Different Cytotoxicity Induced by Hexabromocyclododecanes on Mouse Neuroblastoma N2a Cells via Oxidative Stress and Mitochondrial Apoptotic Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Experiments for Oxidative Stress
2.5. Cell Cycle Analysis
2.6. Quantitative Polymerase Chain Reaction
2.7. Western Blot
2.8. Statistical Analysis
3. Results
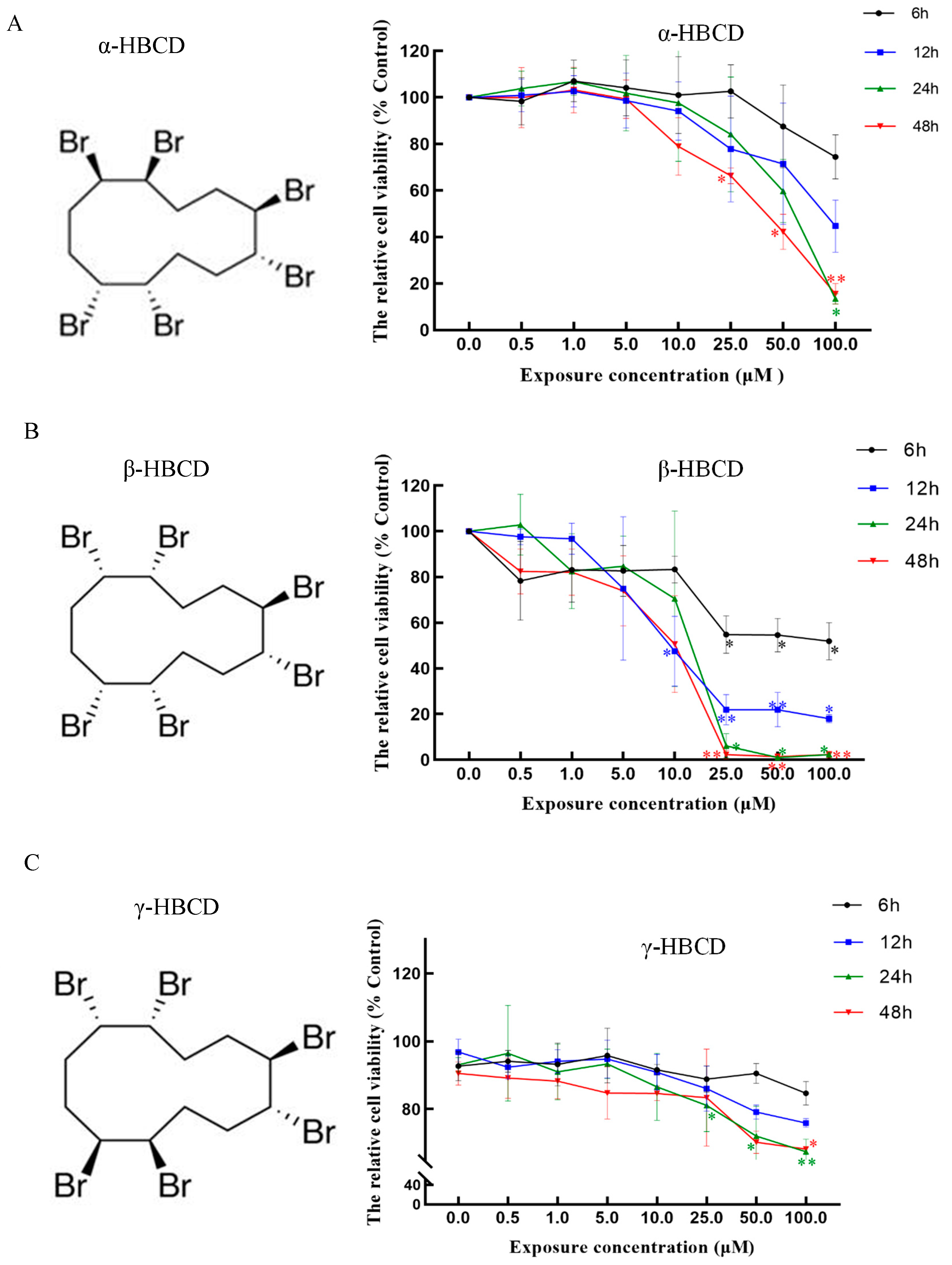
3.1. Effects of HBCD on Cell Viability
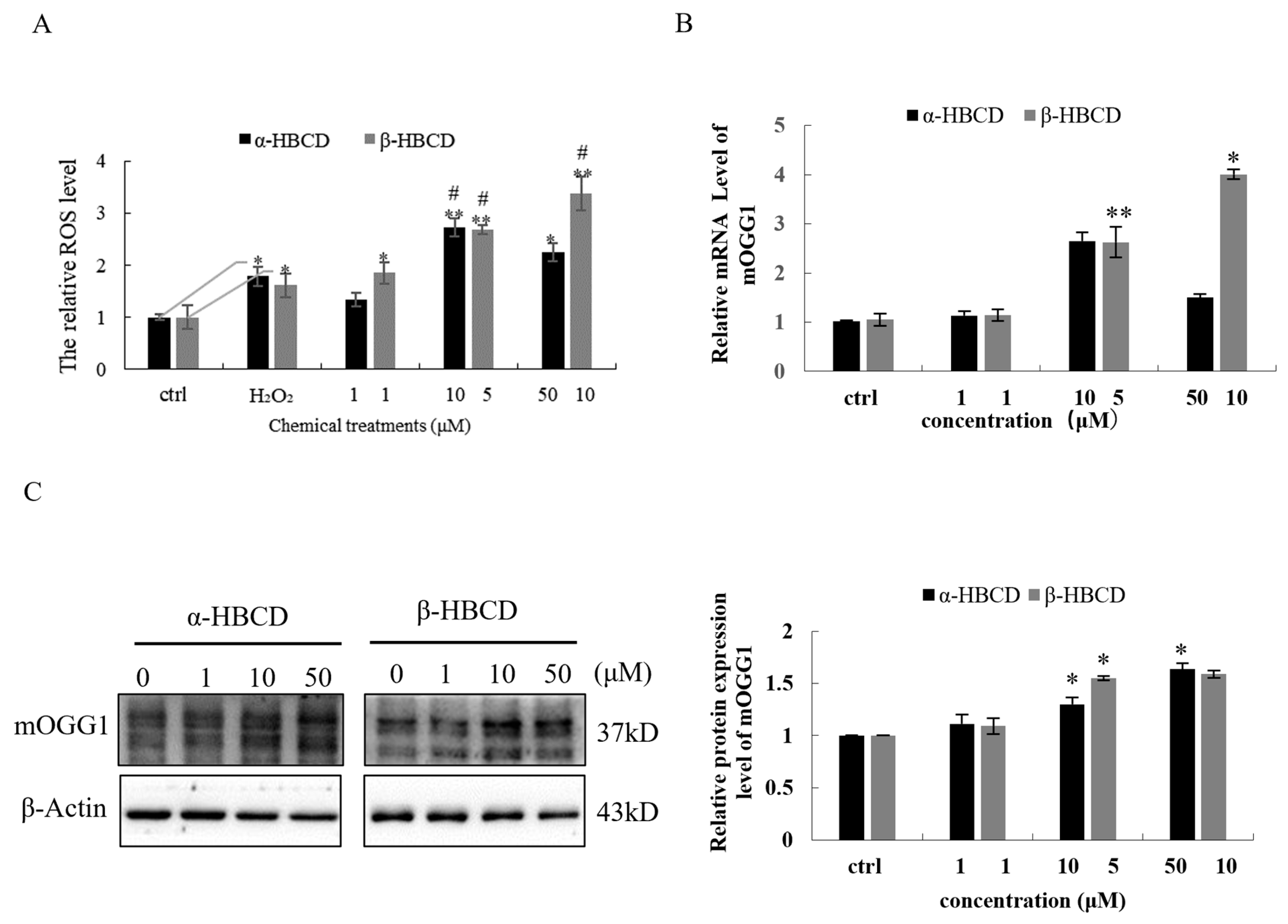
3.2. Oxidative Damage Induced by HBCDs in N2a Cells
3.3. Effects of α-HBCD and β-HBCD on the Cell Cycle of N2a Cells
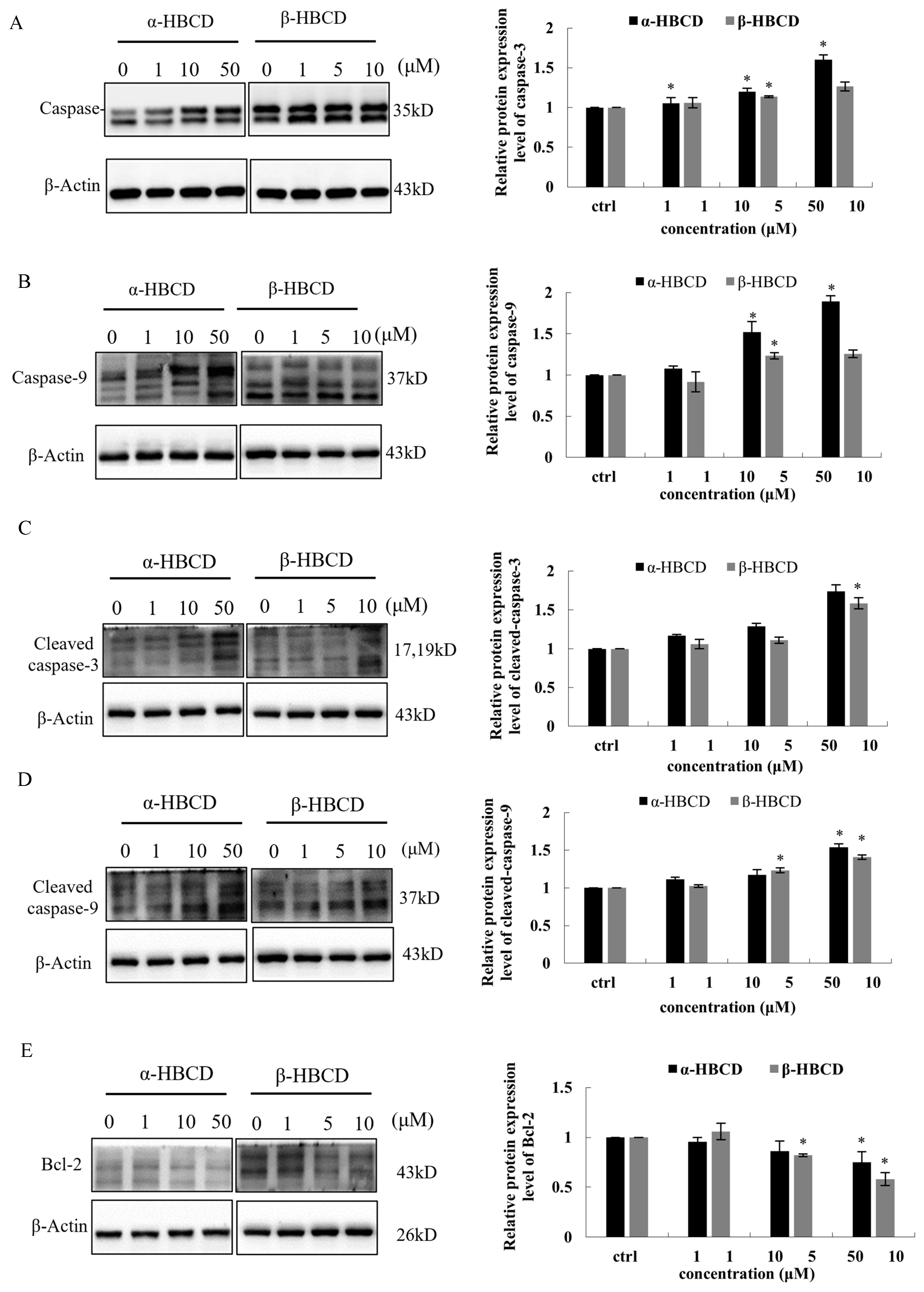
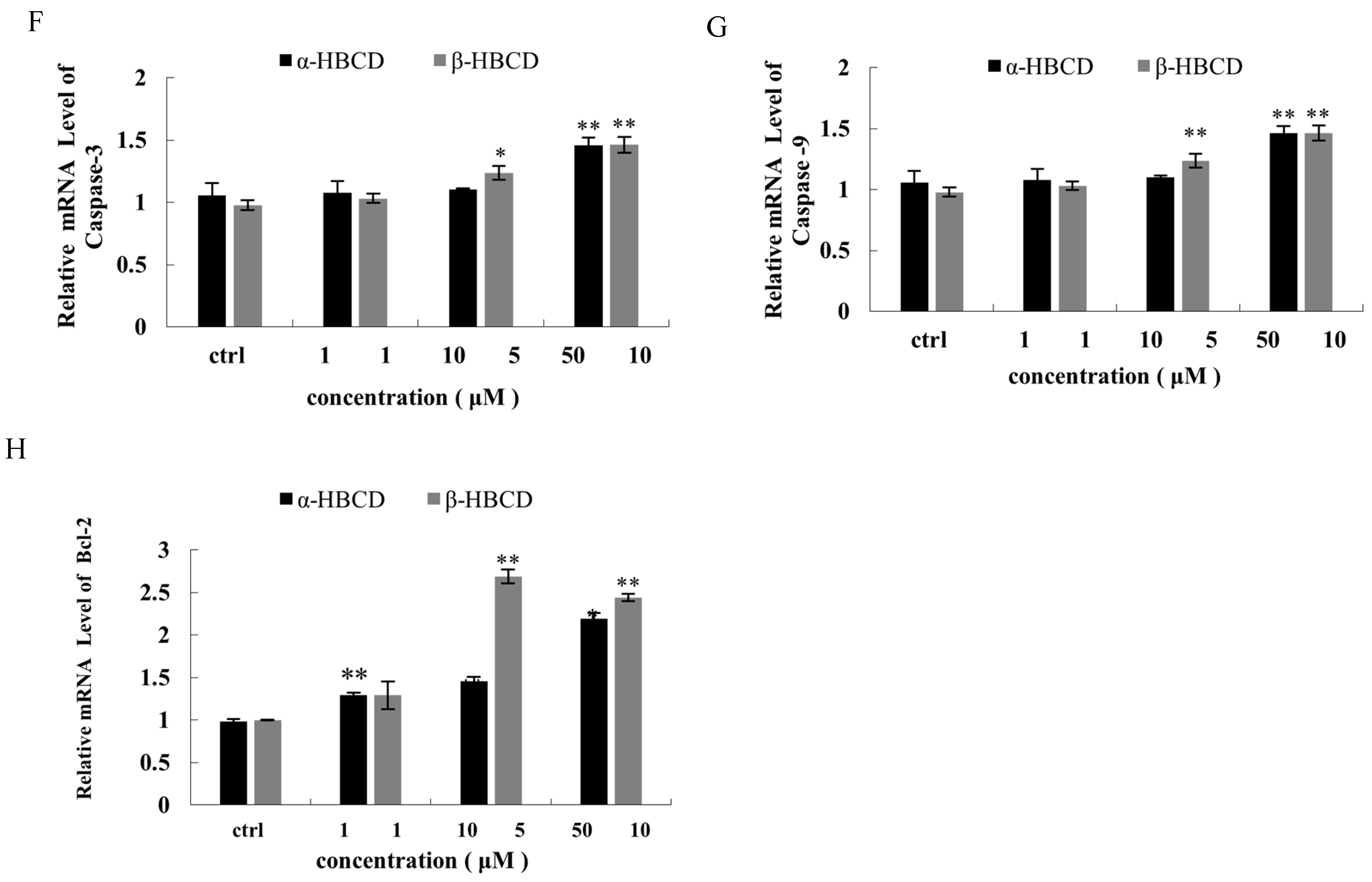
3.4. Effects of α-HBCD and β-HBCD on Mitochondria Function of N2a Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations Environment Programme (UNEP). Report of the Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants on the Work of Its Sixth; UNEP: Nairobi, Kenya, 2013. [Google Scholar]
- Ruan, Y.F.; Zhang, X.H.; Qiu, J.W.; Leung, K.M.Y.; Lam, J.C.W.; Lam, P.K.S. Stereoisomer-specifific Trophodynamics of the Chiral Brominated Flame Retardants HBCD and TBECH in a Marine Food Web, with Implications for Human Exposure. Environ. Sci. Technol. 2018, 52, 8183–8193. [Google Scholar] [CrossRef] [PubMed]
- Marvin, C.H.; Tomy, G.T.; Armitage, J.M.; Arnot, J.A.; McCarty, L.; Covaci, A.; Palace, V. Hexabromocyclododecane: Current understanding of chemistry, environmental fate and toxicology and implications for global management. Environ. Sci. Technol. 2011, 45, 8613–8623. [Google Scholar] [CrossRef] [PubMed]
- Brandsma, S.H.; Van der Ven, L.T.; De Boer, J.; Leonards, P.E. Identification of hydroxylated metabolites of hexabromocyclododecane in wildlife and 28-days exposed Wistar rats. Environ. Sci. Technol. 2009, 43, 6058–6063. [Google Scholar] [CrossRef] [PubMed]
- Germer, S.; Piersma, A.H.; van der Ven, L.; Kamyschnikow, A.; Fery, Y.; Schmitz, H.J.; Schrenk, D. Subacute effects of the brominated flame retardants hexabromocyclododecane and tetrabromobisphenol A on hepatic cytochrome P450 levels in rats. Toxicology 2006, 218, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Crump, D.; Chiu, S.; Egloff, C.; Kennedy, S.W. Effects of hexabromocyclododecane and polybrominated diphenyl ethers on mRNA expression in chicken (Gallus domesticus) hepatocytes. Toxicol. Sci. 2008, 106, 470–487. [Google Scholar] [CrossRef] [PubMed]
- Erratico, C.; Zheng, X.; Eede, N.V.D.; Tomy, G.; Covaci, A. Stereoselective Metabolism of α-, β-, and γ-Hexabromocyclododecanes (HBCDs) by Human Liver Microsomes and CYP3A4. Environ. Sci. Technol. 2016, 50, 8263–8273. [Google Scholar] [CrossRef]
- Hakk, H. Comparative Metabolism Studies of Hexabromocyclododecane (HBCD) Diastereomers in Male Rats Following a Single Oral Dose. Environ. Sci. Technol. 2016, 50, 89–96. [Google Scholar] [CrossRef]
- van der Ven, L.T.; Verhoef, A.; van de Kuil, T.; Slob, W.; Leonards, P.E.; Visser, T.J.; Hamers, T.; Herlin, M.; Hakansson, H.; Olausson, H.; et al. A 28-day oral dose toxicity study enhanced to detect endocrine effects of hexabromocyclododecane in Wistar rats. Toxicol. Sci. 2006, 94, 281–292. [Google Scholar] [CrossRef]
- Ibhazehiebo, K.; Iwasaki, T.; Shimokawa, N.; Koibuchi, N. 1,2,5,6,9,10-αHexabromocyclododecane (HBCD) Impairs Thyroid Hormone-Induced Dendrite Arborization of Purkinje Cells and Suppresses Thyroid Hormone Receptor-Mediated Transcription. Cerebellum 2011, 10, 22–31. [Google Scholar] [CrossRef]
- Yamada-Okabe, T.; Sakai, H.; Kashima, Y.; Yamada-Okabe, H. Modulation at a cellular level of the thyroid hormone receptor-mediated gene expression by 1,2,5,6,9,10-hexabromocyclododecane (HBCD), 4,4′-diiodobiphenyl (DIB), and nitrofen (NIP). Toxicol. Lett. 2005, 155, 127–133. [Google Scholar] [CrossRef]
- Feng, M.; Qu, R.; Wang, C.; Wang, L.; Wang, Z. Comparative antioxidant status in freshwater fish Carassius auratus exposed to six current-use brominated flame retardants: A combined experimental and theoretical study. Aquat. Toxicol. 2013, 140–141, 314–323. [Google Scholar] [CrossRef] [PubMed]
- van der Ven, L.T.; van de Kuil, T.; Leonards, P.E.; Slob, W.; Lilienthal, H.; Litens, S.; Herlin, M.; Håkansson, H.; Cantón, R.F.; van den Berg, M.; et al. Endocrine effects of hexabromocyclododecane (HBCD) in a one-generation reproduction study in Wistar rats. Toxicol. Lett. 2009, 185, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Krivoshiev, B.V.; Dardenne, F.; Covaci, A.; Blust, R.; Husson, S.J. Assessing in-vitro estrogenic effects of currently-used flame retardants. Toxicol. Vitr. 2016, 33, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zainab, B.; Ayaz, Z.; Rashid, U.; Al Farraj, D.A.; Alkufeidy, R.M.; AlQahtany, F.S.; Aljowaie, R.M.; Abbasi, A.M. Role of Persistent Organic Pollutants in Breast Cancer Progression and Identification of Estrogen Receptor Alpha Inhibitors Using In-Silico Mining and Drug-Drug Interaction Network Approaches. Biology 2021, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.A.; Ashraf, M.A.; Sharma, S. Physiology, Thyroid Hormone. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK500006/ (accessed on 5 June 2023).
- Almughamsi, H.; Whalen, M.M. Hexabromocyclododecane and tetrabromobisphenol A alter secretion of interferon gamma (IFN-γ) from human immune cells. Arch. Toxicol. 2016, 90, 1695–1707. [Google Scholar] [CrossRef]
- Wu, M.; Wu, D.; Wang, C.; Guo, Z.; Li, B.; Zuo, Z. Hexabromocyclododecane exposure induces cardiac hypertrophy and arrhythmia by inhibiting miR-1 expression via up-regulation of the homeobox gene Nkx2.5. J. Hazard. Mater. 2016, 302, 304–313. [Google Scholar] [CrossRef]
- Maia, M.L.; Sousa, S.; Pestana, D.; Faria, A.; Teixeira, D.; Delerue-Matos, C.; Domingues, V.F.; Calhau, C. Impact of brominated flame retardants on lipid metabolism: An in vitro approach. Environ. Pollut. 2022, 294, 118639. [Google Scholar] [CrossRef]
- An, J.; Guo, P.; Shang, Y.; Zhong, Y.; Zhang, X.; Yu, Y.; Yu, Z. The “adaptive responses” of low concentrations of HBCD in L02 cells and the underlying molecular mechanisms. Chemosphere 2016, 145, 68–76. [Google Scholar] [CrossRef]
- Szabo, D.T.; Pathmasiri, W.; Sumner, S.; Birnbaum, L.S. Serum Metabolomic Profiles in Neonatal Mice following Oral Brominated Flame Retardant Exposures to Hexabromocyclododecane (HBCD) Alpha, Gamma, and Commercial Mixture. Environ. Health Perspect. 2017, 125, 651–659. [Google Scholar] [CrossRef]
- Shi, X.; Zha, J.; Wen, B.; Zhang, S. Diastereoisomer-specific neurotoxicity of hexabromocyclododecane in human SH-SY5Y neuroblastoma cells. Sci. Total Environ. 2019, 686, 893–902. [Google Scholar] [CrossRef]
- Reistad, T.; Fonnum, F.; Mariussen, E. Neurotoxicity of the pentabrominated diphenyl ether mixture, DE-71, and hexabromocyclododecane (HBCD) in rat cerebellar granule cells in vitro. Arch. Toxicol. 2006, 80, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Al-Mousa, F.; Michelangeli, F. Some commonly used brominated flame retardants cause Ca2+-ATPase inhibition, beta-amyloid peptide release and apoptosis in SH-SY5Y neuronal cells. PLoS ONE 2012, 7, e33059. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.L.; De Sesso, J.M. The potential of selected brominated flame retardants to affect neurological development. J. Toxicol. Environ. Health B Crit. Rev. 2010, 13, 411–448. [Google Scholar] [CrossRef] [PubMed]
- Maranghi, F.; Tassinari, R.; Moracci, G.; Altieri, I.; Rasinger, J.D.; Carroll, T.S.; Hogstrand, C.; Lundebye, A.K.; Mantovani, A. Dietary exposure of juvenile female mice to polyhalogenated seafood contaminants (HBCD, BDE-47, PCB-153, TCDD): Comparative assessment of effects in potential target tissues. Food Chem. Toxicol. 2013, 56, 443–449. [Google Scholar] [CrossRef]
- Rasinger, J.D.; Carroll, T.S.; Lundebye, A.K.; Hogstrand, C. Crossomics gene and protein expression profiling in juvenile female mice highlights disruption of calcium and zinc signalling in the brain following dietary exposure to CB-153, BDE-47, HBCD or TCDD. Toxicology 2014, 321, 1–12. [Google Scholar] [CrossRef]
- Huang, X.; Chen, C.; Shang, Y.; Zhong, Y.; Ren, G.; Yu, Z.; An, J. In vitro study on the biotransformation and cytotoxicity of three hexabromocyclododecane diastereoisomers in liver cells. Chemosphere 2016, 161, 251–258. [Google Scholar] [CrossRef]
- Du, M.; Lin, L.; Yan, C.; Zhang, X. Diastereoisomer- and Enantiomer-Specific Accumulation, Depuration, and Bioisomerization of Hexabromocyclododecanes in Zebrafish (Danio rerio). Environ. Sci. Technol. 2012, 46, 11040–11046. [Google Scholar] [CrossRef]
- Du, M.; Fang, C.; Qiu, L.; Dong, S.; Zhang, X.; Yan, C. Diastereoisomer-specific effects of hexabromocyclododecanes on hepatic aryl hydrocarbon receptors and cytochrome P450s in zebrafish (Danio rerio). Chemosphere 2015, 132, 24–31. [Google Scholar] [CrossRef]
- Reffatto, V.; Rasinger, J.D.; Carroll, T.S.; Ganay, T.; Lundebye, A.K.; Sekler, I.; Hershfinkel, M.; Hogstrand, C. Parallel in vivo and in vitro transcriptomics analysis reveals calcium and zinc signalling in the brain as sensitive targets of HBCD neurotoxicity. Arch. Toxicol. 2018, 92, 1189–1203. [Google Scholar] [CrossRef]
- Genskow, K.R.; Bradner, J.M.; Hossain, M.M.; Richardson, J.R.; Caudle, W.M. Selective damage to dopaminergic transporters following exposure to the brominated flame retardant, HBCDD. Neurotoxicology Teratol. 2015, 52, 162–169. [Google Scholar] [CrossRef]
- Li, H.; Mo, L.; Yu, Z.; Sheng, G.; Fu, J. Levels, isomer profiles and chiral signatures of particle-bound hexabromocyclododecanes in ambient air around Shanghai, China. Environ. Pollut. 2012, 165, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Janák, K.; Covaci, A.; Voorspoels, S.; Becher, G. Hexabromocyclododecane in Marine Species from the Western Scheldt Estuary: Diastereoisomer- and Enantiomer-Specific Accumulation. Environ. Sci. Technol. 2005, 39, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-Z.; Xiang, N.; Duan, Y.-P.; Chen, L.; Zeng, E.Y. Hexabromocyclododecane in consumer fish from South China: Implications for human exposure via dietary intake. Environ. Toxicol. Chem. 2012, 31, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, N.; Chang, H.; Shen, Y.; Li, Z.; Wei, W.; Chen, H.; Lu, H.; Ji, J.; Liu, N. Dual effects of thyroid hormone on neurons and neurogenesis in traumatic brain injury. Cell Death Dis. 2020, 11, 671. [Google Scholar] [CrossRef]
- Chao, H.; Lin, C.; Zuo, Q.; Liu, Y.; Xiao, M.; Xu, X.; Li, Z.; Bao, Z.; Chen, H.; You, Y.; et al. Cardiolipin-Dependent Mitophagy Guides Outcome after Traumatic Brain Injury. J. Neurosci. 2019, 39, 1930–1943. [Google Scholar] [CrossRef]
- Shi, X.; Wen, B.; Huang, H.; Zhang, S. Cytotoxicity of hexabromocyclodode cane,1,2-dibromo-4-(1,2-dibromoethyl) cyclohexane and 1,2,5,6-tetrabromocy clooctane in human SH-SY5Y neuroblastoma cells. Sci. Total Environ. 2020, 739, 139650. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Harrad, S. Tetrabromobisphenol-A, hexabromocyclododecane and its degradation products in UK human milk: Relationship to external exposure. Environ. Int. 2011, 37, 443–448. [Google Scholar] [CrossRef]
- Zheng, X.; Erratico, C.; Abdallah, M.A.; Negreira, N.; Luo, X.; Mai, B.; Covaci, A. In vitro metabolism of BDE-47, BDE-99, and α-, β-, γ-HBCD isomers by chicken liver microsomes. Environ. Res. 2015, 143, 221–228. [Google Scholar] [CrossRef]
- Zheng, X.; Erratico, C.; Luo, X.; Mai, B.; Covaci, A. Oxidative metabolism of BDE-47, BDE-99, and HBCDs by cat liver microsomes: Implications of cats as sentinel species to monitor human exposure to environmental pollutants. Chemosphere 2016, 151, 30–36. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, F.; Xu, C.; Liu, W.; Wen, S.; Xu, Y. Cytotoxicity evaluation of three pairs of hexabromocyclododecane (HBCD) enantiomers on Hep G2 cell. Toxicol. Vitr. 2008, 22, 1520–1527. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, F.; Zhang, X.; Xu, Y.; Liao, T.; Song, S.; Wang, J. Induction of hepatic enzymes and oxidative stress in Chinese rare minnow (Gobiocypris rarus) exposed to waterborne hexabromocyclododecane (HBCDD). Aquat. Toxicol. 2008, 86, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Fa, S.; Pogrmic-Majkic, K.; Samardzija, D.; Hrubik, J.; Glisic, B.; Kovacevic, R.; Andric, N. HBCDD-induced sustained reduction in mitochondrial membrane potential, ATP and steroidogenesis in peripubertal rat Leydig cells. Toxicol. Appl. Pharmacol. 2015, 282, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Lu, G.; Yan, Z.; Liu, J.; Nkoom, M.; Yang, H. Responses of antioxidant and biotransformation enzymes in Carassius carassius exposed to hexabromocyclododecane. Environ. Toxicol. Pharmacol. 2018, 62, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Santabarbara-Ruiz, P.; Lopez-Santillan, M.; Martinez-Rodriguez, I.; Binagui-Casas, A.; Perez, L.; Milan, M.; Corominas, M.; Serras, F. ROS-Induced JNK and p38 Signaling Is Required for Unpaired Cytokine Activation during Drosophila Regeneration. PLoS Genet. 2015, 11, e1005595. [Google Scholar] [CrossRef]
- Xiong, S.; Mu, T.; Wang, G.; Jiang, X. Mitochondria-mediated apoptosis in mammals. Protein Cell 2014, 5, 737–749. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef]
- Manns, J.; Daubrawa, M.; Driessen, S.; Paasch, F.; Hoffmann, N.; Loffler, A.; Lauber, K.; Dieterle, A.; Alers, S.; Iftner, T.; et al. Triggering of a novel intrinsic apoptosis pathway by the kinase inhibitor staurosporine: Activation of caspase-9 in the absence of Apaf-1. FASEB J. 2011, 25, 3250–3261. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Shinoda, N.; Chihara, T.; Miura, M. ROS Regulate Caspase-Dependent Cell Delamination without Apoptosis in the Drosophila Pupal Notum. iScience 2020, 23, 101413. [Google Scholar] [CrossRef]
- Shemorry, A.; Harnoss, J.M.; Guttman, O.; Marsters, S.A.; Kőműves, L.G.; Lawrence, D.A.; Ashkenazi, A. Caspase-mediated cleavage of IRE1 controls apoptotic cell commitment during endoplasmic reticulum stress. eLife 2019, 8, e47084. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Wei, Q.; Gong, Z.; Huang, Y.; Liu, H.; Li, Y.; Peng, X. Effect of norcantharidin on the proliferation, apoptosis, and cell cycle of human mesangial cells. Ren. Fail. 2017, 39, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, X.; Zhu, R.; Dai, M.; Zhu, M.; Shen, Y.; Fang, H.; Sang, A.; Chen, H. Involvement of Fra-1 in Retinal Ganglion Cell Apoptosis in Rat Light-Induced Retina Damage Model. Cell. Mol. Neurobiol. 2017, 37, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Bastos Sales, L.; Kamstra, J.H.; Cenijn, P.H.; van Rijt, L.S.; Hamers, T.; Legler, J. Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation. Toxicol. Vitr. 2013, 27, 1634–1643. [Google Scholar] [CrossRef]
- Schreiber, T.; Gassmann, K.; Götz, C.; Hübenthal, U.; Moors, M.; Krause, G.; Merk, H.F.; Nguyen, N.; Scanlan, T.S.; Abel, J.; et al. Polybrominated Diphenyl Ethers Induce Developmental Neurotoxicity in a Human in Vitro Model: Evidence for Endocrine Disruption. Environ. Health Perspect. 2010, 118, 572–578. [Google Scholar] [CrossRef]
- Lin, C.; Chen, T.; Chen, S.; Hsiao, P.; Yang, R. Activation of Trim17 by PPARγ is involved in Di(2-ethylhexyl) phthalate (DEHP)-induced apoptosis on Neuro-2a cells. Toxicol. Lett. 2011, 206, 245–251. [Google Scholar] [CrossRef]
| Primer Name | Primers Sequence (5′→ 3′) |
|---|---|
| mOGG1 | F: GCATCGTACTCTAGCCTCCAC |
| R: CCTCCGTCTGAGTCAGTGTCC | |
| Caspase-3 | F: CTGACTGGAAAGCCGAAACTC |
| R: CGACCCGTCCTTTGAATTTCT | |
| Caspase-9 | F: GGCTGTTAAACCCCTAGACCA |
| R: TGACGGGTCCAGCTTCACTA | |
| Bcl-2 | F: GTCGCTACCGTCGTGACTTC |
| R: CAGACATGCACCTACCCAGC |
| Diastereomers | Chemical Dose (μM) | MDA (nmol/mg Protein) | GSH (U/mg Protein) |
|---|---|---|---|
| α-HBCD | Control | 5.19 ± 0.19 | 196.71 ± 14.26 |
| 1 | 5.63 ± 0.81 | 184.88 ± 4.72 | |
| 10 | 8.62 ± 0.38 ** | 137.46 ± 29.17 ** | |
| 50 | 11.34 ± 1.22 ** | 109.33 ± 9.01 ** | |
| β-HBCD | Control | 3.67 ± 0.87 | 205.50 ± 58.40 |
| 1 | 3.68 ± 1.06 | 207.33 ± 57.29 | |
| 5 | 7.55 ± 2.11 * | 116.75 ± 14.43 * | |
| 10 | 9.69 ± 2.88 ** | 86.89 ± 28.21 * |
| Diastereomers | Chemical Dose (μM) | G0/G1 (%) | S (%) | G2/M (%) | G2 + S (%) |
|---|---|---|---|---|---|
| α-HBCD | Control | 59.2 ± 4.6 | 33.2 ± 5.6 | 7.6 ± 1.5 | 40.8 ± 4.9 |
| 1 | 60.7 ± 2.5 | 31.0 ± 3.2 | 8.4 ± 1.0 | 39.3 ± 2.5 | |
| 10 | 71.8 ± 4.67 ** | 12.0 ± 5.3 ** | 16.3 ± 5.0 | 28.2 ± 4.7 * | |
| 50 | 41.0 ± 6. 4 ** | 32.8 ± 3.2 | 26.3 ± 6.1 | 59.0 ± 6.4 ** | |
| β-HBCD | Control | 63.2 ± 3.7 | 32.3 ± 4.2 | 4.5 ± 3.9 | 36.8 ± 3.7 |
| 1 | 64.5 ± 4.9 | 30.4 ± 5.2 | 5.1 ± 4.3 | 35.5 ± 4.9 | |
| 5 | 62.0 ± 6.5 | 18.2 ± 4.6 ** | 19.8 ± 5.5 ** | 38.0 ± 6.5 | |
| 10 | 36.0 ± 5.0 ** | 29.8 ± 1.8 | 30.0 ± 3.7 ** | 59.8 ± 5.1 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, K.; Wu, D.; Xie, G.; Li, Y.; Zhang, J. Different Cytotoxicity Induced by Hexabromocyclododecanes on Mouse Neuroblastoma N2a Cells via Oxidative Stress and Mitochondrial Apoptotic Pathway. Toxics 2024, 12, 665. https://doi.org/10.3390/toxics12090665
Wan K, Wu D, Xie G, Li Y, Zhang J. Different Cytotoxicity Induced by Hexabromocyclododecanes on Mouse Neuroblastoma N2a Cells via Oxidative Stress and Mitochondrial Apoptotic Pathway. Toxics. 2024; 12(9):665. https://doi.org/10.3390/toxics12090665
Chicago/Turabian StyleWan, Keyan, Dongting Wu, Guangshan Xie, Yunxiu Li, and Jianqing Zhang. 2024. "Different Cytotoxicity Induced by Hexabromocyclododecanes on Mouse Neuroblastoma N2a Cells via Oxidative Stress and Mitochondrial Apoptotic Pathway" Toxics 12, no. 9: 665. https://doi.org/10.3390/toxics12090665
APA StyleWan, K., Wu, D., Xie, G., Li, Y., & Zhang, J. (2024). Different Cytotoxicity Induced by Hexabromocyclododecanes on Mouse Neuroblastoma N2a Cells via Oxidative Stress and Mitochondrial Apoptotic Pathway. Toxics, 12(9), 665. https://doi.org/10.3390/toxics12090665






