Multiphase Partitioning of Estrogens in a River Impacted by Feedlot Wastewater Discharge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
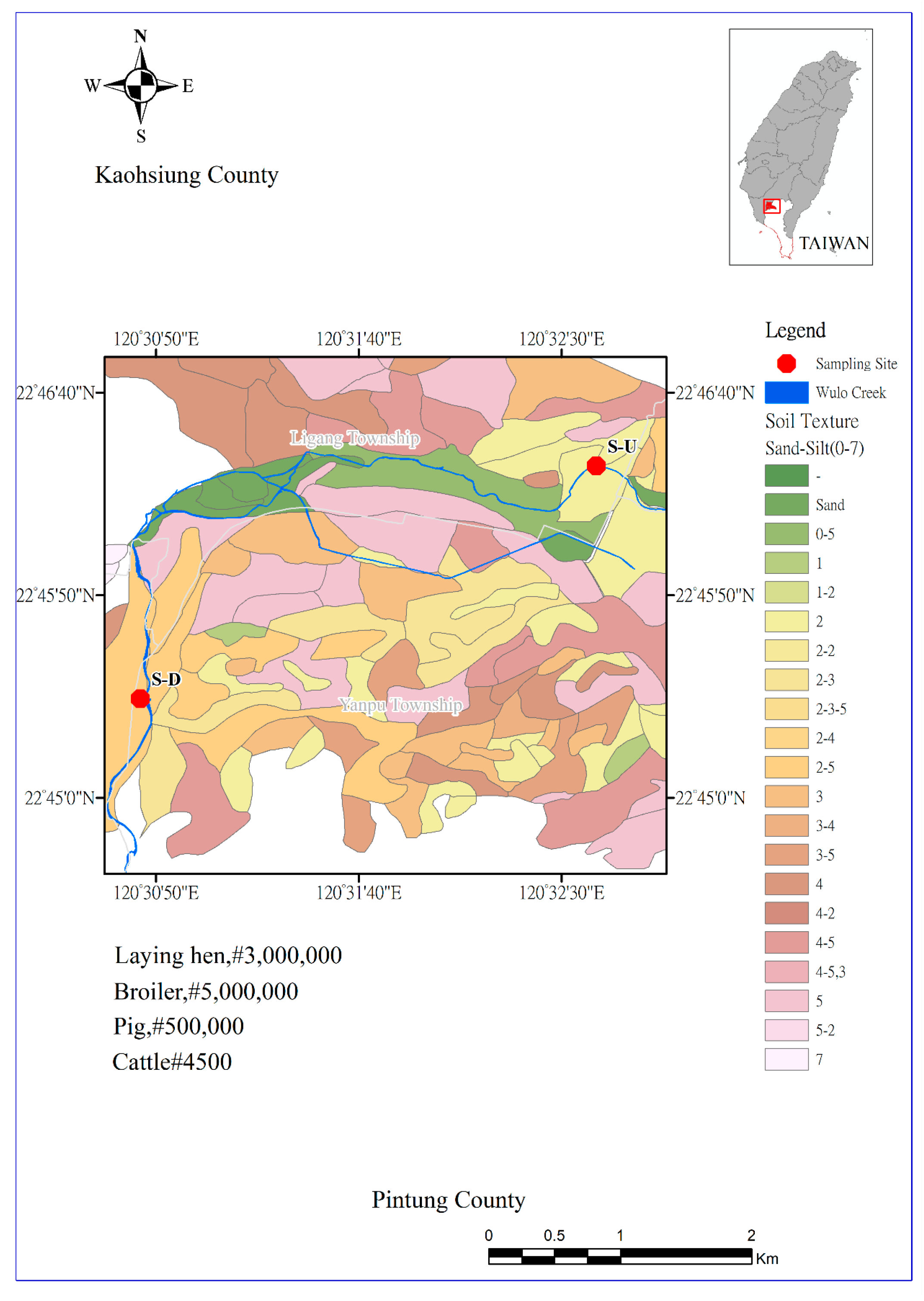
2.2. Sampling Site
2.3. Sampling Procedure and Treatment
2.4. The Separation Process of Water Samples
2.5. Pretreatment of Liquid and SPM Samples for Estrogen Analysis
2.6. LC/MS/MS Analysis Conditions
2.7. Quality Assurance and Control
2.8. Partition Coefficient Calculation
2.9. 17β-Estradiol Equivalent (EEQ) Calculation
2.10. Statistical Analysis
3. Results
3.1. Dissolved Organic Carbon Concentrations in Liquid Samples
3.2. Estrogen Concentrations in Water Samples
3.3. Estrogen Concentrations in SPM
3.4. Multiphase Partition Coefficient of Estrogens
3.5. Risk Assessment of Estrogens in Filtrate Water and SPM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Ciślak, M.; Kruszelnicka, I.; Zembrzuska, J.; Ginter-Kramarczyk, D. Estrogen pollution of the European aquatic environment: A critical review. Water Res. 2023, 229, 119413. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Fan, G.; Yu, W.; Yang, S.; Zhou, J.; Luo, J. Occurrence and risk assessment of steroid estrogens in environmental water samples: A five-year worldwide perspective. Environ. Pollut. 2020, 267, 115405. [Google Scholar] [CrossRef] [PubMed]
- Ojoghoro, J.; Scrimshaw, M.; Sumpter, J. Steroid hormones in the aquatic environment. Sci. Total Environ. 2021, 792, 148306. [Google Scholar] [CrossRef] [PubMed]
- Grzegorzek, M.; Wartalska, K.; Kowalik, R. Occurrence and sources of hormones in water resources—Environmental and health impact. Environ. Sci. Pollut. Res. 2024, 31, 37907–37922. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yates, S.R. Dissolved organic matter and estrogen interactions regulate estrogen removal in the aqueous environment: A review. Sci. Total Environ. 2018, 640, 529–542. [Google Scholar] [CrossRef]
- Zhong, R.; Zou, H.; Gao, J.; Wang, T.; Bu, Q.; Wang, Z.-L.; Hu, M.; Wang, Z. A critical review on the distribution and ecological risk assessment of steroid hormones in the environment in China. Sci. Total Environ. 2021, 786, 147452. [Google Scholar] [CrossRef]
- Arnon, S.; Dahan, O.; Elhanany, S.; Cohen, K.; Pankratov, I.; Gross, A.; Ronen, Z.; Baram, S.; Shore, L.S. Transport of testosterone and estrogen from dairy-farm waste lagoons to groundwater. Environ. Sci. Technol. 2008, 42, 5521–5526. [Google Scholar] [CrossRef]
- Jobling, S.; Williams, R.; Johnson, A.; Taylor, A.; Gross-Sorokin, M.; Nolan, M.; Tyler, C.R.; van Aerle, R.; Santos, E.; Brighty, G. Predicted exposures to steroid estrogens in UK rivers correlate with widespread sexual disruption in wild fish populations. Environ. Health Perspect. 2006, 114, 32–39. [Google Scholar] [CrossRef]
- Johnson, A.; Williams, R.; Matthiessen, P. The potential steroid hormone contribution of farm animals to freshwaters, the United Kingdom as a case study. Sci. Total Environ. 2006, 362, 166–178. [Google Scholar] [CrossRef]
- Hutchins, S.R.; White, M.V.; Hudson, F.M.; Fine, D.D. Analysis of lagoon samples from different concentrated animal feeding operations for estrogens and estrogen conjugates. Environ. Sci. Technol. 2007, 41, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Lafrance, P.; Caron, E. Impact of recent manure applications on natural estrogen concentrations in streams near agricultural fields. Environ. Res. 2013, 126, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Rechsteiner, D.; Wettstein, F.E.; Pfeiffer, N.; Hollender, J.; Bucheli, T.D. Natural estrogen emissions to subsurface tile drains from experimental grassland fields in Switzerland after application of livestock slurries and free compounds. Sci. Total Environ. 2021, 779, 146351. [Google Scholar] [CrossRef] [PubMed]
- Casey, F.X.; Hakk, H.; DeSutter, T.M. Free and conjugated estrogens detections in drainage tiles and wells beneath fields receiving swine manure slurry. Environ. Pollut. 2020, 256, 113384. [Google Scholar] [CrossRef] [PubMed]
- Havens, S.M.; Hedman, C.J.; Hemming, J.D.; Mieritz, M.G.; Shafer, M.M.; Schauer, J.J. Occurrence of estrogens, androgens and progestogens and estrogenic activity in surface water runoff from beef and dairy manure amended crop fields. Sci. Total Environ. 2020, 710, 136247. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, X.; Xu, D.; Xiang, Y.; Ling, W.; Chen, M. Contamination and risk assessment of estrogens in livestock manure: A case study in Jiangsu Province, China. Int. J. Environ. Res. Public Health 2018, 15, 125. [Google Scholar] [CrossRef]
- Gomes, F.B.R.; Fernandes, P.A.A.; Bottrel, S.E.C.; Brandt, E.M.F.; Pereira, R.d.O. Fate, occurrence, and removal of estrogens in livestock wastewaters. Water Sci. Technol. 2022, 86, 814–833. [Google Scholar] [CrossRef]
- Tang, Z.; Wan, Y.-p.; Liu, Z.-h.; Wang, H.; Dang, Z.; Liu, Y. Twelve natural estrogens in urines of swine and cattle: Concentration profiles and importance of eight less-studied. Sci. Total Environ. 2022, 803, 150042. [Google Scholar] [CrossRef]
- Combalbert, S.; Hernandez-Raquet, G. Occurrence, fate, and biodegradation of estrogens in sewage and manure. Appl. Microbiol. Biot. 2010, 86, 1671–1692. [Google Scholar] [CrossRef]
- Hanselman, T.A.; Graetz, D.A.; Wilkie, A.C. Manure-borne estrogens as potential environmental contaminants: A review. Environ. Sci. Technol. 2003, 37, 5471–5478. [Google Scholar] [CrossRef]
- Khanal, S.K.; Xie, B.; Thompson, M.L.; Sung, S.; Ong, S.-K.; Van Leeuwen, J. Fate, transport, and biodegradation of natural estrogens in the environment and engineered systems. Environ. Sci. Technol. 2006, 40, 6537–6546. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yang, Y.; Zhou, J.; Nie, M.; Liu, M.; Hochella Jr, M.F. Selected emerging organic contaminants in the Yangtze Estuary, China: A comprehensive treatment of their association with aquatic colloids. J. Hazard. Mater. 2015, 283, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, R.; Wilding, A.; Hibberd, A. Sorption of selected endocrine disrupting chemicals to different aquatic colloids. Environ. Sci. Technol. 2007, 41, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wilding, A.; Hibberd, A.; Zhou, J.L. Partition of endocrine-disrupting chemicals between colloids and dissolved phase as determined by cross-flow ultrafiltration. Environ. Sci. Technol. 2005, 39, 2753–2761. [Google Scholar] [CrossRef]
- Carballa, M.; Fink, G.; Omil, F.; Lema, J.M.; Ternes, T. Determination of the solid–water distribution coefficient (Kd) for pharmaceuticals, estrogens and musk fragrances in digested sludge. Water Res. 2008, 42, 287–295. [Google Scholar] [CrossRef]
- Lee, J.; Cho, J.; Kim, S.H.; Kim, S.D. Influence of 17β-estradiol binding by dissolved organic matter isolated from wastewater effluent on estrogenic activity. Ecotoxicol. Environ. Saf. 2011, 74, 1280–1287. [Google Scholar] [CrossRef]
- Yan, C.; Nie, M.; Yang, Y.; Zhou, J.; Liu, M.; Baalousha, M.; Lead, J.R. Effect of colloids on the occurrence, distribution and photolysis of emerging organic contaminants in wastewaters. J. Hazard. Mater. 2015, 299, 241–248. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, X.; Zhou, L.J.; Ji, X.; Gao, B.; Xu, G.Z.; Li, A. Multi-phase distribution and risk assessment of endocrine disrupting chemicals in the surface water of the Shaying River,-Huai River Basin, China. Ecotoxicol. Environ. Saf. 2019, 173, 45–53. [Google Scholar] [CrossRef]
- Nie, M.; Yang, Y.; Liu, M.; Yan, C.; Shi, H.; Dong, W.; Zhou, J.L. Environmental estrogens in a drinking water reservoir area in Shanghai: Occurrence, colloidal contribution and risk assessment. Sci. Total Environ. 2014, 487, 785–791. [Google Scholar] [CrossRef]
- Chen, T.-C.; Yeh, K.-J.C.; Kuo, W.-C.; Chao, H.-R.; Sheu, S.-C. Estrogen degradation and sorption onto colloids in a constructed wetland with different hydraulic retention times. J. Hazard. Mater. 2014, 277, 62–68. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Qin, L.; Xie, X.; Gao, B.; Sun, J.; Li, A. Distribution of endocrine-disrupting chemicals in colloidal and soluble phases in municipal secondary effluents and their removal by different advanced treatment processes. Chemosphere 2019, 219, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Gudda, F.O.; Ateia, M.; Waigi, M.G.; Wang, J.; Gao, Y. Ecological and human health risks of manure-borne steroid estrogens: A 20-year global synthesis study. J. Environ. Manag. 2022, 301, 113708. [Google Scholar] [CrossRef] [PubMed]
- Neale, P.A.; Escher, B.I.; Schäfer, A.I. pH dependence of steroid hormone—Organic matter interactions at environmental concentrations. Sci. Total Environ. 2009, 407, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Huang, Y.; Huang, W.; Ran, Y.; Chen, D. Multiphase partitioning and risk assessment of endocrine-disrupting chemicals in the Pearl River, China. Environ. Toxicol. Chem. 2016, 35, 2474–2482. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-S.; Chen, T.-C.; Yeh, K.-J.C.; Chao, H.-R.; Liaw, E.-T.; Hsieh, C.-Y.; Chen, K.-C.; Hsieh, L.-T.; Yeh, Y.-L. High estrogen concentrations in receiving river discharge from a concentrated livestock feedlot. Sci. Total Environ. 2010, 408, 3223–3230. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-S.; Yeh, K.-J.C.; Chen, T.-C. Investigation of free and conjugated estrogen fate and emission coefficients in three duck farms. Environ. Sci. Pollut. Res. 2023, 30, 9874–9885. [Google Scholar] [CrossRef]
- Hung, H.-S.; Yeh, K.-J.C.; Hsieh, C.-Y.; Chen, T.-C. Occurrence and Degradation of Free and Conjugated Estrogens in a River Receiving Feedlot Animal Discharge. Appl. Sci. 2022, 12, 11961. [Google Scholar] [CrossRef]
- Chuang, C.-W.; Hsu, L.-F.; Tsai, H.-C.; Liu, Y.-Y.; Huang, W.-S.; Chen, T.-C. Nickel Binding Affinity with Size-Fractioned Sediment Dissolved and Particulate Organic Matter and Correlation with Optical Indicators. Appl. Sci. 2020, 10, 8995. [Google Scholar] [CrossRef]
- Isobe, T.; Shiraishi, H.; Yasuda, M.; Shinoda, A.; Suzuki, H.; Morita, M. Determination of estrogens and their conjugates in water using solid-phase extraction followed by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2003, 984, 195–202. [Google Scholar] [CrossRef]
- Kumar, V.; Nakada, N.; Yasojima, M.; Yamashita, N.; Johnson, A.C.; Tanaka, H. Rapid determination of free and conjugated estrogen in different water matrices by liquid chromatography–tandem mass spectrometry. Chemosphere 2009, 77, 1440–1446. [Google Scholar] [CrossRef]
- Bartelt-Hunt, S.L.; Snow, D.D.; Kranz, W.L.; Mader, T.L.; Shapiro, C.A.; Donk, S.J.v.; Shelton, D.P.; Tarkalson, D.D.; Zhang, T.C. Effect of growth promotants on the occurrence of endogenous and synthetic steroid hormones on feedlot soils and in runoff from beef cattle feeding operations. Environ. Sci. Technol. 2012, 46, 1352–1360. [Google Scholar] [CrossRef]
- Bevacqua, C.E.; Rice, C.P.; Torrents, A.; Ramirez, M. Steroid hormones in biosolids and poultry litter: A comparison of potential environmental inputs. Sci. Total Environ. 2011, 409, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yates, S.R.; Bradford, S.A. Analysis of steroid hormones in a typical dairy waste disposal system. Environ. Sci. Technol. 2008, 42, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ying, G.; Chen, F.; Liu, Y.; Wang, L.; Yang, B.; Liu, S.; Tao, R. Estrogenic activity profiles and risks in surface waters and sediments of the Pearl River system in south China assessed by chemical analysis and in vitro bioassay. J. Environ. Monit. 2011, 13, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Yang, F.; Xu, Y.; Dai, H.; Liu, W. Risk assessment of xenoestrogens in a typical sewage-holding lake in China. Chemosphere 2013, 93, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Beck, I.-C.; Bruhn, R.; Gandrass, J. Analysis of estrogenic activity in coastal surface waters of the Baltic Sea using the yeast estrogen screen. Chemosphere 2006, 63, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-W.; Huang, W.-S.; Chen, H.-S.; Hsu, L.-F.; Liu, Y.-Y.; Chen, T.-C. Sorption Constant of Bisphenol A and Octylphenol Onto Size-Fractioned Dissolved Organic Matter Using a Fluorescence Method. Int. J. Environ. Res. Public Health 2021, 18, 1102. [Google Scholar] [CrossRef]
- Ilina, S.M.; Lapitskiy, S.A.; Alekhin, Y.V.; Viers, J.; Benedetti, M.; Pokrovsky, O.S. Speciation, size fractionation and transport of trace elements in the continuum soil water–mire–humic lake–river–large oligotrophic lake of a Subarctic watershed. Aquat. Geochem. 2016, 22, 65–95. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Lin, Y.-S.; Yen, C.-H.; Miaw, C.-L.; Chen, T.-C.; Wu, M.-C.; Hsieh, C.-Y. Identification, contribution, and estrogenic activity of potential EDCs in a river receiving concentrated livestock effluent in Southern Taiwan. Sci. Total Environ. 2018, 636, 464–476. [Google Scholar] [CrossRef]
- Sutaswiriya, N.; Homklin, S.; Kreetachat, T.; Vaithanomsat, P.; Kreetachat, N. Monitoring estrogen and androgen residues from livestock farms in Phayao Lake, Thailand. Environ. Monit. Assess. 2021, 193, 812. [Google Scholar] [CrossRef]
- Yamamoto, H.; Liljestrand, H.M.; Shimizu, Y.; Morita, M. Effects of physical− chemical characteristics on the sorption of selected endocrine disruptors by dissolved organic matter surrogates. Environ. Sci. Technol. 2003, 37, 2646–2657. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, R.D.; Love, N.G.; Novak, J.T. Sorption of 17β-estradiol and 17α-ethinylestradiol by colloidal organic carbon derived from biological wastewater treatment systems. Environ. Sci. Technol. 2004, 38, 3322–3329. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.-G.; Williams, B.; Kookana, R. Environmental fate of alkylphenols and alkylphenol ethoxylates—A review. Environ. Int. 2002, 28, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Holthaus, K.I.; Johnson, A.C.; Jürgens, M.D.; Williams, R.J.; Smith, J.J.; Carter, J.E. The potential for estradiol and ethinylestradiol to sorb to suspended and bed sediments in some English rivers. Environ. Toxicol. Chem. Int. J. 2002, 21, 2526–2535. [Google Scholar] [CrossRef]
- Nie, M.; Yan, C.; Dong, W.; Liu, M.; Zhou, J.; Yang, Y. Occurrence, distribution and risk assessment of estrogens in surface water, suspended particulate matter, and sediments of the Yangtze Estuary. Chemosphere 2015, 127, 109–116. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Zhang, S.-H.; Ji, G.-X.; Wu, S.-M.; Guo, R.-X.; Cheng, J.; Yan, Z.-Y.; Chen, J.-Q. Occurrence, distribution and risk assessment of suspected endocrine-disrupting chemicals in surface water and suspended particulate matter of Yangtze River (Nanjing section). Ecotoxicol. Environ. Saf. 2017, 135, 90–97. [Google Scholar] [CrossRef]
- Holbrook, R.D.; Love, N.G.; Novak, J.T. Biological wastewater treatment and estrogenic endocrine disrupting compounds: Importance of colloid organic carbon. Pract. Period. Hazard. Toxic. Radioact. Waste Manag. 2003, 7, 289–296. [Google Scholar] [CrossRef]
- Qiao, X.; Carmosini, N.; Li, F.; Lee, L.S. Probing the primary mechanisms affecting the environmental distribution of estrogen and androgen isomers. Environ. Sci. Technol. 2011, 45, 3989–3995. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, X.; Shen, Y.; Di, M.; Wang, J. Occurrence, behavior and risk assessment of estrogens in surface water and sediments from Hanjiang River, Central China. Ecotoxicology 2019, 28, 143–153. [Google Scholar] [CrossRef]
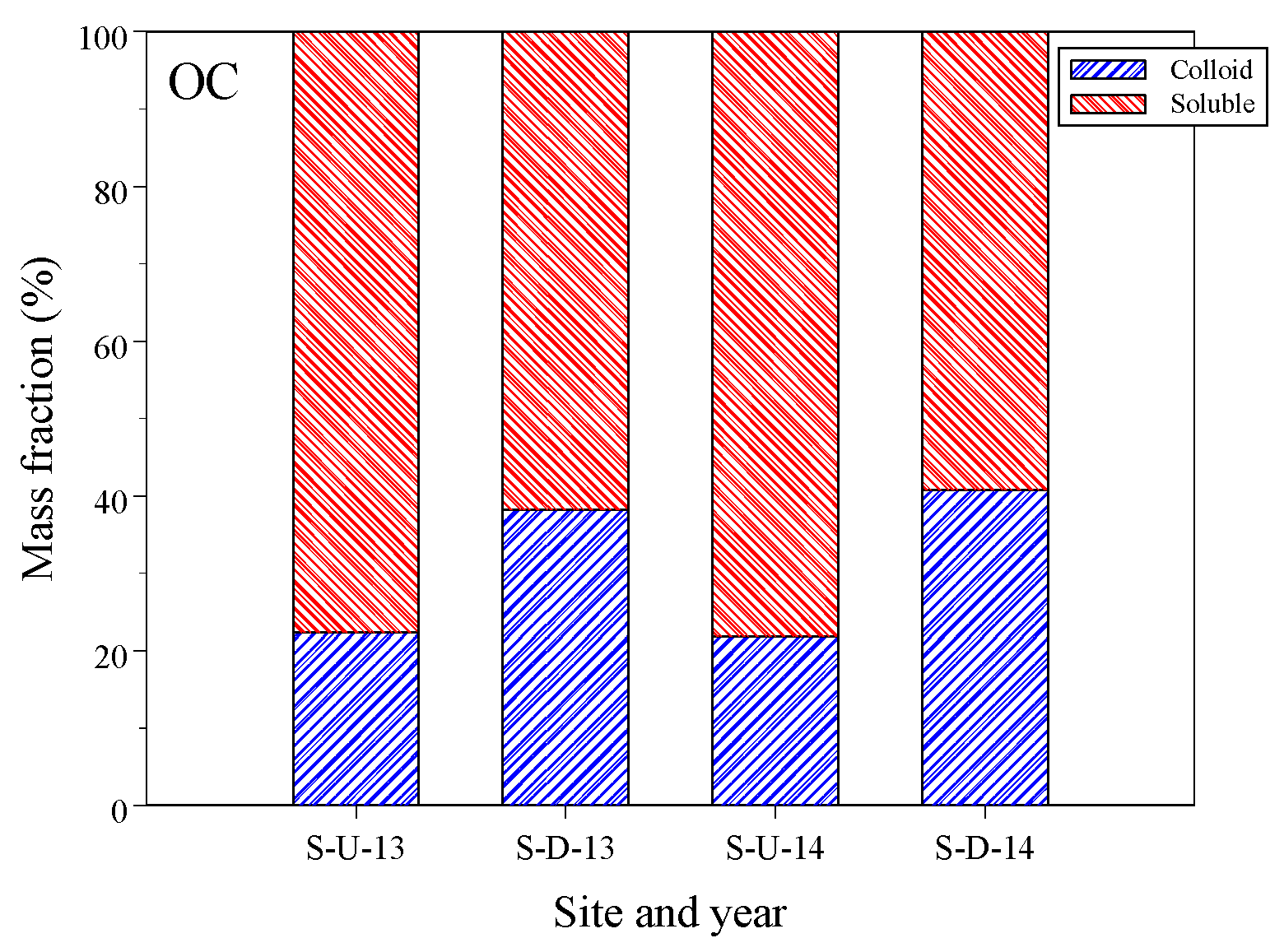

| Site | Phase | E1, ng/L | E2, ng/L | E3, ng/L | DOC, mg/L |
|---|---|---|---|---|---|
| S-U13 | Filtrate | 22.8 ± 11.9 | 4.1 ± 2.6 | 5.0 ± 1.6 | 27.8 ± 9.4 |
| Colloid | 25.6 ± 14.2 | 5.8 ± 4.8 | 6.8 ± 3.6 | 49.2 ± 15.2 | |
| Soluble | 16.4 ± 6.7 | 5.3 ± 4.4 | 3.8 ± 1.9 | 20.5 ± 9.7 | |
| S-D13 | Filtrate | 46.8 ± 32.5 | 4.2 ± 3.8 | 4.0 ± 3.8 | 11.6 ± 8.6 |
| Colloid | 53.0 ± 47.6 | 7.9 ± 4.4 | 6.0 ± 4.6 | 31.4 ± 13.6 | |
| Soluble | 36.7 ± 26.0 | 5.9 ± 2.7 | 4.4 ± 3.0 | 9.0 ± 9.1 | |
| S-U14 | Filtrate | 164.7 ± 108.6 | 6.09 ± 4.50 | 3.53 ± 1.01 | 12.4 ± 4.9 |
| Colloid | 239 ± 103 | 7.8 ± 2.5 | 5.5 ± 4.3 | 28.9 ± 19.9 | |
| Soluble | 201 ± 93 | 7.0 ± 5.6 | 4.0 ± 2.3 | 12.8 ± 5.0 | |
| S-D14 | Filtrate | 328.3 ± 171.0 | 6.48 ± 2.77 | 4.63 ± 1.05 | 4.84 ± 0.6 |
| Colloid | 377.1 ± 219.1 | 7.9 ± 3.6 | 6.5 ± 1.6 | 18.5 ± 3.9 | |
| Soluble | 275.3 ± 120.5 | 4.4 ± 1.4 | 5.0 ± 0.4 | 3.0 ± 0.4 |
| Site | SPM (mg/L) | TOC (%) | E1 (μg/kg) | E2 (μg/kg) | E3 (μg/kg) |
|---|---|---|---|---|---|
| S-U13 | 98 ± 14 | 33.5 ± 0.1 | 1.05 ± 1.21 | 0.30 ± 0.35 | 0.70 ± 0.81 |
| S-D13 | 35 ± 18 | 22.3 ± 11.3 | 2.27 ± 1.34 | 1.27 ± 1.04 | 2.13 ± 1.80 |
| S-U14 | 140 ± 32 | 16.4 ± 1.2 | 0.72 ± 1.11 | ND | 0.64 ± 1.28 |
| S-D14 | 40 ± 22 | 23.1 ± 5.9 | 1.65 ± 0.44 | ND | 1.40 ± 0.86 |
| Site | Coefficients | E1 | E2 | E3 |
|---|---|---|---|---|
| S-U13 | log KOC | 2.41 ± 0.02 | 2.58 ± 0.03 | 2.95 ± 0.03 |
| log KPOC | 2.58 ± 0.02 | 2.42 ± 0.05 | 3.09 ± 0.11 | |
| log KCOC | 4.48 ± 0.19 | 4.36 ± 0.19 | 4.57 ± 0.14 | |
| S-D13 | log KOC | 2.54 ± 0.57 | 3.46 ± 0.05 | 4.00 ± 0.07 |
| log KPOC | 2.64 ± 0.58 | 3.52 ± 0.01 | 4.02 ± 0.11 | |
| log KCOC | 4.70 ± 0.13 | 4.68 ± 0.08 | 4.70 ± 0.14 | |
| S-U14 | log KOC | 2.30 ± 0.12 | NA | 3.60 ± 0.07 |
| log KPOC | NA | NA | 3.53 ± 0.11 | |
| log KCOC | 4.75 ± 0.41 | 4.81 ± 0.54 | 4.94 ± 0.47 | |
| S-D14 | log KOC | 1.39 ± 0.31 | NA | 3.25 ± 0.21 |
| log KPOC | 1.46 ± 0.29 | NA | 3.18 ± 0.23 | |
| log KCOC | 4.85 ± 0.19 | 4.99 ± 0.29 | 4.85 ± 0.17 |
| E1 | E2 | E3 | Matrix and Method | Ref. | |
|---|---|---|---|---|---|
| log KOW | 3.43 | 3.94 | 2.81 | [50] | |
| log KOC | 1.08–2.96 (2.02, 17) * | 2.43–3.05 (2.74, 4) * | 2.80–4.07 (3.38, 11) * | In situ river water (CFUF) | PS |
| log KOC | 3.21 | 3.38 | 2.84 | In situ river water (CFUF) | [31] |
| log KOC | 1.67–3.46 | 2.27–2.46 | 3.53–4.07 | In situ river water (CFUF) | [29] |
| log KPOC | 1.23–2.97 (2.06, 14) * | 2.26–3.09 (2.69, 4) * | 2.85–4.19 (3.40, 13) * | In situ river water (CFUF) | PS |
| log KPOC | 1.75–3.60 | 2.48–2.60 | 3.79–4.49 | In situ river water (CFUF) | [29] |
| log KPOC | 3.35 | 3.45 | 2.94 | In situ river water (CFUF) | [31] |
| log KCOC | 4.23–5.26 (4.72, 22) * | 4.17–5.59 (4.75, 20) * | 4.08–5.45 (4.77, 20) * | In situ river water (CFUF) | PS |
| log KCOC | 4.18–4.85 | 3.96–4.2 | In situ river water (CFUF) | [24] | |
| log KCOC | 4.18–4.23 | 3.96–4.20 | River water (CFUF) | [23] | |
| log KCOC | 6.60–6.81 | 6.42–6.78 | 7.09–7.85 | In situ river water (CFUF) | [22] |
| log KCOC | 5.03 | 4.76 | 4.25 | In situ river water (CFUF) | [31] |
| log KCOC | 5.4 | 6.11 | In situ river water (CFUF) | [22] | |
| log KCOC | 7.09 | 7.58 | 7.8 | In situ river water (CFUF) | [27] |
| log KCOC | 4.08 | 4.04 | 4.11 | In situ river water (CFUF) | [30] |
| log KCOC | 4.57–4.94 | Commercial DOM (FQ) | [48] | ||
| log KCOC | 4.08–4.68 | Biological wastewater (FQ) | [54] | ||
| log KCOC | <3–5.25 | Biological wastewater (FQ) | [49] | ||
| log KCOC | 3.98 | 3.93–4.12 | Commercial DOM (FQ) | [55] | |
| log KCOC | 4.52–6.02 | 3.42–5.11 | Commercial DOM (SPME) | [33] |
| Species | EEQ of Filtrate | EEQ of SPM | ||
|---|---|---|---|---|
| Mean ± SD | Ranges | Mean ± SD | Ranges | |
| E1 | 38.2 ± 38.4 | 3.1–132.3 | 9.1 ± 12.4 | 0.4–40.6 |
| E2 | 5.4 ± 3.6 | 0.0–16.7 | 3.6 ± 1.5 | 2.2–5.1 |
| E3 | 0.0 ± 0.0 | 0.0–0.1 | 0.0 ± 0.0 | 0.0–0.0 |
| Sum EEQ | 43.7 ± 40.6 | 3.6–142.1 | 6.8 ± 11.0 | 0.0–40.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.-H.; Hung, H.-S.; Huang, W.-H.; Hsieh, C.-Y.; Chen, T.-C. Multiphase Partitioning of Estrogens in a River Impacted by Feedlot Wastewater Discharge. Toxics 2024, 12, 671. https://doi.org/10.3390/toxics12090671
Yang K-H, Hung H-S, Huang W-H, Hsieh C-Y, Chen T-C. Multiphase Partitioning of Estrogens in a River Impacted by Feedlot Wastewater Discharge. Toxics. 2024; 12(9):671. https://doi.org/10.3390/toxics12090671
Chicago/Turabian StyleYang, Kuo-Hui, Hao-Shen Hung, Wei-Hsiang Huang, Chi-Ying Hsieh, and Ting-Chien Chen. 2024. "Multiphase Partitioning of Estrogens in a River Impacted by Feedlot Wastewater Discharge" Toxics 12, no. 9: 671. https://doi.org/10.3390/toxics12090671







