Integrated Biomarker Response Emphasizing Neuronal Oxidative Stress and Genotoxicity Induced by Oxamyl in Sprague Dawley Rats: Ameliorative Effect of Ginseng as a Neuroprotective Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Animals
2.2. Methods
2.2.1. Experimental Design
2.2.2. Sample Preparation
2.2.3. Tissue Homogenates
2.2.4. Genotoxicity Potential
Micronucleus Test
Alkaline Single-Cell Gel Electrophoresis (Comet Assay)
Gene Expression Analysis
2.2.5. Biochemical Analyses of Cholinesterase Activity and Oxidative Stress Markers in Serum and Tissue Homogenate
Cholinesterase (ChE)
Malondialdehyde (MDA)
Superoxide Dismutase (SOD)
Nitric Oxide (NO)
2.2.6. Histopathology and Immunohistochemistry
Histopathology
Immunohistochemistry
2.2.7. Statistical Analysis
3. Results
3.1. Acute Oral LD50 of Oxamyl
3.2. Repeated Exposure to Oxamyl Induces DNA Damage, as Indicated by Micronucleus and Comet Assays
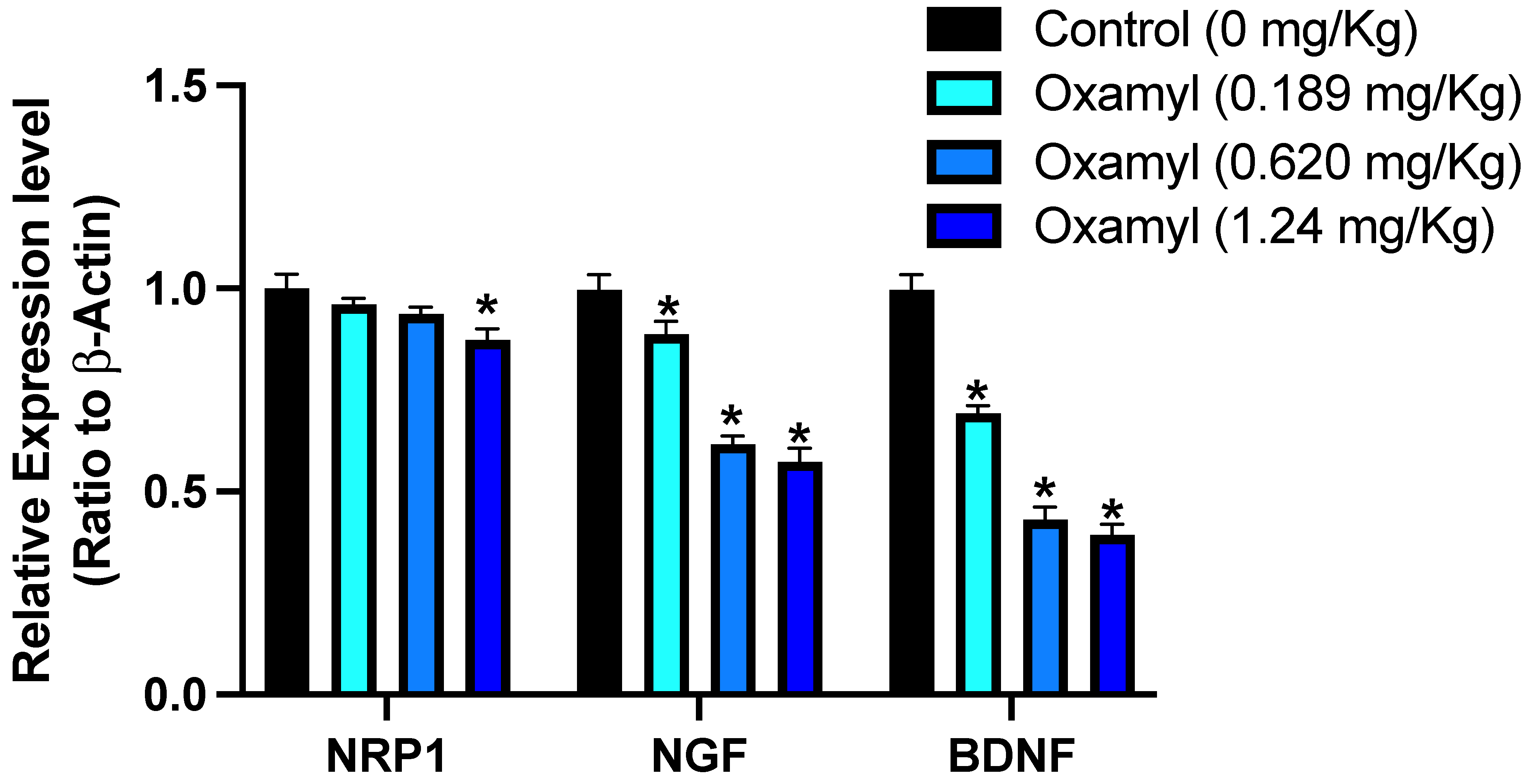
3.3. Impact of Subacute Exposure to Oxamyl on Neuronal Gene Expression
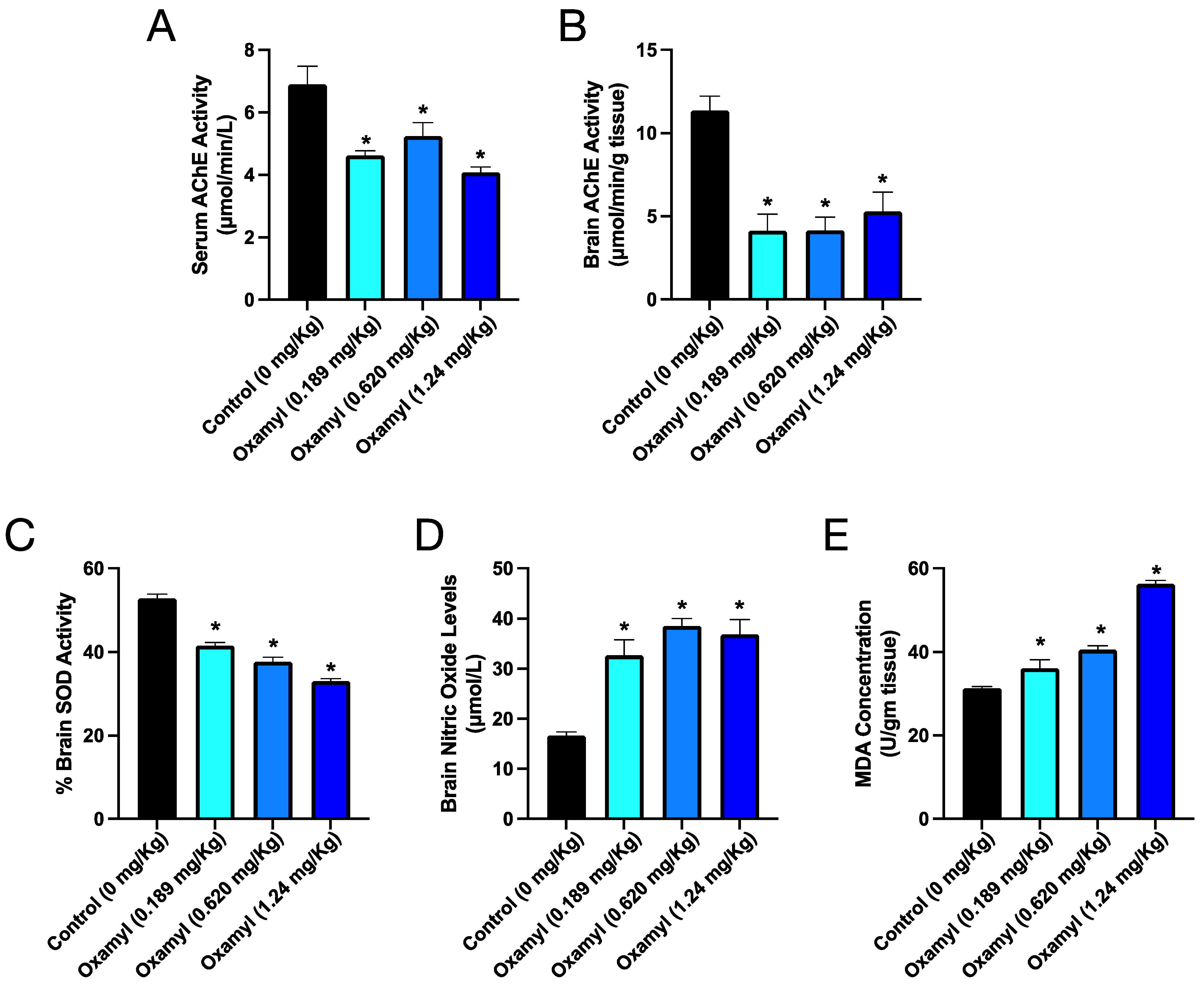
3.4. Repeated Exposure to Oxamyl Triggers a Reduction in Brain and Serum Cholinesterase Activity and an Increase in Brain Oxidative Stress
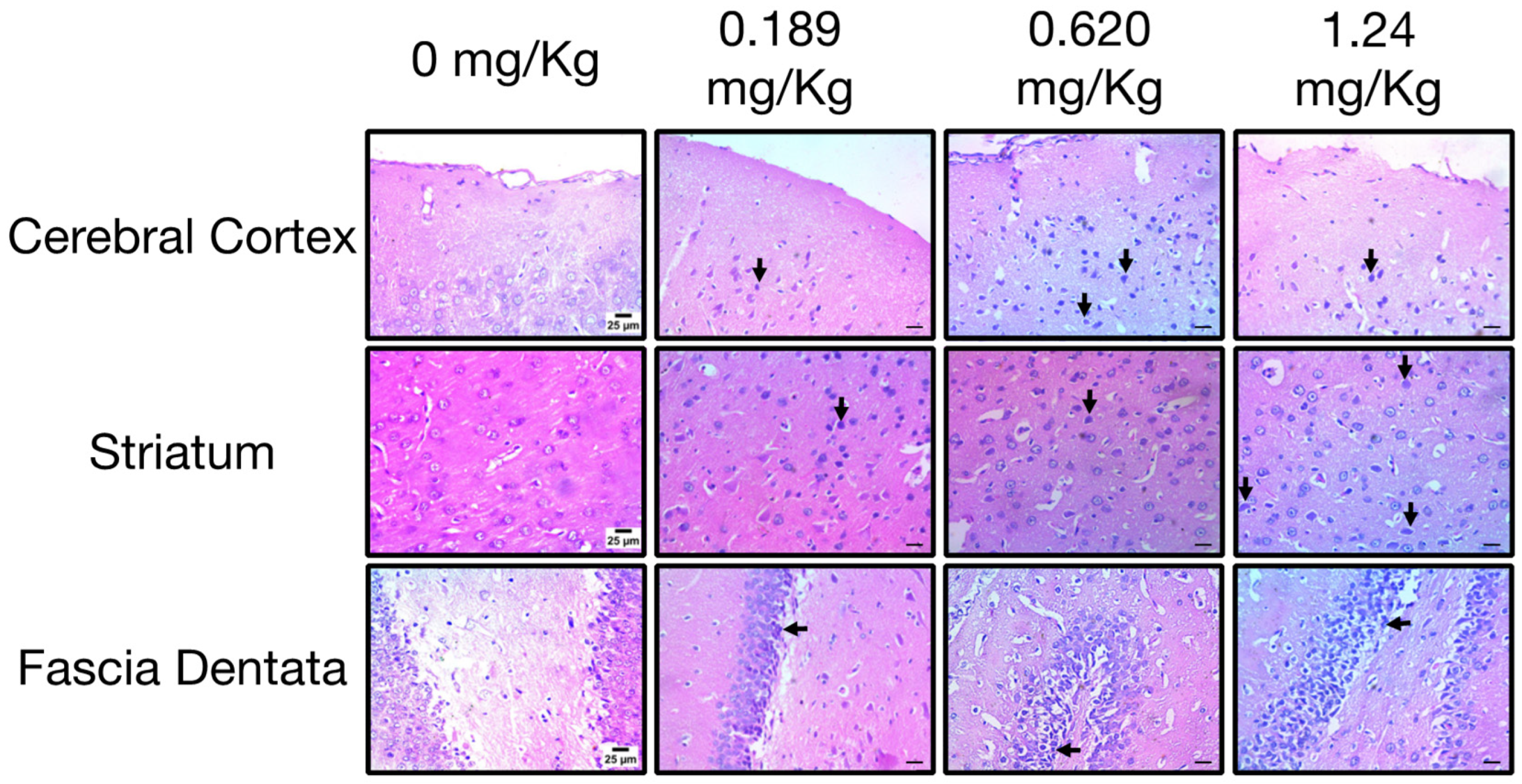
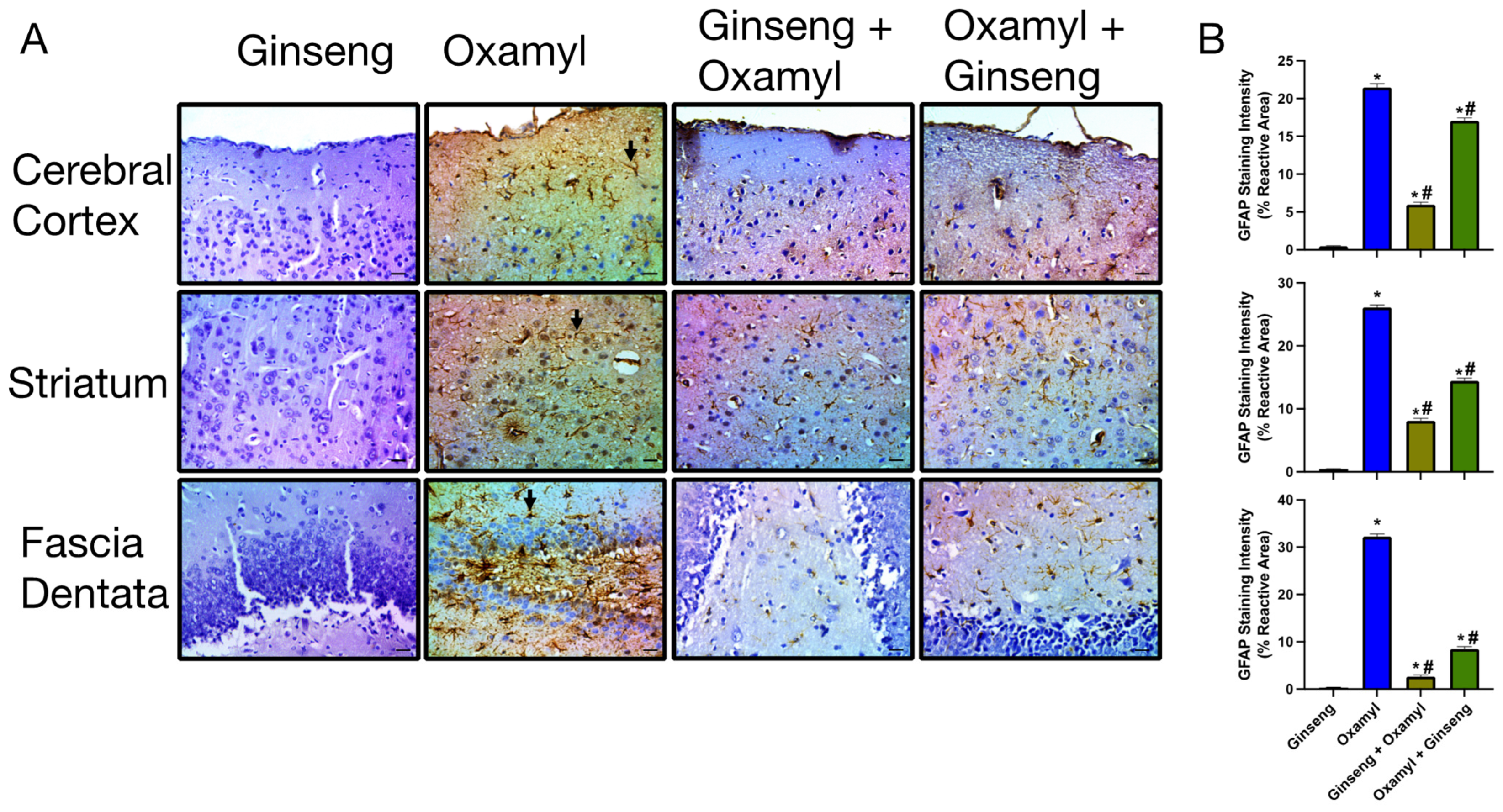
3.5. Repeated Exposure to Oxamyl Induces Structural and Molecular Changes Consistent with Neuroinflammation
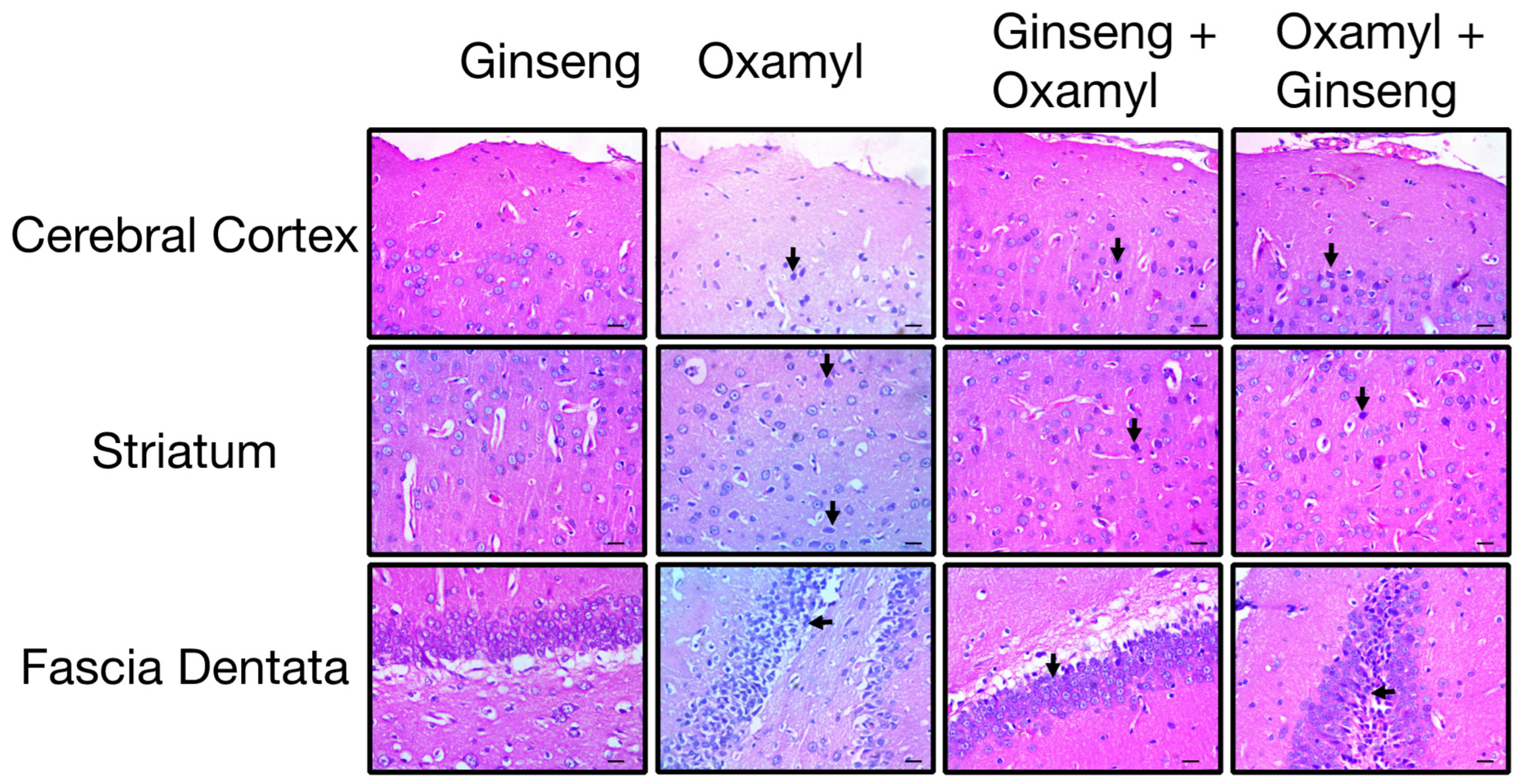
3.6. Protection or Treatment Impact of Ginseng on Genotoxicity, Brain Oxidative Stress, Biochemical Parameters, and Neuroinflammation Following Oxamyl Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The Impact of Climate Change on Agricultural Insect Pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- America Pesticide Action Network (PAN). Pesticides and Climate Change: A Vicious Cycle; Pesiticide Action Network: Berkely, CA, USA, 2023. [Google Scholar]
- Mergia, M.T.; Weldemariam, E.D.; Eklo, O.M.; Yimer, G.T. Small-Scale Farmer Pesticide Knowledge and Practice and Impacts on the Environment and Human Health in Ethiopia. J. Health Pollut. 2021, 11, 210607. [Google Scholar] [CrossRef] [PubMed]
- Zinyemba, C.; Archer, E.; Rother, H.-A. Climate Change, Pesticides and Health: Considering the Risks and Opportunities of Adaptation for Zimbabwean Smallholder Cotton Growers. Int. J. Environ. Res. Public Health 2020, 18, 121. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; AlShahrani, A.M.; Muzammil, K.; Saati, A.A.; Wahab, S.; Elbendary, E.Y.; Kambal, N.; et al. Pesticides Impacts on Human Health and the Environment with Their Mechanisms of Action and Possible Countermeasures. Heliyon 2024, 10, e29128. [Google Scholar] [CrossRef]
- Semu, E.; Tindwa, H.; Singh, B.R. Heavy Metals and Organopesticides: Ecotoxicology, Health Effects and Mitigation Options with Emphasis on Sub-Saharan Africa. J. Toxicol. Curr. Res. 2019, 3, 010. [Google Scholar] [CrossRef]
- Parra-Arroyo, L.; González-González, R.B.; Castillo-Zacarías, C.; Melchor Martínez, E.M.; Sosa-Hernández, J.E.; Bilal, M.; Iqbal, H.M.N.; Barceló, D.; Parra-Saldívar, R. Highly Hazardous Pesticides and Related Pollutants: Toxicological, Regulatory, and Analytical Aspects. Sci. Total Environ. 2022, 807, 151879. [Google Scholar] [CrossRef]
- World Health Organization (WHO). The WHO Recommended Classii Cation of Pesticides by Hazard and Guidelines to Classification 2019; World Health Organization (WHO): Geneva, Switzerland, 2020. [Google Scholar]
- Pohanish Richard, P. Sittig’s Handbook of Pesticides and Agricultural Chemicals, 2nd ed.; Pohanish, R.P., Ed.; William Andrew Publishing: Oxford, UK, 2015; ISBN 9781455731480. [Google Scholar]
- Biancardi, A.; Aimo, C.; Piazza, P.; Lo Chiano, F.; Rubini, S.; Baldini, E.; Vertuani, S.; Manfredini, S. Acetylcholinesterase (AChE) Reversible Inhibitors: The Role of Oxamyl in the Production of Poisoned Baits. Toxics 2022, 10, 432. [Google Scholar] [CrossRef]
- Peter, J.V.; Sudarsan, T.I.; Moran, J.L. Clinical Features of Organophosphate Poisoning: A Review of Different Classification Systems and Approaches. Indian J. Crit. Care Med. 2014, 18, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-H.; Lein, P.J. Mechanisms of Organophosphate Neurotoxicity. Curr. Opin. Toxicol. 2021, 26, 49–60. [Google Scholar] [CrossRef]
- Ndonwi, E.N.; Atogho-Tiedeu, B.; Lontchi-Yimagou, E.; Shinkafi, T.S.; Nanfa, D.; Balti, E.V.; Indusmita, R.; Mahmood, A.; Katte, J.-C.; Mbanya, A.; et al. Gestational Exposure to Pesticides Induces Oxidative Stress and Lipid Peroxidation in Offspring That Persist at Adult Age in an Animal Model. Toxicol. Res. 2019, 35, 241–248. [Google Scholar] [CrossRef]
- Sajad, M.; Shabir, S.; Singh, S.K.; Bhardwaj, R.; Alsanie, W.F.; Alamri, A.S.; Alhomrani, M.; Alsharif, A.; Vamanu, E.; Singh, M.P. Role of Nutraceutical against Exposure to Pesticide Residues: Power of Bioactive Compounds. Front. Nutr. 2024, 11, 1342881. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, W.; Wen, Y.; Lu, S.; Zhao, C. Potential Therapeutic Natural Compounds for the Treatment of Alzheimer’s Disease. Phytomedicine 2024, 132, 155822. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.-S.; Li, X.; Pang, L.; Deng, H.; Chen, F.; Joon Lee, J.; Quan, Z.-S.; Liu, P.; Guo, H.-Y.; Shen, Q.-K. Panaxadiol Carbamate Derivatives: Synthesis and Biological Evaluation as Potential Multifunctional Anti-Alzheimer Agents. Bioorg. Chem. 2024, 143, 106977. [Google Scholar] [CrossRef]
- OECD. Guidance Document on Aquatic Toxicity Testing of Pesticides; Organisation for Economic Co-operation and Development: Paris, France, 2022. [Google Scholar]
- US Environmental Protection Agency Office of Pesticide Programs. Reregistration Eligibility Decision for Oxamyl; US Environmental Protection Agency: Washington, DC, USA, 2007.
- Chou, T.-W.; Huang, H.-S.; Panyod, S.; Huang, Y.-J.; Sheen, L.-Y. Korean Red Ginseng Water Extract Produces Antidepressant-like Effects through Involving Monoamines and Brain-Derived Neurotrophic Factor in Rats. J. Ginseng Res. 2023, 47, 552–560. [Google Scholar] [CrossRef]
- Iqbal, H.; Kim, S.-K.; Cha, K.-M.; Jeong, M.-S.; Ghosh, P.; Rhee, D.-K. Korean Red Ginseng Alleviates Neuroinflammation and Promotes Cell Survival in the Intermittent Heat Stress-Induced Rat Brain by Suppressing Oxidative Stress via Estrogen Receptor Beta and Brain-Derived Neurotrophic Factor Upregulation. J. Ginseng Res. 2020, 44, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.K.; Chauhan, L.K.S. An Improved Chemical Substitute for Fetal Calf Serum for the Micronucleus Test. Biotech. Histochem. 1993, 68, 187–188. [Google Scholar] [CrossRef]
- Schmid, W. The Micronucleus Test. Mutat. Res./Environ. Mutagen. Relat. Subj. 1975, 31, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Khalil, W.K.B.; Ahmed, K.A.; Park, M.H.; Kim, Y.T.; Park, H.H.; Abdel-Wahhab, M.A. The Inhibitory Effects of Garlic and Panax Ginseng Extract Standardized with Ginsenoside Rg3 on the Genotoxicity, Biochemical, and Histological Changes Induced by Ethylenediaminetetraacetic Acid in Male Rats. Arch. Toxicol. 2008, 82, 183–195. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single Cell Gel/Comet Assay: Guidelines for in Vitro and in Vivo Genetic Toxicology Testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Khalil, W.; Roshdy, H.; Kassem, S. The Potential Therapeutic Role of Fenugreek Saponin against Alzheimers Disease: Evaluation of Apoptotic and Acetylcholinesterase Inhibitory Activities. J. Appl. Pharm. Sci. 2016, 6, 166–173. [Google Scholar] [CrossRef][Green Version]
- Refaie, A.A.; Ramadan, A.; Sabry, N.M.; Khalil, W.K.B.; Mossa, A.-T.H. Over-Gene Expression in the Apoptotic, Oxidative Damage and Liver Injure in Female Rats Exposed to Butralin. Environ. Sci. Pollut. Res. 2020, 27, 31383–31393. [Google Scholar] [CrossRef] [PubMed]
- Elhinnawi, M.A.; Mohareb, R.M.; Rady, H.M.; Khalil, W.K.B.; Abd Elhalim, M.M.; Elmegeed, G.A. Novel Pregnenolone Derivatives Modulate Apoptosis via Bcl-2 Family Genes in Hepatocellular Carcinoma in Vitro. J. Steroid Biochem. Mol. Biol. 2018, 183, 125–136. [Google Scholar] [CrossRef]
- Patel, A.B. Simple and Rapid Colorimetric Method for Determination of Erythrocyte and Plasma Cholinesterase Activities and Comparison with the Standard Ellman’s Method. Public Health Toxicol. 2023, 3, 14. [Google Scholar] [CrossRef]
- Al meqbaali, F.T.; Habib, H.; Othman, A.; Al-Marzooqi, S.; Al-Bawardi, A.; Pathan, J.Y.; Hilary, S.; Souka, U.; Al-Hammadi, S.; Ibrahim, W.; et al. The Antioxidant Activity of Date Seed: Preliminary Results of a Preclinical in Vivo Study. Emir. J. Food Agric. 2017, 29, 822–832. [Google Scholar] [CrossRef]
- Platat, C.; Habib, H.; Othman, A.; Al-Marzooqi, S.; Al-Bawardi, A.; Pathan, J.Y.; Hilary, S.; Al-Maqbali, F.; Souka, U.; Al-Hammadi, S.; et al. Safety and protective effect of date (phoenix dactylifera) seed extract against oxidative damage in rat. Int. J. Food Nutr. Sci. 2015, 4, 21–28. [Google Scholar]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of Nitric Oxide Production in Biological Systems by Using Griess Reaction Assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Suzuki, Y.; Imada, T.; Yamaguchi, I.; Yoshitake, H.; Sanada, H.; Kashiwagi, T.; Takaba, K. Effects of Prolonged Water Washing of Tissue Samples Fixed in Formalin on Histological Staining. Biotech. Histochem. 2012, 87, 241–248. [Google Scholar] [CrossRef]
- Hayashi, M. The Micronucleus Test—Most Widely Used in Vivo Genotoxicity Test. Genes Environ. 2016, 38, 18. [Google Scholar] [CrossRef]
- Schwarz, Q.; Maden, C.H.; Vieira, J.M.; Ruhrberg, C. Neuropilin 1 Signaling Guides Neural Crest Cells to Coordinate Pathway Choice with Cell Specification. Proc. Natl. Acad. Sci. USA 2009, 106, 6164–6169. [Google Scholar] [CrossRef]
- Rose, R.; Peschke, N.; Nigi, E.; Gelléri, M.; Ritz, S.; Cremer, C.; Luhmann, H.J.; Sinning, A. Chromatin Compaction Precedes Apoptosis in Developing Neurons. Commun. Biol. 2022, 5, 797. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current Status of Pesticide Effects on Environment, Human Health and It’s Eco-Friendly Management as Bioremediation: A Comprehensive Review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef] [PubMed]
- Roldan-Tapia, L.; Nieto-Escamez, F.A.; del Aguila, E.M.; Laynez, F.; Parron, T.; Sanchez-Santed, F. Neuropsychological Sequelae from Acute Poisoning and Long-Term Exposure to Carbamate and Organophosphate Pesticides. Neurotoxicol. Teratol. 2006, 28, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Haydock, P.P.J.; Ambrose, E.L.; Wilcox, A.; Deliopoulos, T. Degradation of the Nematicide Oxamyl under Field and Laboratory Conditions. Nematology 2012, 14, 339–352. [Google Scholar] [CrossRef]
- Kennedy, G. Chronic Toxicity, Reproductive, and Teratogenic Studies with Oxamyl. Fundam. Appl. Toxicol. 1986, 7, 106–118. [Google Scholar] [CrossRef]
- Karslı, S.; Yazar, S.; İncedere Düzdağ, E.; Yurdun, T. Assessment of Genotoxic Effects of Organophosphate and Carbamate Pesticides by Comet Assay. İstanb. J. Pharm. 2022, 52, 136–142. [Google Scholar] [CrossRef]
- Broz, M.; Kolarič, A.; Jukič, M.; Bren, U. Neuropilin (NRPs) Related Pathological Conditions and Their Modulators. Int. J. Mol. Sci. 2022, 23, 8402. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, G.; Zhou, H.; Liu, Y.; Ding, H.; Wang, Z.; Shen, H.; Li, X.; Li, H. Downregulation of Nrp1 Transcription Promotes Blood–Brain Barrier Disruption Following Experimental Cerebral Ischemia–Reperfusion. Neurosci. Lett. 2024, 818, 137553. [Google Scholar] [CrossRef]
- Aloe, L.; Rocco, M.; Balzamino, B.; Micera, A. Nerve Growth Factor: A Focus on Neuroscience and Therapy. Curr. Neuropharmacol. 2015, 13, 294–303. [Google Scholar] [CrossRef]
- Connor, B.; Dragunow, M. The Role of Neuronal Growth Factors in Neurodegenerative Disorders of the Human Brain. Brain Res. Rev. 1998, 27, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Hasan, H.; Habashy, K.J.; Fakih, W.; Abdelhady, S.; Ahmad, F.; Zibara, K.; Eid, A.H.; El-Yazbi, A.F.; Kobeissy, F.H. Western Diet Aggravates Neuronal Insult in Post-Traumatic Brain Injury: Proposed Pathways for Interplay. eBioMedicine 2020, 57, 102829. [Google Scholar] [CrossRef] [PubMed]
- Fakih, W.; Mroueh, A.; Salah, H.; Eid, A.H.; Obeid, M.; Kobeissy, F.; Darwish, H.; El-Yazbi, A.F. Dysfunctional Cerebrovascular Tone Contributes to Cognitive Impairment in a Non-Obese Rat Model of Prediabetic Challenge: Role of Suppression of Autophagy and Modulation by Anti-Diabetic Drugs. Biochem. Pharmacol. 2020, 178, 114041. [Google Scholar] [CrossRef]
- Al-Assi, O.; Ghali, R.; Mroueh, A.; Kaplan, A.; Mougharbil, N.; Eid, A.H.; Zouein, F.A.; El-Yazbi, A.F. Cardiac Autonomic Neuropathy as a Result of Mild Hypercaloric Challenge in Absence of Signs of Diabetes: Modulation by Antidiabetic Drugs. Oxid. Med. Cell. Longev. 2018, 2018, 9389784. [Google Scholar] [CrossRef]
- Chidambaram, S.B.; Anand, N.; Varma, S.R.; Ramamurthy, S.; Vichitra, C.; Sharma, A.; Mahalakshmi, A.M.; Essa, M.M. Superoxide Dismutase and Neurological Disorders. IBRO Neurosci. Rep. 2024, 16, 373–394. [Google Scholar] [CrossRef]
- Liy, P.M.; Puzi, N.N.A.; Jose, S.; Vidyadaran, S. Nitric Oxide Modulation in Neuroinflammation and the Role of Mesenchymal Stem Cells. Exp. Biol. Med. 2021, 246, 2399–2406. [Google Scholar] [CrossRef]
- Cupic Miladinovic, D.; Borozan, S.; Ivanovic, S. Involvement of Cholinesterases in Oxidative Stress Induced by Chlorpyrifos in the Brain of Japanese Quail. Poult. Sci. 2018, 97, 1564–1571. [Google Scholar] [CrossRef]
- Selmi, S.; El-Fazaa, S.; Gharbi, N. Oxidative Stress and Cholinesterase Inhibition in Plasma, Erythrocyte and Brain of Rats’ Pups Following Lactational Exposure to Malathion. Environ. Toxicol. Pharmacol. 2012, 34, 753–760. [Google Scholar] [CrossRef]
- Santos, D.; Milatovic, D.; Andrade, V.; Batoreu, M.C.; Aschner, M.; Marreilha dos Santos, A.P. The Inhibitory Effect of Manganese on Acetylcholinesterase Activity Enhances Oxidative Stress and Neuroinflammation in the Rat Brain. Toxicology 2012, 292, 90–98. [Google Scholar] [CrossRef]
- Mangelus, M.; Kroyter, A.; Galron, R.; Sokolovsky, M. Reactive Oxygen Species Regulate Signaling Pathways Induced by M1 Muscarinic Receptors in PC12M1 Cells. J. Neurochem. 2001, 76, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.H.; Bhilare, K.D.; In, G.; Park, C.-K.; Kim, J.-H. Effects of Panax Ginseng and Ginsenosides on Oxidative Stress and Cardiovascular Diseases: Pharmacological and Therapeutic Roles. J. Ginseng Res. 2022, 46, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.R.; Ma, J.Y.; Sung, C.K. Chronic Dietary Ginseng Extract Administration Ameliorates Antioxidant and Cholinergic Systems in the Brains of Aged Mice. J. Ginseng Res. 2017, 41, 615–619. [Google Scholar] [CrossRef] [PubMed]





| Gene | Primer Sequences | NCBI Reference |
|---|---|---|
| NRP1 | F: ttt cct ccc tca atc gtg ct, R: ccg gga gat gta agg tac cc | BC085689.1 |
| NGF | F: cag tgt cag tgt gtg ggt tg, R: gcc tgt ttg tcg tct gtt gt | NM_001277055.1 |
| BDNF | F: att acc tgg atg ccg caa ac, R: cct gca gcc ttc ttt tgt gt | AY176065.1 |
| β-actin | F: tct tcc agc ctt cct tcc tg, R: cac aca gag tac ttg cgc tc | EF156276.1 |
| Treatment (mg/kg) | PCE Screened | MnPCEs/2000 PCE | Mean ± SEM | NCE | PCE/NCE | |
|---|---|---|---|---|---|---|
| Number | Screened | Ratio | Mean ± SEM | |||
| Control | 2000 | 9 | 7.33 ± 0.88 d | 781 | 2.56 | 2.62 ± 0.03 |
| 2000 | 7 | 764 | 2.62 | |||
| 2000 | 6 | 747 | 2.68 | |||
| Oxamyl High | 2000 | 33 | 30.67 ± 1.46 a | 533 | 3.75 | 3.54 ± 0.12 |
| 2000 | 31 | 569 | 3.51 | |||
| 2000 | 28 | 597 | 3.35 | |||
| Ginseng | 2000 | 9 | 7.65 ± 0.66 d | 741 | 2.70 | 2.67 ± 0.03 |
| 2000 | 7 | 768 | 2.60 | |||
| 2000 | 7 | 737 | 2.71 | |||
| Ginseng + Oxamyl (28 d) | 2000 | 22 | 20.34 ± 0.89 b | 636 | 3.14 | 3.06 ± 0.05 |
| 2000 | 19 | 651 | 3.07 | |||
| 2000 | 20 | 675 | 2.96 | |||
| Oxamyl + Ginseng (28 d) + (10 d) | 2000 | 18 | 16.70 ± 1.34 c | 683 | 2.93 | 2.99 ± 0.04 |
| 2000 | 14 | 669 | 2.99 | |||
| 2000 | 18 | 655 | 3.05 | |||
| Treatment | DNA Damage | ||
|---|---|---|---|
| Tail Length | DNA% in Tail | Tail Moment | |
| High-dose Oxamyl | 9.242 ± 0.862 | 15.023 ± 2.081 | 14.095 ± 1.852 |
| Control + Ginseng group | 0.967 ± 0.061 | 3.333 ± 0.426 | 1.078 ± 0.215 |
| Ginseng + Oxamyl group | 3.148 ± 0.271 | 9.670 ± 0.502 | 6.489 ± 0.419 |
| Oxamyl + Ginseng group | 2.265 ± 0.159 | 6.333 ± 0.674 | 4.726 ± 0.715 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdallah, S.M.; Muhammed, R.E.; Mohamed, R.E.; Khalil, W.K.B.; Taha, D.A.; Shalaby, M.B.; Elgohary, I.; Abdallah, A.A.; Habib, H.M.; El-Yazbi, A.F. Integrated Biomarker Response Emphasizing Neuronal Oxidative Stress and Genotoxicity Induced by Oxamyl in Sprague Dawley Rats: Ameliorative Effect of Ginseng as a Neuroprotective Agent. Toxics 2024, 12, 655. https://doi.org/10.3390/toxics12090655
Abdallah SM, Muhammed RE, Mohamed RE, Khalil WKB, Taha DA, Shalaby MB, Elgohary I, Abdallah AA, Habib HM, El-Yazbi AF. Integrated Biomarker Response Emphasizing Neuronal Oxidative Stress and Genotoxicity Induced by Oxamyl in Sprague Dawley Rats: Ameliorative Effect of Ginseng as a Neuroprotective Agent. Toxics. 2024; 12(9):655. https://doi.org/10.3390/toxics12090655
Chicago/Turabian StyleAbdallah, Salwa M., Reham E. Muhammed, Reda E. Mohamed, Wagdy K. B. Khalil, Dalia A. Taha, Mohamed B. Shalaby, Islam Elgohary, Amr A. Abdallah, Hosam M. Habib, and Ahmed F. El-Yazbi. 2024. "Integrated Biomarker Response Emphasizing Neuronal Oxidative Stress and Genotoxicity Induced by Oxamyl in Sprague Dawley Rats: Ameliorative Effect of Ginseng as a Neuroprotective Agent" Toxics 12, no. 9: 655. https://doi.org/10.3390/toxics12090655
APA StyleAbdallah, S. M., Muhammed, R. E., Mohamed, R. E., Khalil, W. K. B., Taha, D. A., Shalaby, M. B., Elgohary, I., Abdallah, A. A., Habib, H. M., & El-Yazbi, A. F. (2024). Integrated Biomarker Response Emphasizing Neuronal Oxidative Stress and Genotoxicity Induced by Oxamyl in Sprague Dawley Rats: Ameliorative Effect of Ginseng as a Neuroprotective Agent. Toxics, 12(9), 655. https://doi.org/10.3390/toxics12090655







