Occupational Exposure of On-Shift Ottawa Firefighters to Flame Retardants and Polycyclic Aromatic Hydrocarbons
Abstract
1. Introduction
2. Materials and Methods
2.1. Fire Station Sample Collection
2.2. Firefighter Sample Collection
2.3. Chemicals and Standards
2.4. Materials
2.5. Sample Preparation
2.6. Analysis
2.7. Quality Assurance/Quality Control
2.8. Statistical Analysis
3. Results
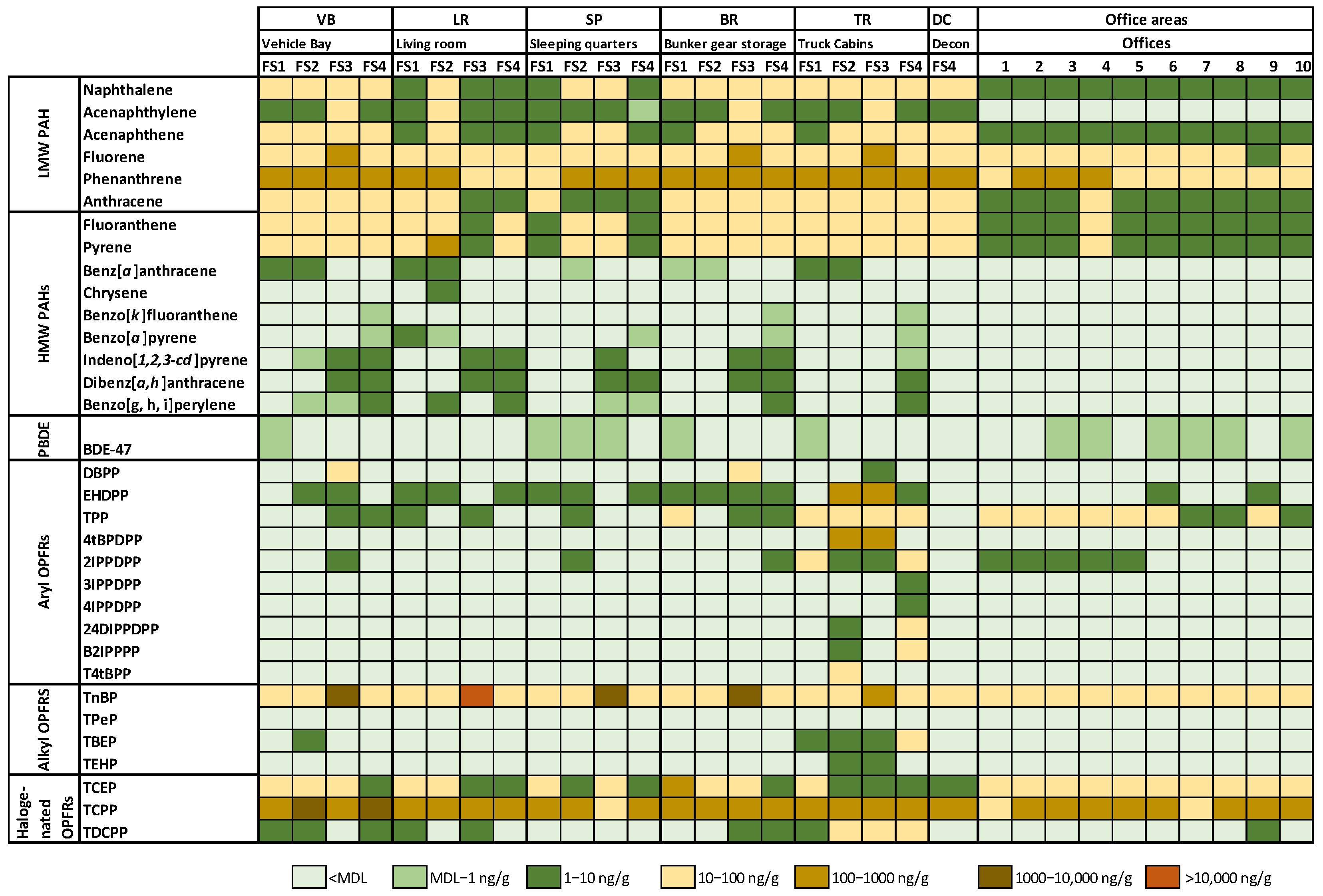
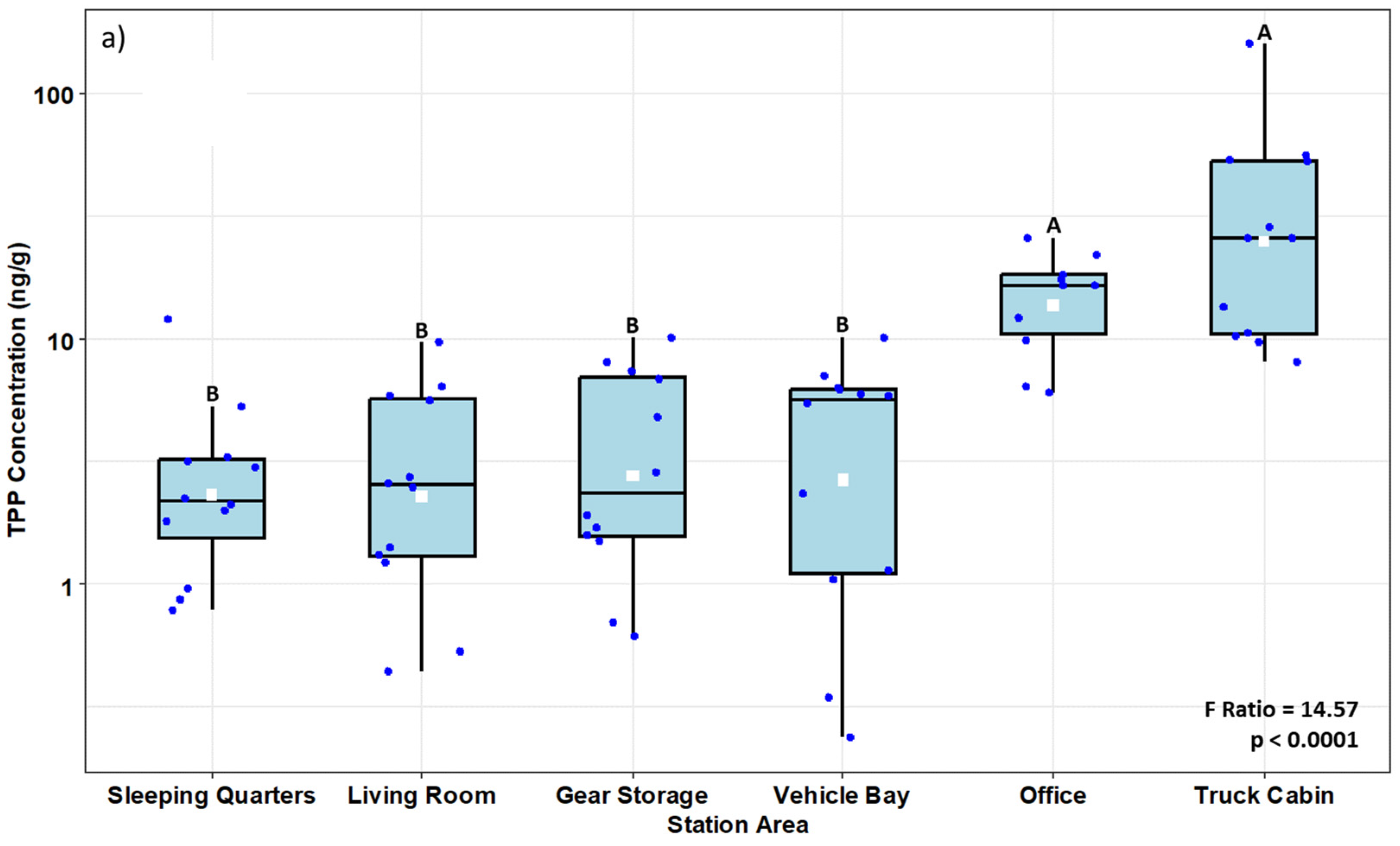
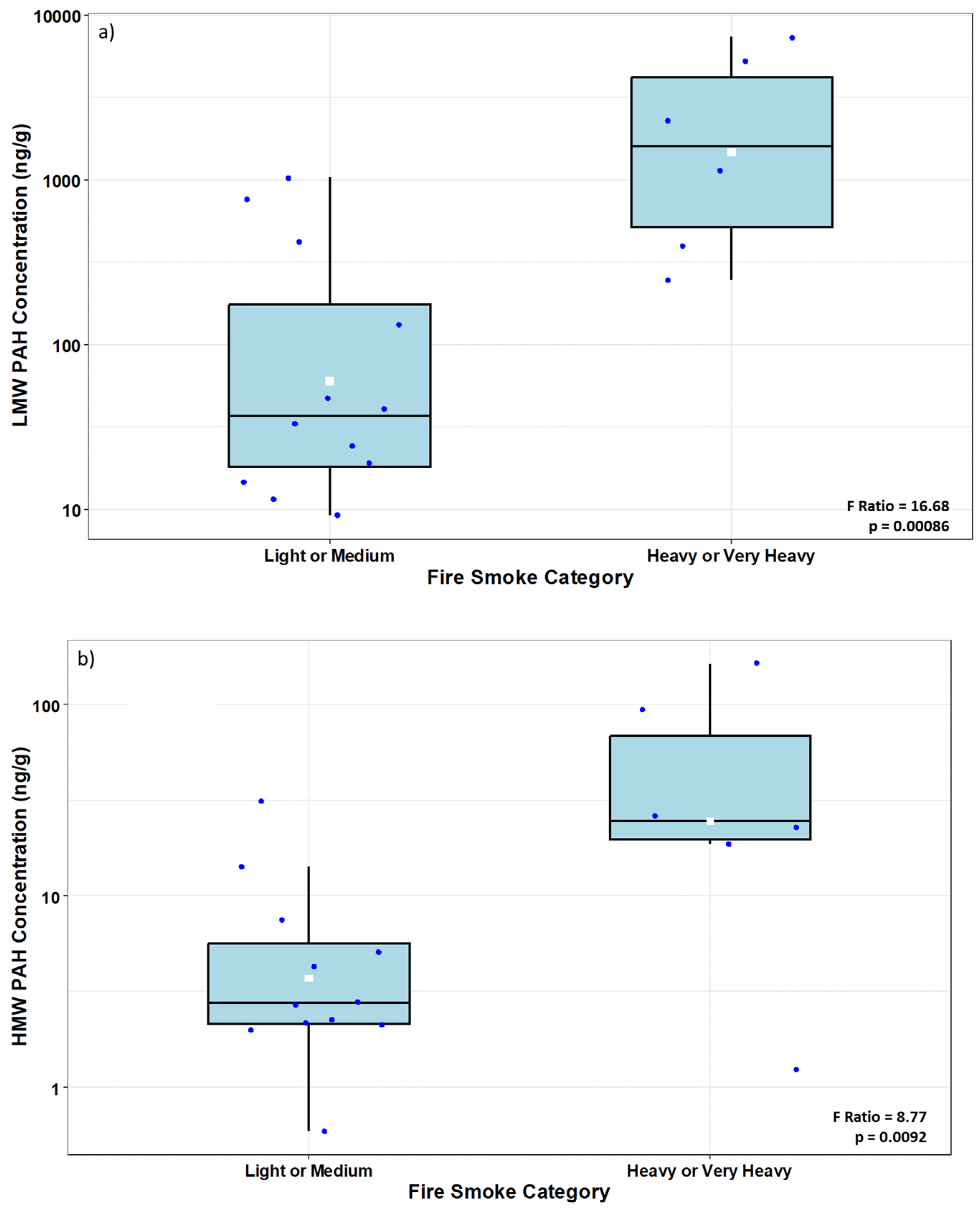
3.1. Fire Stations
3.1.1. Polycyclic Aromatic Hydrocarbons (PAHs)
3.1.2. Flame Retardants
3.2. Firefighter Exposure
3.2.1. PAHs
3.2.2. Flame Retardants
4. Discussion
4.1. Fire Stations
4.2. Firefighter Exposure
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Pinkerton, L.; Bertke, S.J.; Yiin, J.; Dahm, M.; Kubale, T.; Hales, T.; Purdue, M.; Beaumont, J.J.; Daniels, R. Mortality in a cohort of US firefighters from San Francisco, Chicago and Philadelphia: An update. Occup. Environ. Med. 2020, 77, 84. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Occupational Exposure as a Firefighter. IARC Monographs on the Identification of Carcinogenic Hazards to Humans; International Agency for Research on Cancer: Lyon, France, 2023; Volume 132; pp. 1–730.
- Stec, A.A.; Dickens, K.E.; Salden, M.; Hewitt, F.E.; Watts, D.P.; Houldsworth, P.E.; Martin, F.L. Occupational exposure to polycyclic aromatic hydrocarbons and elevated cancer incidence in firefighters. Sci. Rep. 2018, 8, 2476. [Google Scholar] [CrossRef]
- Jalilian, H.; Ziaei, M.; Weiderpass, E.; Rueegg, C.S.; Khosravi, Y.; Kjaerheim, K. Cancer incidence and mortality among firefighters. Int. J. Cancer 2019, 145, 2639. [Google Scholar] [CrossRef] [PubMed]
- European Union (EU). Directive 2003/11/EC of the European Parliament. Available online: https://eur-lex.europa.eu/eli/dir/2003/11/oj (accessed on 23 August 2024).
- Blum, A.; Behl, M.; Birnbaum, L.S.; Diamond, M.L.; Phillips, A.; Singla, V.; Sipes, N.S.; Stapleton, H.M.; Venier, M. Organophosphate ester flame retardants: Are they a regrettable substitution for polybrominated diphenyl ethers? Environ. Sci. Technol. Lett. 2019, 6, 638. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zeng, X.; Dong, G.; Venier, M.; Xie, Q.; Yang, M.; Wu, Q.; Zhao, F.; Chen, D. Plastic additives in ambient fine particulate matter in the Pearl River Delta, China: High-throughput characterization and health implications. Environ. Sci. Technol. 2021, 55, 4474. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, S.; Jin, Y.; Wu, Y.; Liu, L.; Qian, H.; Fu, Z. TPP and TCEP induce oxidative stress and alter steroidogenesis in TM3 Leydig cells. Reprod. Toxicol. 2015, 57, 100. [Google Scholar] [CrossRef]
- Demers, P.A.; DeMarini, D.M.; Fent, K.W.; Glass, D.C.; Hansen, J.; Adetona, O.; Andersen, M.H.; Freeman, L.E.B.; Caban-Martinez, A.J.; Daniels, R.D.; et al. Carcinogenicity of occupational exposure as a firefighter. Lancet Oncol. 2020, 23, 985–986. [Google Scholar]
- International Agency for Research on Cancer (IARC). Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures. IARC Monographs on the Identification of Carcinogenic Hazards to Humans; Volume 92; International Agency for Research on Cancer: Lyon, France, 2010; pp. 1–853.
- Keir, J.L.A.; Akhtar, U.S.; Matschke, D.M.J.; White, P.A.; Kirkham, T.L.; Chan, H.M.; Blais, J.M. Polycyclic aromatic hydrocarbon (PAH) and metal contamination of air and surfaces exposed to combustion emissions during emergency fire suppression: Implications for firefighters’ exposures. Sci. Total Environ. 2020, 698, 134211. [Google Scholar] [CrossRef]
- Choi, S.; Ekpe, O.D.; Sim, W.; Choo, G.; Oh, J. Exposure and risk assessment of Korean firefighters to PBDEs and PAHs via fire vehicle dust and personal protective equipment. Environ. Sci. Technol. 2022, 57, 520. [Google Scholar] [CrossRef]
- Fent, K.W.; Toennis, C.; Sammons, D.; Robertson, S.; Bertke, S.; Calafat, A.M.; Pleil, J.D.; Wallace, M.A.G.; Kerber, S.; Smith, D.; et al. Firefighters’ absorption of PAHs and VOCs during controlled residential fires by job assignment and fire attack tactic. J. Expo. Sci. Environ. Epidemiol. 2019, 30, 338. [Google Scholar] [CrossRef]
- Keir, J.L.A.; Akhtar, U.S.; Matschke, D.M.J.; Kirkham, T.L.; Chan, H.M.; Ayotte, P.; White, P.A.; Blais, J.M. Elevated exposures to polycyclic aromatic hydrocarbons and other organic mutagens in Ottawa firefighters participating in emergency, on-shift fire suppression. Environ. Sci. Technol. 2017, 51, 12745. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.C.; Fent, K.W.; Chen, I.; Sammons, D.; Toennis, C.; Robertson, S.; Kerber, S.; Horn, G.P.; Smith, D.L.; Calafat, A.M.; et al. Characterizing exposures to flame retardants, dioxins, and furans among firefighters responding to controlled residential fires. Int. J. Hyg. Environ. Health 2021, 236, 113782. [Google Scholar] [CrossRef] [PubMed]
- Ekpe, O.D.; Sim, W.; Choi, S.; Choo, G.; Oh, J. Assessment of exposure of Korean firefighters to polybrominated diphenyl ethers and polycyclic aromatic hydrocarbons via their measurement in serum and polycyclic aromatic hydrocarbon metabolites in urine. Environ. Sci. Technol. 2021, 55, 14015–14025. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.W.; LaGuardia, M.; Luellen, D.; McCormick, S.; Mayer, A.; Chen, I.; Kerber, S.; Smith, D.; Horn, G.P. Flame retardants, dioxins, and furans in air and on firefighters’ protective ensembles during controlled residential firefighting. Environ. Int. 2020, 140, 105756. [Google Scholar] [CrossRef]
- Horn, G.P.; Fent, K.W.; Kerber, S.; Smith, D.L. Hierarchy of contamination control in the fire service: Review of exposure control options to reduce cancer risk. J. Occup. Environ. Hyg. 2022, 19, 538–557. [Google Scholar] [CrossRef]
- Banks, A.P.W.; Engelsman, M.; He, C.; Wang, X.; Mueller, J.F. The occurrence of PAHs and flame-retardants in air and dust from Australian fire stations. J. Occup. Environ. Hyg. 2020, 17, 73. [Google Scholar] [CrossRef]
- Gill, R.; Hurley, S.; Brown, R.; Tarrant, D.; Dhaliwal, J.; Sarala, R.; Park, J.; Patton, S.; Petreas, M. Polybrominated diphenyl ether and organophosphate flame retardants in Canadian fire station dust. Chemosphere 2020, 253, 126669. [Google Scholar] [CrossRef]
- O’Connell, S.G.; Kincl, L.D.; Anderson, K.A. Silicone wristbands as personal passive samplers. Environ. Sci. Technol. 2014, 48, 3327–3335. [Google Scholar] [CrossRef]
- Paulik, L.B.; Hobbie, K.A.; Rohlman, D.; Smith, B.W.; Scott, R.P.; Kincl, L.; Haynes, E.N.; Anderson, K.A. Environmental and individual PAH exposures near rural natural gas extraction. Environ. Pollut. 2018, 241, 397–405. [Google Scholar] [CrossRef]
- Hendryx, M.; Wang, S.; Romanak, K.A.; Salamova, A.; Venier, M. Personal exposure to polycyclic aromatic hydrocarbons in Appalachian mining communities. Environ. Pollut. 2019, 257, 113501. [Google Scholar] [CrossRef]
- Baum, J.L.R.; Bakali, U.; Killawala, C.; Santiago, K.M.; Dikici, E.; Kobetz, E.N.; Solle, N.S.; Deo, S.; Bachas, L.; Daunert, S. Evaluation of silicone-based wristbands as passive sampling systems using PAHs as an exposure proxy for carcinogen monitoring in firefighters: Evidence from the firefighter cancer initiative. Ecotoxicol. Environ. Saf. 2020, 205, 111100. [Google Scholar] [CrossRef] [PubMed]
- Caban-Martinez, A.J.; Feliciano, P.L.; Baum, J.; Bakali, U.F.; Santiago, K.M.; Solle, N.S.; Rivera, G.; Ramirez, C.E.; Deo, S.; Miric, M.; et al. Objective measurement of carcinogens among Dominican Republic firefighters using silicone-based wristbands. JCO Glob. Oncol. 2020, 6, 15. [Google Scholar] [CrossRef]
- Levasseur, J.L.; Hoffman, K.; Herkert, N.J.; Cooper, E.; Hay, D.; Stapleton, H.M. Characterizing firefighter’s exposure to over 130 SVOCs using silicone wristbands: A pilot study comparing on-duty and off-duty exposures. Sci. Total Environ. 2022, 834, 155237. [Google Scholar] [CrossRef] [PubMed]
- Bakali, U.; Baum, J.L.R.; Killawala, C.; Kobetz, E.N.; Solle, N.S.; Deo, S.K.; Caban-Martinez, A.J.; Bachas, L.G.; Daunert, S. Mapping carcinogen exposure across urban fire incident response arenas using passive silicone-based samplers. Ecotoxicol. Environ. Saf. 2021, 228, 112929. [Google Scholar] [CrossRef] [PubMed]
- Bonner, E.M.; Horn, G.P.; Smith, D.L.; Kerber, S.; Fent, K.W.; Tidwell, L.G.; Scott, R.P.; Adams, K.T.; Anderson, K.A. Silicone passive sampling used to identify novel dermal chemical exposures of firefighters and assess PPE innovations. Int. J. Hyg. Environ. Health 2023, 248, 114095. [Google Scholar] [CrossRef]
- Keir, J.L.A.; Papas, W.; Wawrzynczak, A.; Aranda-Rodriguez, R.; Blais, J.M.; White, P.A. Use of silicone wristbands to measure firefighters’ exposures to polycyclic aromatic hydrocarbons (PAHs) during live fire training. Environ. Res. 2023, 239, 117306. [Google Scholar] [CrossRef]
- Romanak, K.A.; Wang, S.; Stubbings, W.A.; Hendryx, M.; Venier, M.; Salamova, A. Analysis of brominated and chlorinated flame retardants, organophosphate esters, and polycyclic aromatic hydrocarbons in silicone wristbands used as personal passive samplers. J. Chromatogr. A 2019, 1588, 41–47. [Google Scholar] [CrossRef]
- Helsel, D. Much ado about next to nothing: Incorporating nondetects in science. Ann. Occup. Hyg. 2009, 54, 257–262. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons; Office of Health and Environmental Assessment: Washington, DC, USA, 1993.
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Mackay, D.; Shiu, W.-Y.; Ma, K.-C.; Lee, S.C. Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Jarvis, I.W.H.; Dreij, K.; Mattsson, Å.; Jernström, B.; Stenius, U. Interactions between polycyclic aromatic hydrocarbons in complex mixtures and implications for cancer risk assessment. Toxicology 2014, 321, 27–39. [Google Scholar] [CrossRef]
- Murcia-Morales, M.; Díaz-Galiano, F.J.; Gómez-Ramos, M.J.; Fernández-Alba, A.R. Human exposure to PAHs through silicone-based passive samplers: Methodological aspects and main findings. TrAC Trends Anal. Chem. 2024, 173, 117643. [Google Scholar] [CrossRef]
- Bralewska, K. Air pollution inside fire stations: State-of-the-art and future challenges. Int. J. Hyg. Environ. Health 2024, 255, 114289. [Google Scholar] [CrossRef] [PubMed]
- Engelsman, M.; Snoek, M.F.; Banks, A.P.W.; Cantrell, P.; Wang, X.; Toms, L.; Koppel, D.J. Exposure to metals and semivolatile organic compounds in Australian fire stations. Environ. Res. 2019, 179, 108745. [Google Scholar] [CrossRef] [PubMed]
- Rogula-Kozłowska, W.; Bralewska, K.; Rogula-Kopiec, P.; Makowski, R.; Majder-Łopatka, M.; Łukawski, A.; Brandyk, A.; Majewski, G. Respirable particles and polycyclic aromatic hydrocarbons at two Polish fire stations. Build. Environ. 2020, 184, 107255. [Google Scholar] [CrossRef]
- Hoehn, R.M.; Jahl, L.G.; Herkert, N.J.; Hoffman, K.; Soehl, A.; Diamond, M.L.; Blum, A.; Stapleton, H.M. Flame retardant exposure in vehicles is influenced by use in seat foam and temperature. Environ. Sci. Technol. 2024, 58, 8825–8834. [Google Scholar] [CrossRef]
- de la Torre, A.; Navarro, I.; Sanz, P.; de los Ángeles Martínez, M. Organophosphate compounds, polybrominated diphenyl ethers and novel brominated flame retardants in European indoor house dust: Use, evidence for replacements and assessment of human exposure. J. Hazard. Mater. 2020, 382, 121009. [Google Scholar] [CrossRef]
- Okeme, J.O.; Yang, C.; Abdollahi, A.; Dhal, S.; Harris, S.A.; Jantunen, L.M.; Tsirlin, D.; Diamond, M.L. Passive air sampling of flame retardants and plasticizers in Canadian homes using PDMS, XAD-coated PDMS and PUF samplers. Environ. Pollut. 2018, 239, 109–117. [Google Scholar] [CrossRef]
- 49 CFR §571.302 – Standard No. 302; Flammability of Interior Materials, Federal Motor Vehicle Safety Standards. Department of Transportation: Hongkong, China, 1998; pp. 1–32.
- Poutasse, C.M.; Poston, W.S.C.; Jahnke, S.A.; Haddock, C.K.; Tidwell, L.G.; Hoffman, P.D.; Anderson, K.A. Discovery of firefighter chemical exposures using military-style silicone dog tags. Environ. Int. 2020, 142, 105818. [Google Scholar] [CrossRef]
- Phillips, A.L.; Hammel, S.C.; Konstantinov, A.; Stapleton, H.M. Characterization of individual isopropylated and tert-butylated triarylphosphate (ITP and TBPP) isomers in several commercial flame retardant mixtures and house dust standard reference material SRM 2585. Environ Sci Technol 2017, 51, 13443–13449. [Google Scholar] [CrossRef]
- O’Connell, S.G.; Anderson, K.A.; Epstein, M.I. Determining chemical air equivalency using silicone personal monitors. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 268–279. [Google Scholar] [CrossRef]
- Tromp, P.C.; Beeltje, H.; Okeme, J.O.; Vermeulen, R.; Pronk, A.; Diamond, M.L. Calibration of polydimethylsiloxane and polyurethane foam passive air samplers for measuring semi volatile organic compounds using a novel exposure chamber design. Chemosphere 2019, 227, 435. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 His Majesty the King in Right of Canada. Reproduced with the permission of the Minister of Health Canada. 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papas, W.; Aranda-Rodriguez, R.; Fan, X.; Kubwabo, C.; Lee, J.S.L.; Fantin, E.; Zheng, E.D.; Keir, J.L.A.; Matschke, D.; Blais, J.M.; et al. Occupational Exposure of On-Shift Ottawa Firefighters to Flame Retardants and Polycyclic Aromatic Hydrocarbons. Toxics 2024, 12, 677. https://doi.org/10.3390/toxics12090677
Papas W, Aranda-Rodriguez R, Fan X, Kubwabo C, Lee JSL, Fantin E, Zheng ED, Keir JLA, Matschke D, Blais JM, et al. Occupational Exposure of On-Shift Ottawa Firefighters to Flame Retardants and Polycyclic Aromatic Hydrocarbons. Toxics. 2024; 12(9):677. https://doi.org/10.3390/toxics12090677
Chicago/Turabian StylePapas, William, Rocio Aranda-Rodriguez, Xinghua Fan, Cariton Kubwabo, Janet S. L. Lee, Emma Fantin, Elita D. Zheng, Jennifer L. A. Keir, Dave Matschke, Jules M. Blais, and et al. 2024. "Occupational Exposure of On-Shift Ottawa Firefighters to Flame Retardants and Polycyclic Aromatic Hydrocarbons" Toxics 12, no. 9: 677. https://doi.org/10.3390/toxics12090677
APA StylePapas, W., Aranda-Rodriguez, R., Fan, X., Kubwabo, C., Lee, J. S. L., Fantin, E., Zheng, E. D., Keir, J. L. A., Matschke, D., Blais, J. M., & White, P. A. (2024). Occupational Exposure of On-Shift Ottawa Firefighters to Flame Retardants and Polycyclic Aromatic Hydrocarbons. Toxics, 12(9), 677. https://doi.org/10.3390/toxics12090677





