.L.R.; Rawat, S.; Adisa, I.O.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Plant uptake and translocation of contaminants of emerging concern in soil. Sci. Total Environ. 2018, 636, 1585–1596. [Google Scholar] [CrossRef]
- Paris, A.; Ledauphin, J.; Poinot, P.; Gaillard, J.L. Polycyclic aromatic hydrocarbons in fruits and vegetables: Origin, analysis, and occurrence. Environ. Pollut. 2018, 234, 96–106. [Google Scholar] [CrossRef]
- Jia, J.; Bi, C.; Jin, X.; Zeng, Y.; Deng, L.; Wang, X.; Chen, Z. Uptake, translocation, and risk assessment of PAHs in con-taminated soil-air-vegetable systems based on a field simulation experiment. Environ. Pollut. 2021, 271, 116361. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, L. Prediction of organic contaminant uptake by plants: Modified partition-limited model based on a sequential ultrasonic extraction procedure. Environ. Pollut. 2019, 246, 124–130. [Google Scholar] [CrossRef]
- Fatma, M.; Asgher, M.; Iqbal, N.; Rasheed, F.; Sehar, Z.; Sofo, A.; Khan, N.A. Ethylene Signaling under Stressful Environments: Analyzing Collaborative Knowledge. Plants 2022, 11, 2211. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xu, X.; Guo, L.; Jin, R.; Lu, Y. Uptake and transport of micro/nanoplastics in terrestrial plants: Detection, mechanisms, and influencing factors. Sci. Total Environ. 2024, 907, 168155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zeng, N.; Feng, Q.; Xu, S.; Cheng, J.; Wang, J.; Zhan, X. New mechanistic insights into PAHs transport across wheat root cell membrane: Evidence for ABC transporter mediation. Sci. Total Environ. 2023, 859 Pt 1, 160251. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, H.; Lu, Y.; Yu, X.; Wang, J.; Sun, S.; Liu, M.; Li, D.; Li, Y.F.; Zhang, D. Uptake and translocation of polycyclic aromatic hydrocarbons (PAHs) and heavy metals by maize from soil irrigated with wastewater. Sci. Rep. 2017, 7, 12165. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz-Walec, E.; Krzebietke, S.J.; Sienkiewicz, S. The Influence of Crops on the Content of Polycyclic Aromatic Hydrocarbons in Soil Fertilized with Manure and Mineral Fertilizers. Int. J. Environ. Res. Public Health 2022, 19, 13627. [Google Scholar] [CrossRef]
- Molina, L.; Segura, A. Biochemical and Metabolic Plant Responses toward Polycyclic Aromatic Hydrocarbons and Heavy Metals Present in Atmospheric Pollution. Plants 2021, 10, 2305. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2019, 42, 33–43. [Google Scholar] [CrossRef]
- Zandi, P.; Schnug, E. Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective. Biology 2022, 11, 155. [Google Scholar] [CrossRef]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef]
- Krzyszczak, A.; Dybowski, M.; Jośko, I.; Kusiak, M.; Sikora, M.; Czech, B. The antioxidant defense responses of Hordeum vulgare L. to polycyclic aromatic hydrocarbons and their derivatives in biochar-amended soil. Environ. Pollut. 2022, 294, 118664. [Google Scholar] [CrossRef]
- Paková, V.; Hilscherová, K.; Feldmannová, M.; Bláha, L. Toxic effects and oxidative stress in higher plants exposed to polycyclic aromatic hydrocarbons and their N-heterocyclic derivatives. Environ. Toxicol. Chem. 2006, 25, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- And Jajoo, A.; Mekala, N.R.; Tomar, R.S.; Grieco, M.; Tikkanen, M.; Aro, E.M. Inhibitory effects of polycyclic aromatic hydrocarbons (PAHs) on photosynthetic performance are not related to their aromaticity. J. Photochem. Photobiol. B Biol. 2014, 137, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, J.; Wang, W.; Zhu, L. Mechanism of growth inhibition mediated by disorder of chlorophyll metabolism in rice (Oryza sativa) under the stress of three polycyclic aromatic hydrocarbons. Chemosphere 2023, 329, 138554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Rengel, Z.; Meney, K.; Pantelic, L.; Tomanovic, R. Polynuclear aromatic hydrocarbons (PAHs) mediate cadmium toxicity to an emergent wetland species. J. Hazard. Mater. 2011, 189, 119–126. [Google Scholar] [CrossRef]
- Lin, F.; Sun, J.; Liu, N.; Zhu, L. Phytotoxicity and metabolic responses induced by tetrachlorobiphenyl and its hydroxylated and methoxylated derivatives in rice (Oryza sative L.). Environ. Int. 2020, 139, 105695. [Google Scholar] [CrossRef]
- Weisman, D.; Alkio, M.; Colón-Carmona, A. Transcriptional responses to polycyclic aromatic hydrocarbon-induced stress in Arabidopsis thaliana reveal the involvement of hormone and defense signaling pathways. BMC Plant Biol. 2010, 10, 59. [Google Scholar] [CrossRef]
- Liu, H.; Weisman, D.; Tang, L.; Tan, L.; Zhang, W.K.; Wang, Z.H.; Huang, Y.H.; Lin, W.X.; Liu, X.M.; Colón-Carmona, A. Stress signaling in response to polycyclic aromatic hydrocarbon exposure in Arabidopsis thaliana involves a nucleoside diphosphate kinase, NDPK-3. Planta 2015, 241, 95–107. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Bao, H.; Li, J.; Li, J.; Xing, W.; Hong, H.; Wu, F. Dynamic distribution and accumulation of PAHs in winter wheat during whole plant growth: Field investigation. Ecotoxicol. Environ. Saf. 2020, 202, 110886. [Google Scholar] [CrossRef]
- Zhang, A.; Ye, X.; Yang, X.; Li, J.; Zhu, H.; Xu, H.; Meng, J.; Xu, T.; Sun, J. Elevated urbanization-driven plant accumulation and human intake risks of polycyclic aromatic hydrocarbons in crops of peri-urban farmlands. Environ. Sci. Pollut. Res. Int. 2022, 29, 68143–68151. [Google Scholar] [CrossRef]
- Lian, M.Q.; Chng, W.H.; Liang, J.; Yeo, H.Q.; Lee, C.K.; Belaid, M.; Tollemeto, M.; Wacker, M.G.; Czarny, B.; Pastorin, G. Plant-derived extracellular vesicles: Recent advancements and current challenges on their use for biomedical applications. J. Extracell. Vesicles 2022, 11, e12283. [Google Scholar] [CrossRef]
- Sall, I.M.; Flaviu, T.A. Plant and mammalian-derived extracellular vesicles: A new therapeutic approach for the future. Front. Bioeng. Biotechnol. 2023, 11, 1215650. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Y.; Li, C.Q.; Zhang, Y.L.; Ma, M.W.; Cheng, W.; Zhang, G.J. Emerging Drug Delivery Vectors: Engineering of Plant-Derived Nanovesicles and Their Applications in Biomedicine. Int. J. Nanomed. 2024, 19, 2591–2610. [Google Scholar] [CrossRef]
- An, Q.; van Bel, A.J.; Hückelhoven, R. Do plant cells secrete exosomes derived from multivesicular bodies? Plant Signal. Behav. 2007, 2, 4–7. [Google Scholar] [CrossRef]
- Regente, M.; Corti-Monzón, G.; Maldonado, A.M.; Pinedo, M.; Jorrín, J.; de la Canal, L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009, 583, 3363–3366. [Google Scholar] [CrossRef]
- Bai, C.; Liu, J.; Zhang, X.; Li, Y.; Qin, Q.; Song, H.; Yuan, C.; Huang, Z. Research status and challenges of plant-derived exosome-like nanoparticles. Biomed. Pharmacother. 2024, 174, 116543. [Google Scholar] [CrossRef] [PubMed]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Liu, X.; Yan, F.; Zhou, Z. Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal. CCS 2022, 20, 69. [Google Scholar] [CrossRef]
- Johnson, S.M.; Banyard, A.; Smith, C.; Mironov, A.; McCabe, M.G. Large Extracellular Vesicles Can be Characterised by Multiplex Labelling Using Imaging Flow Cytometry. Int. J. Mol. Sci. 2020, 21, 8723. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Khan, M.I.; Kameli, N.; Alsahafi, E.; Riza, Y.M. Plant-Derived Extracellular Vesicles and Their Exciting Potential as the Future of Next-Generation Drug Delivery. Biomolecules 2023, 13, 839. [Google Scholar] [CrossRef]
- Liu, N.; Hou, L.; Chen, X.; Bao, J.; Chen, F.; Cai, W.; Zhu, H.; Wang, L.; Chen, X. Arabidopsis TETRASPANIN8 mediates exosome secretion and glycosyl inositol phosphoceramide sorting and trafficking. Plant Cell 2024, 36, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Latella, R.; Calzoni, E.; Urbanelli, L.; Cerrotti, G.; Porcellati, S.; Emiliani, C.; Buratta, S.; Tancini, B. Isolation of Extracellular Vesicles from Agri-Food Wastes: A Novel Perspective in the Valorization of Agri-Food Wastes and By-Products. Foods 2024, 13, 1492. [Google Scholar] [CrossRef] [PubMed]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant Extracellular Vesicles Contain Diverse Small RNA Species and Are Enriched in 10- to 17-Nucleotide “Tiny” RNAs. Plant Cell 2019, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jang, H.; Kim, W.; Kim, D.; Park, J.H. Therapeutic Applications of Plant-Derived Extracellular Vesicles as Antioxidants for Oxidative Stress-Related Diseases. Antioxidants 2023, 12, 1286. [Google Scholar] [CrossRef]
- Lo, K.J.; Wang, M.H.; Ho, C.T.; Pan, M.H. Plant-Derived Extracellular Vesicles: A New Revolutionization of Modern Healthy Diets and Biomedical Applications. J. Agric. Food Chem. 2024, 72, 2853–2878. [Google Scholar] [CrossRef]
- Ambrosone, A.; Barbulova, A.; Cappetta, E.; Cillo, F.; De Palma, M.; Ruocco, M.; Pocsfalvi, G. Plant Extracellular Vesicles: Current Landscape and Future Directions. Plants 2023, 12, 4141. [Google Scholar] [CrossRef]
- Shen, Y.; Li, J.; Gu, R.; Yue, L.; Wang, H.; Zhan, X.; Xing, B. Carotenoid and superoxide dismutase are the most effective antioxidants participating in ROS scavenging in phenanthrene accumulated wheat leaf. Chemosphere 2018, 197, 513–525. [Google Scholar] [CrossRef]
- Hao, S.; Yang, H.; Hu, J.; Luo, L.; Yuan, Y.; Liu, L. Bioactive compounds and biological functions of medicinal plant-derived extracellular vesicles. Pharmacol. Res. 2024, 200, 107062. [Google Scholar] [CrossRef]
- Kumar, M.; Bolan, N.S.; Hoang, S.A.; Sawarkar, A.D.; Jasemizad, T.; Gao, B.; Keerthanan, S.; Padhye, L.P.; Singh, L.; Kumar, S.; et al. Remediation of soils and sediments polluted with polycyclic aromatic hydrocarbons: To immobilize, mobilize, or degrade? J. Hazard. Mater. 2021, 420, 126534. [Google Scholar] [CrossRef]
- Liu, S.; Liu, N.; Lu, H.; Zhu, L. Disturbed phospholipid metabolism by three polycyclic aromatic hydrocarbons in Oryza sativa. Environ. Pollut. 2021, 283, 117073. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Alkio, M.; Tabuchi, T.M.; Wang, X.; Colón-Carmona, A. Stress responses to polycyclic aromatic hydrocarbons in Arabidopsis include growth inhibition and hypersensitive response-like symptoms. J. Exp. Bot. 2005, 56, 2983–2994. [Google Scholar] [CrossRef]
- Zhao, Z.; He, W.; Wu, R.; Xu, F. Distribution and Relationships of Polycyclic Aromatic Hydrocarbons (PAHs) in Soils and Plants near Major Lakes in Eastern China. Toxics 2022, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, C. Exploring and exploiting cuticle biosynthesis for abiotic and biotic stress tolerance in wheat and barley. Front. Plant Sci. 2022, 13, 1064390. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Ding, N.Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef]
- Zhou, P.; Graether, S.P.; Hu, L.; Zhang, W. Editorial: The role of stress proteins in plants under abiotic stress. Front. Plant Sci. 2023, 14, 1193542. [Google Scholar] [CrossRef]
- Ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.X.; Zhang, H.X.; Wei, A.M.; Gong, Z.H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ Response to Abiotic Stress: Mechanisms and Strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Alagić, S.Č.; Maluckov, B.S.; Radojičić, V.B. How can plants manage polycyclic aromatic hydrocarbons? May these effects represent a useful tool for an effective soil remediation? A review. Clean Technol. Environ. Policy 2015, 17, 597–614. [Google Scholar] [CrossRef]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 760689. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.R.; Armour, B.; Thirsk, J. Comment on “Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application”. J. Nutr. Metab. 2015, 2015, 934070. [Google Scholar] [CrossRef] [PubMed]
- Hussain, O.A.; Abdel Rahim, E.A.; Badr, A.N.; Hathout, A.S.; Rashed, M.M.; Fouzy, A.S.M. Total phenolics, flavonoids, and antioxidant activity of agricultural wastes, and their ability to remove some pesticide residues. Toxicol. Rep. 2022, 9, 628–635. [Google Scholar] [CrossRef]
- Correa-García, S.; Pande, P.; Séguin, A.; St-Arnaud, M.; Yergeau, E. Rhizoremediation of petroleum hydrocarbons: A model system for plant microbiome manipulation. Microb. Biotechnol. 2018, 11, 819–832. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Košnář, Z.; Mercl, F.; Aranda, E.; Tlustoš, P. A comparative study to evaluate natural attenuation, mycoaugmentation, phytoremediation, and microbial-assisted phytoremediation strategies for the bioremediation of an aged PAH-polluted soil. Ecotoxicol. Environ. Saf. 2018, 147, 165–174. [Google Scholar] [CrossRef]
- Kadri, T.; Rouissi, T.; Kaur Brar, S.; Cledon, M.; Sarma, S.; Verma, M. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: A review. J. Environ. Sci. 2017, 51, 52–74. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Liang, H.; Li, M.; Liu, Y.; Wang, L.; Lai, W.; Tang, T.; Diao, Y.; Bai, Y.; et al. Distinct laccase expression and activity profiles of Trametes versicolor facilitate degradation of benzo[a]pyrene. Front. Bioeng. Biotechnol. 2023, 11, 1264135. [Google Scholar] [CrossRef]
- Kakoulidou, I.; Avramidou, E.V.; Baránek, M.; Brunel-Muguet, S.; Farrona, S.; Johannes, F.; Kaiserli, E.; Lieberman-Lazarovich, M.; Martinelli, F.; Mladenov, V.; et al. Epigenetics for Crop Improvement in Times of Global Change. Biology 2021, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Kang, G.; Wang, S.; Huang, Y.; Cai, Q. Extracellular Vesicles: Emerging Players in Plant Defense Against Pathogens. Front. Plant Sci. 2021, 12, 757925. [Google Scholar] [CrossRef] [PubMed]
- Subha, D.; AnuKiruthika, R.; Sreeraj, H.; Tamilselvi, K.S. Plant exosomes: Nano conveyors of pathogen resistance. Discov. Nano 2023, 18, 146. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yang, Z.; Liu, L.; Duan, L. DNA Methylation in Plant Responses and Adaption to Abiotic Stresses. Int. J. Mol. Sci. 2022, 23, 6910. [Google Scholar] [CrossRef] [PubMed]

| Study Location | Top 3 PAHs | Average Concentration | Range |
|---|---|---|---|
| Nam Son Landfill Area | Naphthalene (Nap) | 10.33 ng/g | nd–26.16 ng/g |
| Phenanthrene (Phe) | 8.43 ng/g | 3.16–14.53 ng/g | |
| Benzo[b]fluoranthene (BbF) | 6.63 ng/g | 0.60–30.94 ng/g | |
| Shandong Province, China | Phenanthrene (Phe) | 16.3% of total PAH concentration | - |
| Acenaphthene (Ace) | 13.1% of total PAH concentration | - | |
| Fluorene (Fl) | 10.5% of total PAH concentration | - | |
| Cluj-Napoca, Romania | Naphthalene (Nap) | 1.23 μg/kg | - |
| Benzo[a]anthracene (BaA) | 1.38 μg/kg | - | |
| Benzo[b]fluoranthene (BbF) | 1.71 μg/kg | - | |
| Taiyuan, Northern China | Naphthalene (Nap) | 28.39% of total PAH concentration | - |
| Benzo[g,h,i]perylene (BgP) | 0.65% of total PAH concentration | - | |
| Benzo[b]fluoranthene (BbF) | 28.19% of total carcinogenic PAHs | - | |
| Zhiwu Park, China | Dibenz[a,h]anthracene (DahN) | 1.469 mg/kg | - |
| Bursa, Turkey | Benzo[a]pyrene (BaP) | - | 13–189.4 ng/g dry matter |
| Antalya Aksu Region, Turkey | Benzo[a]pyrene (BaP) | 2.31 μg/kg | - |
| Uzbekistan (Industrial Area) | Phenanthrene (Phe) | 21.53 ng/g | 4.25–41.03% of total PAHs |
| Chrysene (Chr) | - | 3.4–24.1% of total PAHs | |
| Hamedan County, Iran | PAHs with four or more rings | 78% of total PAH mass | - |
| Antarctic, Arctic, Tibetan Plateau | Phenanthrene (Phe) | Most abundant PAH | - |
| Uptake Pathway | Description | Key Points | References |
|---|---|---|---|
| Root Uptake | Diffusion of PAH molecules through the soil solution into root cells. | PAHs may undergo transformations or be transported within the plant. | [77,78,79,80] |
| Stomatal Uptake | Diffusion of gaseous PAH molecules through stomatal openings on leaves into internal leaf tissues. | Major source of PAHs in vegetable leaves in polluted environments. | [81] |
| Cuticular Penetration | Diffusion of PAHs through the waxy cuticle covering plant aerial parts, accumulating in leaf tissues. | Significant for aerial parts of plants in contact with PAH-contaminated environments. | [81] |
| Hydroponic Uptake | Absorption of PAHs dissolved in water directly from hydroponic solutions in controlled environments. | Used in studies to evaluate PAH uptake in controlled conditions. | [82] |
| Effect of PAHs on Plants | Mechanism | Consequence | References |
|---|---|---|---|
| Oxidative Stress | ROS-induced lipid peroxidation, protein oxidation, and DNA damage | Impaired cell functionality, compromised growth | [89,90,91] |
| Antioxidant Response | Elevated SOD, CAT, and APX activity | Enhanced defense against oxidative stress | [92,93] |
| Genotoxicity | Interaction with plant DNA | Mutations, chromosomal aberrations | [94,99] |
| Photosynthesis Disruption | Inhibition of chlorophyll synthesis, Rubisco down-regulation | Reduced photosynthetic efficiency and biomass yield | [95,96,97] |
| Hormonal Dysregulation | Interference with hormonal signaling pathways | Abnormal development, disrupted metabolism | [98] |
| Aspect | Potential Effects |
|---|---|
| Cellular Stress Responses | PAH-induced oxidative stress, membrane damage, and signaling disruptions could affect the production and release mechanisms of plant EVs. |
| Cargo Composition | PAH-induced stress responses may lead to changes in the composition of EVs, altering the packaging of proteins, lipids, and nucleic acids. |
| Intercellular Communication | EVs could serve as carriers of stress-related molecules, such as antioxidants, signaling proteins, or small RNAs, facilitating intercellular communication and stress response mediation. |
| Detoxification Mechanisms | EVs may participate in the detoxification of PAHs by transporting and secreting these compounds or their metabolites as a defense mechanism to remove toxic substances. |
| Membrane Properties | PAHs can disrupt plant cell membranes, potentially affecting the lipid composition and properties of EV membranes, influencing their stability and interactions with recipient cells. |
| Role in Signaling Pathways | PAH-induced stress responses might disrupt the release and uptake mechanisms of EVs involved in stress-related signaling pathways, impacting plant stress resilience. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barathan, M.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Plant Defense Mechanisms against Polycyclic Aromatic Hydrocarbon Contamination: Insights into the Role of Extracellular Vesicles. Toxics 2024, 12, 653. https://doi.org/10.3390/toxics12090653
Barathan M, Ng SL, Lokanathan Y, Ng MH, Law JX. Plant Defense Mechanisms against Polycyclic Aromatic Hydrocarbon Contamination: Insights into the Role of Extracellular Vesicles. Toxics. 2024; 12(9):653. https://doi.org/10.3390/toxics12090653
Chicago/Turabian StyleBarathan, Muttiah, Sook Luan Ng, Yogeswaran Lokanathan, Min Hwei Ng, and Jia Xian Law. 2024. "Plant Defense Mechanisms against Polycyclic Aromatic Hydrocarbon Contamination: Insights into the Role of Extracellular Vesicles" Toxics 12, no. 9: 653. https://doi.org/10.3390/toxics12090653







