Characteristics and Mechanism of Hematite Dissolution and Release on Arsenic Migration in Heterogeneous Materials
Abstract
1. Introduction
2. Experimental Materials and Analytical Methods
2.1. Sand Trough Structure and Operation Mode
2.2. Reagents and Samples
2.3. Tests and Methods
2.4. Statistical Analysis and Plotting Software
3. Results and Discussion
3.1. Migration Characteristics of As
3.2. Fe Ion Release and Migration Characteristics
3.3. Morphology and Distribution Characteristics of Solid As
4. Reaction Mechanism Analysis
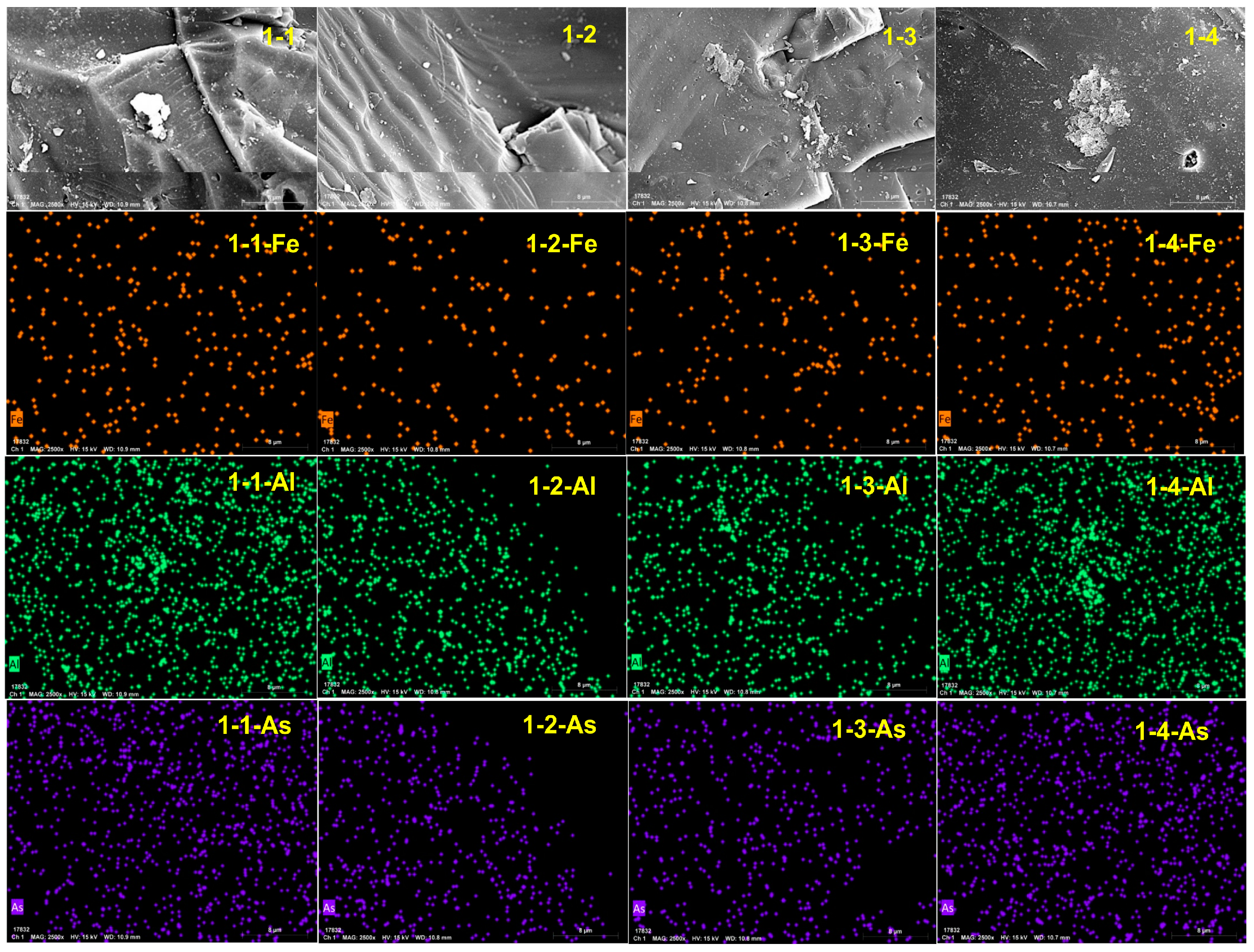
4.1. Surface Morphology and Element Distribution
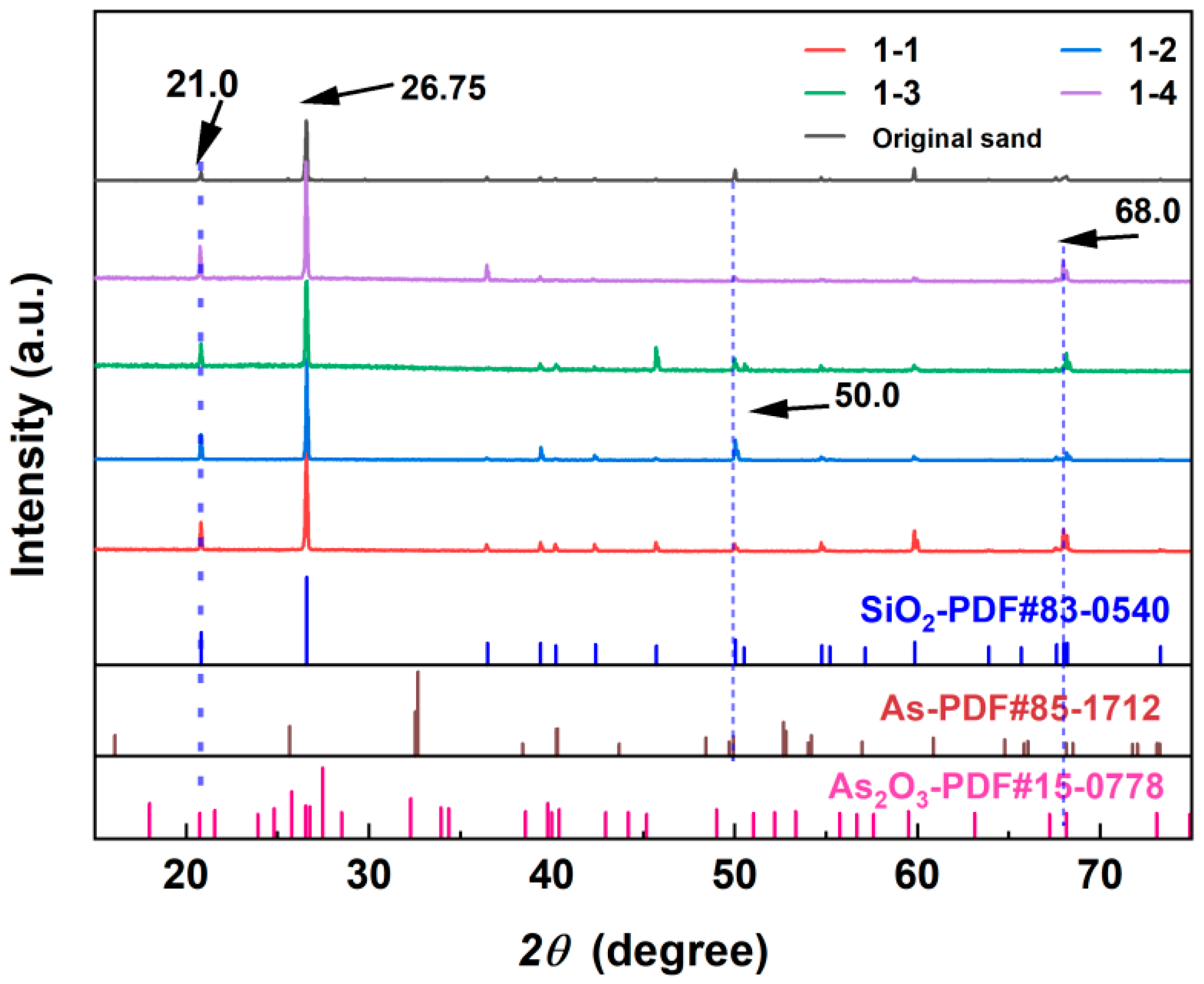
4.2. Main Crystalline Phase on the Surface
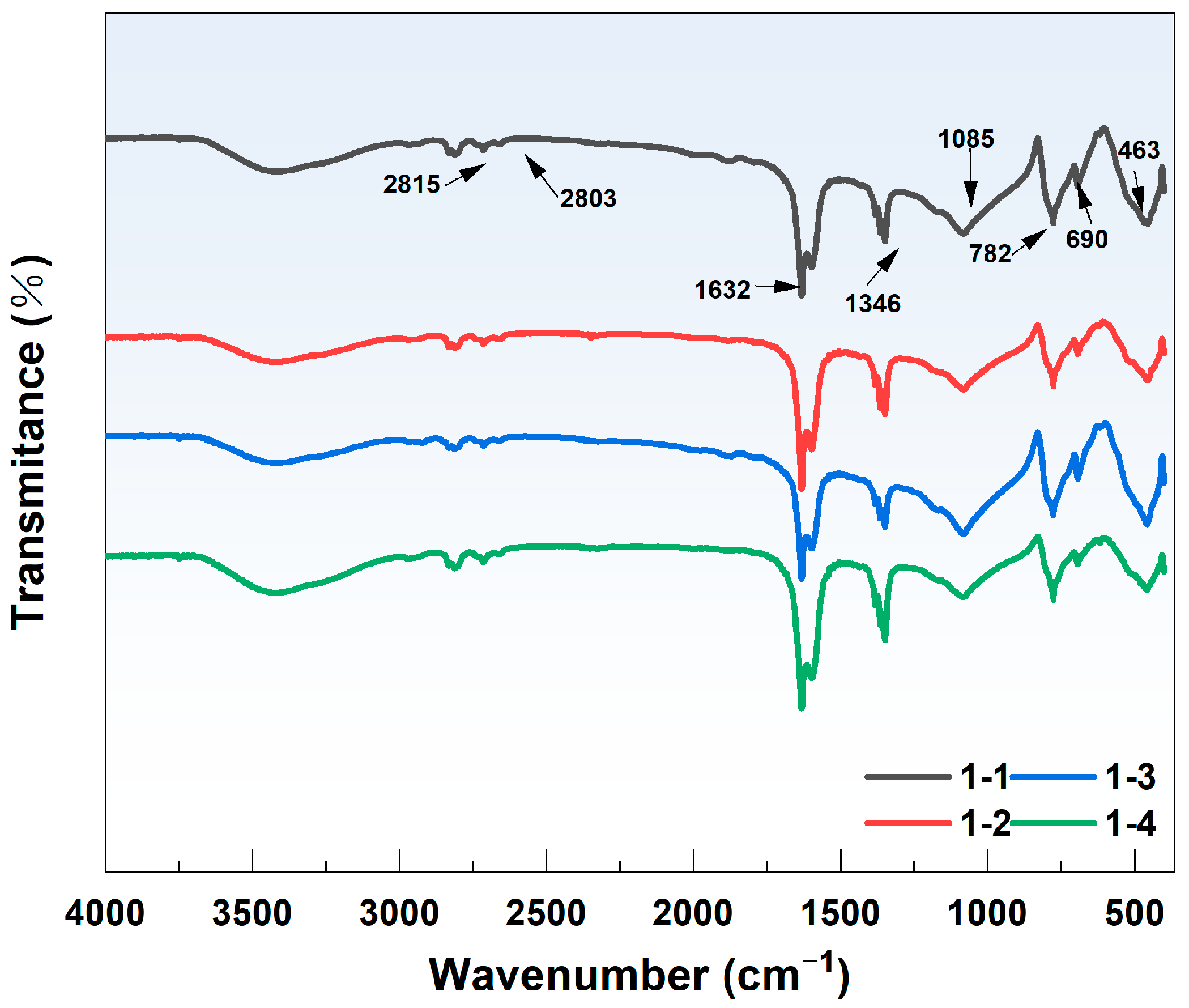
4.3. Changes in the Surface Functional Groups
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Fu, Y.; Cheng, Y.; Zhai, W.; Sun, X.; Ren, Y.; Pan, D. Modeling potential arsenic enrichment and distribution using stacking ensemble learning in the lower Yellow River Plain, China. J. Hydrol. 2023, 625, 129985. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Guidelines for Drinking Water Quality: Fourth Edition Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Guo, J.; Cao, W.; Lang, G.; Sun, Q.; Nan, T.; Li, X.; Ren, Y.; Li, Z. Worldwide Distribution, Health Risk, Treatment Technology, and Development Tendency of Geogenic High-Arsenic Groundwater. Water 2024, 16, 478. [Google Scholar] [CrossRef]
- Sinha, D.; Prasad, P. Health effects inflicted by chronic low-level arsenic contamination in groundwater: A global public health challenge. J. Appl. Toxicol. 2020, 40, 87–131. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhang, F.; Xu, N.; Li, L.; Liu, L. Chemical and mineralogical variability of sediment in a quaternary aquifer from Huaihe River Basin, China: Implications for groundwater arsenic source and its mobilization. Sci. Total Environ. 2023, 865, 160864. [Google Scholar] [CrossRef]
- Yuan, R.; Li, Z.; Guo, S. Health risks of shallow groundwater in the five basins of Shanxi, China: Geographical, geological and human activity roles. Environ. Pollut. 2023, 316, 120524. [Google Scholar] [CrossRef]
- Biswas, T.; Pal, S.C.; Saha, A.; Ruidas, D. Arsenic and fluoride exposure in drinking water caused human health risks in coastal groundwater aquifers. Environ. Res. 2023, 238, 117257. [Google Scholar] [CrossRef]
- McArthur, J.M.; Nath, B.; Banerjee, D.M.; Purohit, R.; Grassinea, N. Palaeosol control on groundwater flow and pollutant distribution: The example of arsenic. Environ. Sci. Technol. 2011, 45, 1376–1383. [Google Scholar] [CrossRef]
- Brikowski, T.H.; Neku, A.; Shrestha, S.D.; Smith, L.S. Hydrologic control of temporal variability in groundwater arsenic on the Ganges floodplain of Nepal. J. Hydrol. 2014, 518, 342–353. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Zhu, Q.; Wang, C.; Tang, X. Review on arsenic environment behaviors in aqueous solution and soil. Chemosphere 2023, 333, 138869. [Google Scholar] [CrossRef]
- Fischel, M.H.H.; Clarke, C.E.; Sparks, D.L. Arsenic sorption and oxidation by natural manganese-oxide-enriched soils: Reaction kinetics respond to varying environmental conditions. Geoderma 2024, 441, 116715. [Google Scholar] [CrossRef]
- Li, C.; Bundschuh, J.; Gao, X.; Li, Y.; Zhang, X.; Luo, W.; Pan, Z. Occurrence and behavior of arsenic in groundwater-aquifer system of irrigated areas. Sci. Total Environ. 2022, 838, 155991. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hu, Y.; Luo, J.; Xu, B.; Zhao, J. Geochemical processes controlling fate and transport of arsenic in acid mine drainage (AMD) and natural systems. J. Hazard. Mater. 2009, 165, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, R.; Gan, Y.; Yu, K.; Tong, J.; Zeng, G.; Ke, D.; Wu, W.; Liu, C. Impact of physico-chemical heterogeneity on arsenic sorption and reactive transport underwater extraction. Environ. Sci. Technol. 2020, 54, 14974–14983. [Google Scholar] [CrossRef]
- Duan, Y.; Li, R.; Yu, K.; Zeng, G.; Liu, C. Effects of geochemical and hydrodynamic transiency on desorption and transport of As in heterogeneous systems. Sci. Total Environ. 2022, 835, 155381. [Google Scholar] [CrossRef]
- Zou, Q.; Wei, H.; Chen, Z.; Ye, P.; Zhang, J.; Sun, M.; Huang, L.; Li, J. Soil particle size fractions affect arsenic (As) release and speciation: Insights into dissolved organic matter and functional genes. J. Hazard. Mater. 2023, 443, 130100. [Google Scholar] [CrossRef]
- Aftabtalab, A.; Rinklebe, J.; Shaheen, S.M.; Niazi, N.K.; Moreno-Jiménez, E.; Schaller, J.; Knorr, K.H. Review on the interactions of arsenic, iron (oxy)(hydr) oxides, and dissolved organic matter in soils, sediments, and groundwater in a ternary system. Chemosphere 2022, 286, 131790. [Google Scholar] [CrossRef]
- Cai, X.; Yin, N.; Liu, X.; Wang, P.; Du, H.; Cui, Y.; Hu, Z. Biogeochemical processes of arsenic transformation and redistribution in contaminated soils: Combined effects of iron, sulfur, and organic matter. Geoderma 2022, 422, 115948. [Google Scholar] [CrossRef]
- Lu, S.; Su, X.; Feng, X.; Sun, C. Study on the formation and influencing factors of arsenic in nearshore groundwater during river water infiltration. Earth Sci. Front. 2022, 29, 455–467, (In Chinese with English abstract). [Google Scholar]
- Zhang, D.; Guo, H.; Xiu, W.; Ni, P.; Zheng, H.; Wei, C. In-situ mobilization and transformation of iron oxides-adsorbed arsenate in natural groundwater. J. Hazard. Mater. 2017, 321, 228–237. [Google Scholar] [CrossRef]
- Nath, B.; Berner, Z.; Mallik, S.B.; Chatterjee, D.; Charlet, L.; Stueben, D. Characterization of aquifers conducting groundwaters with low and high arsenic concentrations: A comparative case study from West Bengal, India. Mineral. Mag. 2005, 69, 841–854. [Google Scholar] [CrossRef]
- Fendorf, S.; Michael, H.A.; Van Geen, A. Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 2010, 328, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic fractionation in soils using an improved sequential extraction procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Shepley, M.G.; Schmidt, N. Utility trench water level recessions in an aquitard: Findings from analytical and numerical analyses. Hydrogeol. J. 2022, 30, 2495–2507. [Google Scholar] [CrossRef]
- Glose, T.J.; Zipper, S.; Hyndman, D.W.; Stingeder, G.; Lombi, E.; Adriano, D.C. Quantifying the impact of lagged hydrological responses on the effectiveness of groundwater conservation. Water Resour. Res. 2022, 58, e2022WR032295. [Google Scholar] [CrossRef]
- Qin, R.; Wu, Y.; Xu, Z.; Xie, D.; Zhang, C. Numerical modeling of contaminant transport in a stratified heterogeneous aquifer with dipping anisotropy. Hydrogeol. J. 2013, 21, 1235. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, J.; Lin, W.; Wang, G. Dye tracing of soil water flow pattern and evaluation of preferential flow degree. Trans. Chin. Soc. Agric. Eng. 2014, 30, 82–90, (In Chinese with English abstract). [Google Scholar]
- Yan, Y.; Xie, X.; Zheng, W.; Chi, Z.; Liu, Y. Effects of irrigation activities on arsenic migration in surface soil of Datong Basin. Geol. Sci. Technol. Inf. 2017, 36, 235–241, (In Chinese with English abstract). [Google Scholar]
- Li, H.; Ding, S.; Song, W.; Wang, X.; Ding, J.; Lu, J. The degradation of dissolved organic matter in black and odorous water by humic substance-mediated Fe (II)/Fe (III) cycle under redox fluctuation. J. Environ. Manag. 2022, 321, 115942. [Google Scholar] [CrossRef]
- Xu, F.; Li, P. Biogeochemical mechanisms of iron (Fe) and manganese (Mn) in groundwater and soil profiles in the Zhongning section of the Weining Plain (northwest China). Sci. Total Environ. 2024, 939, 173506. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Chen, J.; Zhuang, G. Heterogeneous reaction mechanism of SO2 and Fe2O3 to form Fe(II)(aq) and sulfate. J. Chem. Univ. 2006, 1347–1350, (In Chinese with English abstract). [Google Scholar]
- Peng, X.; Yang, B.; Li, X.; Dai, X.; Wei, C.; Lu, Z.; Deng, Z.; Li, M.; Fan, G. Dissolution behavior of hematite in H2SO4 solution: A kinetic analysis and its importance on the zinc hydrometallurgical hematite process. Miner. Eng. 2024, 215, 108811. [Google Scholar] [CrossRef]
- Zeng, J.; Tabelin, C.B.; Gao, W.; Tang, L.; Luo, X.; Ke, W.; Jiang, J.; Xue, S. Heterogeneous distributions of heavy metals in the soil-groundwater system empowers the knowledge of the pollution migration at a smelting site. Chem. Eng. J. 2023, 454, 140307. [Google Scholar] [CrossRef]
- Yao, Y.; Mi, N.; He, C.; Yin, L.; Zhou, D.; Zhang, Y.; Sun, H.; Yang, S.; Li, S.; He, H. Transport of arsenic loaded by ferric humate colloid in saturated porous media. Chemosphere 2020, 240, 124987. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Liu, M.; Zhang, J.; Huang, L.; Chen, Z. Research progress on the sources of arsenic in soil and factors affecting its migration and release. Soils 2020, 52, 234–246, (In Chinese with English abstract). [Google Scholar]
- Zhang, Y.; Xie, X.; Sun, S.; Wang, Y. Arsenic transformation and redistribution in groundwater induced by the complex geochemical cycling of iron and sulfur. Sci. Total Environ. 2023, 894, 164941. [Google Scholar] [CrossRef]
- Zhong, S.; Yin, G.; He, H.; Huang, R.; Chen, Z.; Lin, Q.; Peng, H.; Wang, K. Stabilization effect and mechanism of different iron minerals on arsenic in paddy soil. J. Environ. Sci. 2017, 37, 1931–1938, (In Chinese with English abstract). [Google Scholar]
- Park, J.H.; Han, Y.S.; Ahn, J.S. Comparison of arsenic co-precipitation and adsorption by iron minerals and the mechanism of arsenic natural attenuation in a mine stream. Water Res. 2016, 106, 295–303. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, X.; Sun, B.; Su, S.; Wang, Y.; Zhang, N.; Zhang, Y.; Wu, C. Research progress on fixation of soil heavy metals by iron oxides. Soil Bull. 2021, 52, 728–735, (In Chinese with English abstract). [Google Scholar]
- Yao, D.; Shi, Y.; Pan, H.; Zhong, D.; Hou, H.; Wu, X.; Chen, J.; Wang, L.; Hu, Y.; Crittenden, J.C. Promotion mechanism of natural clay colloids in the adsorption of arsenite on iron oxide particles in water. Chem. Eng. J. 2020, 392, 123637. [Google Scholar] [CrossRef]
- Inchaurrondo, N.; Di Luca, C.; Haure, P.; Žerjav, G.; Pintar, A.; Palet, C. Evaluation of low-cost geo-adsorbents for As (V) removal. Environ. Technol. Innov. 2021, 21, 101341. [Google Scholar] [CrossRef]
- Wang, H.; Tsang, Y.F.; Wang, Y.; Sun, Y.; Zhang, D.; Pan, X. Adsorption capacities of poorly crystalline Fe minerals for antimonate and arsenate removal from water: Adsorption properties and effects of environmental and chemical conditions. Clean Technol. Environ. Policy 2018, 20, 2169–2179. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, S.; Wei, Y.; Zhou, L. Schwertmannite modified with ethanol: A simple and feasible method for improving As (III) adsorption capacity. J. Environ. Chem. Eng. 2022, 10, 107412. [Google Scholar] [CrossRef]
- Li, Z.; Peng, S.; Shan, H.; Liao, Q.; Zhou, H.; Zhao, Z. The Influence of Aqueous Iron on River Sand’s Arsenic Adsorption: Characteristics and Mechanisms. Water 2024, 16, 1107. [Google Scholar] [CrossRef]
- Du, H.; Shan, H.; Huang, J.; Zeng, C.; Zhang, X.; Liu, Y. Experimental study on the effects of flow velocity and medium particle size on As(III) migration. Earth Sci. 2024, 49, 1459–1469, (In Chinese with English abstract). [Google Scholar]
- Itamiya, H.; Sugita, R.; Sugai, T. Analysis of the surface microtextures and morphologies of beach quartz grains in Japan and implications for provenance research. Prog. Earth Planet. Sci. 2019, 6, 43. [Google Scholar] [CrossRef]
- Ding, K.; Ruan, L.; Wang, H.; Deng, X.; Yang, W.; Jiang, N.; Zhang, J. Research progress on the application of FeOOH in adsorption of water pollutants. Appl. Chem. Ind. 2024, 53, 894–899, (In Chinese with English abstract). [Google Scholar]
- Zhang, Y.; Hou, Z.; Fu, P.; Wang, X.; Xue, T.; Chen, Y. Simultaneous stabilization of arsenic and antimony co-contaminated mining soil by Fe(II) activated-Fenton sludge: Behavior and mechanisms. Environ. Pollut. 2023, 337, 122538. [Google Scholar] [CrossRef]
- Li, J.; Zhan, M.; Zhong, X.; Wang, Y.; Ou, Y.; Zhao, X. Accumulation characteristics and influencing factors of heavy metals in soil-crop systems in typical karst areas of Guangxi. J. Environ. Sci. 2021, 41, 597–606, (In Chinese with English abstract). [Google Scholar]
- Nguyen, K.T.; Navidpour, A.H.; Ahmed, M.B.; Mojiri, A.; Huang, Y.; Zhou, J.L. Adsorption and desorption behavior of arsenite and arsenate at river sediment-water interface. J. Environ. Manag. 2022, 317, 115497. [Google Scholar] [CrossRef]
- Hou, Q.; Zhang, Y.; Yu, K.; Han, D.; Chen, J. Dynamic transformation of soil arsenic binding forms driven by flooding-drying cycles. Sci. Technol. Eng. 2023, 23, 9728–9736, (In Chinese with English abstract). [Google Scholar]
- Chen, H.S.; Sun, Z.Y.; Shao, J.C. Investigation on FT-IR Spectroscopy for Eight Different Sources of SiO2. Bull. Chin. Ceram. Soc. 2011, 30, 934–937, (In Chinese with English abstract). [Google Scholar]
- Sun, S.Y.; Wen, K.; Yang, B.; Zhou, Q.; Dong, F.; Nie, X.; Liu, L.; Fan, S. The preparation and adsorption properties of novel active carbon/diatomite. Acta Petrol. Mineral. 2013, 32, 941–946, (In Chinese with English abstract). [Google Scholar]
- Wang, Y.; Yu, W.; Chang, Z.; Gao, C.; Yang, Y.; Zhang, B.; Wang, Y.; Xing, B. Effects of dissolved organic matter on the adsorption of norfloxacin on a sandy soil (fraction)from the Yellow River of Northern China. Sci. Total Environ. 2022, 848, 157495. [Google Scholar] [CrossRef]
- Zhang, L.; Song, L.T.; Zheng, X.D.; Teng, Y.; Wang, J. The remobilization of heavy metals influenced by interaction of DOM and iron oxides. Chin. J. Ecol. 2014, 33, 2193–2198, (In Chinese with English abstract). [Google Scholar]
- Adames-Montero, Y.; López-Guerra, S.; Marrero-Águila, R.; Cueli-Corugedo, A.; Davis-Harriett, J. Transformaciones físico-químicas de productos de corrosión el hierro en instalaciones petroleras. Tecnol. Química 2020, 40, 627–639. [Google Scholar]
- Wang, H.M.; Ma, Y.P.; Chen, X.Y.; Xu, S.; Chen, D.; Zhang, L.; Zhao, B.; Ning, P. Promoting effect of SO42− functionalization on the performance of Fe2O3 catalyst in the selective catalytic reduction of NOx with NH3. J. Fuel Chem. Technol. 2020, 48, 584–593, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Zulfikar, M.A.; Utami, A.R.; Handayani, N.; Wahyuningrum, D.; Setiyanto, H.; Azis, M.Y. Removal of phthalate ester compound from PVC plastic samples using magnetic molecularly imprinted polymer on the surface of superparamagnetic Fe3O4 (Fe3O4@ MIPs). Environ. Nanotechnol. Monit. Manag. 2022, 17, 100646. [Google Scholar] [CrossRef]
- Kefirov, R.; Ivanova, E.; Hadjiivanov, K.; Dzwigaj, S.; Che, M. FTIR characterization of Fe3+–OH groups in Fe–H–BEA zeolite: Interaction with, CO and NO. Catal. Lett. 2008, 125, 209–214. [Google Scholar] [CrossRef]
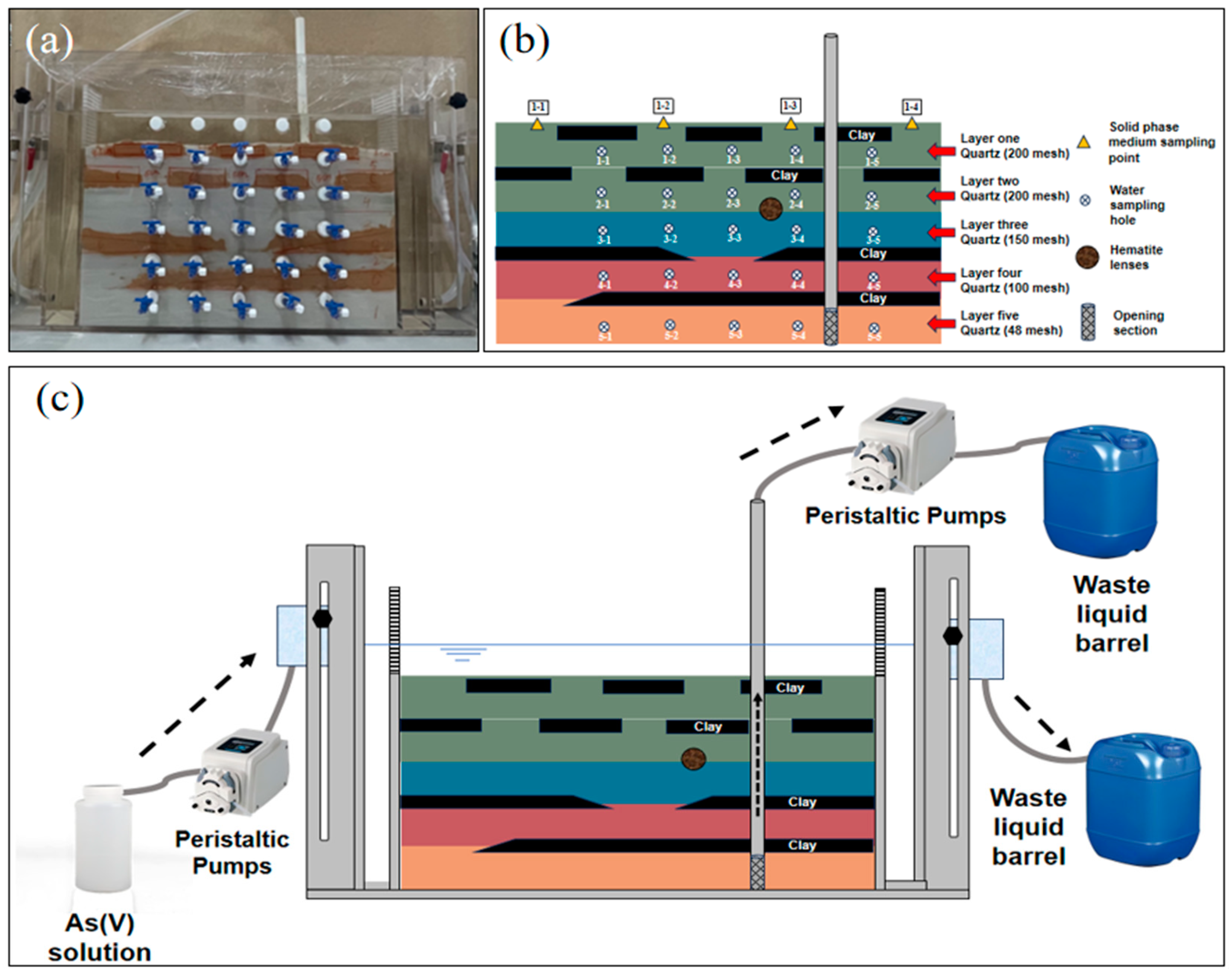
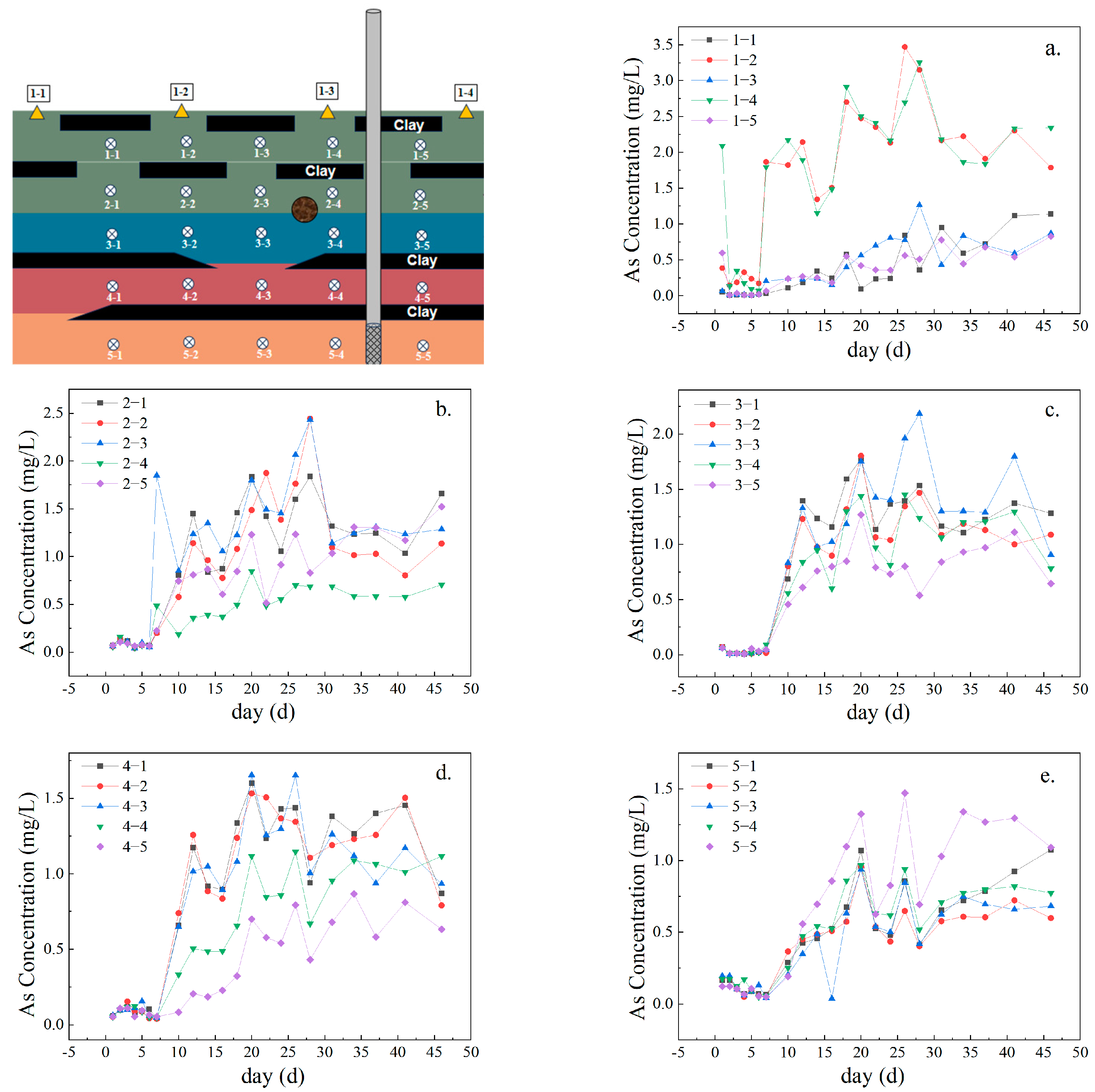
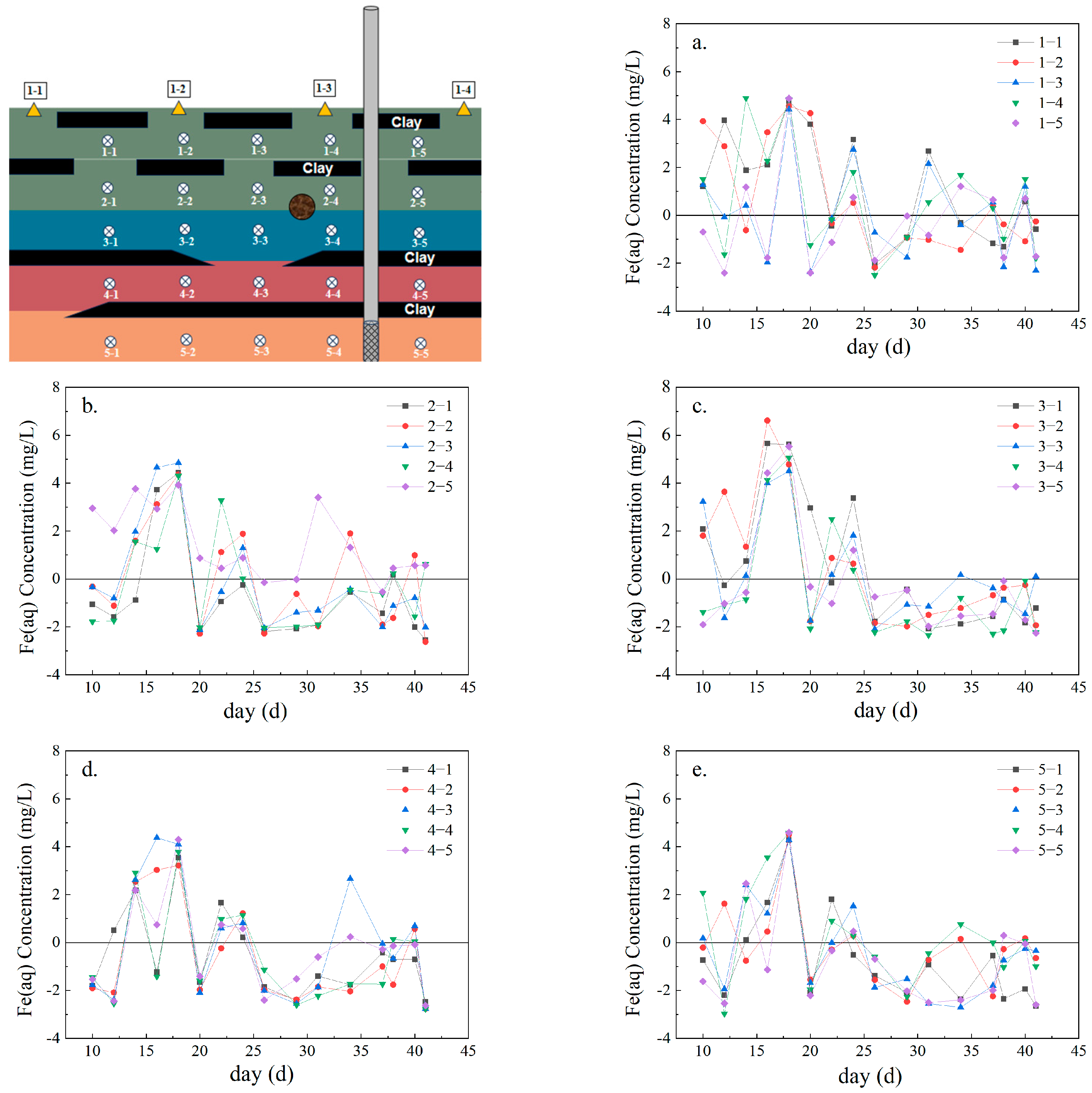
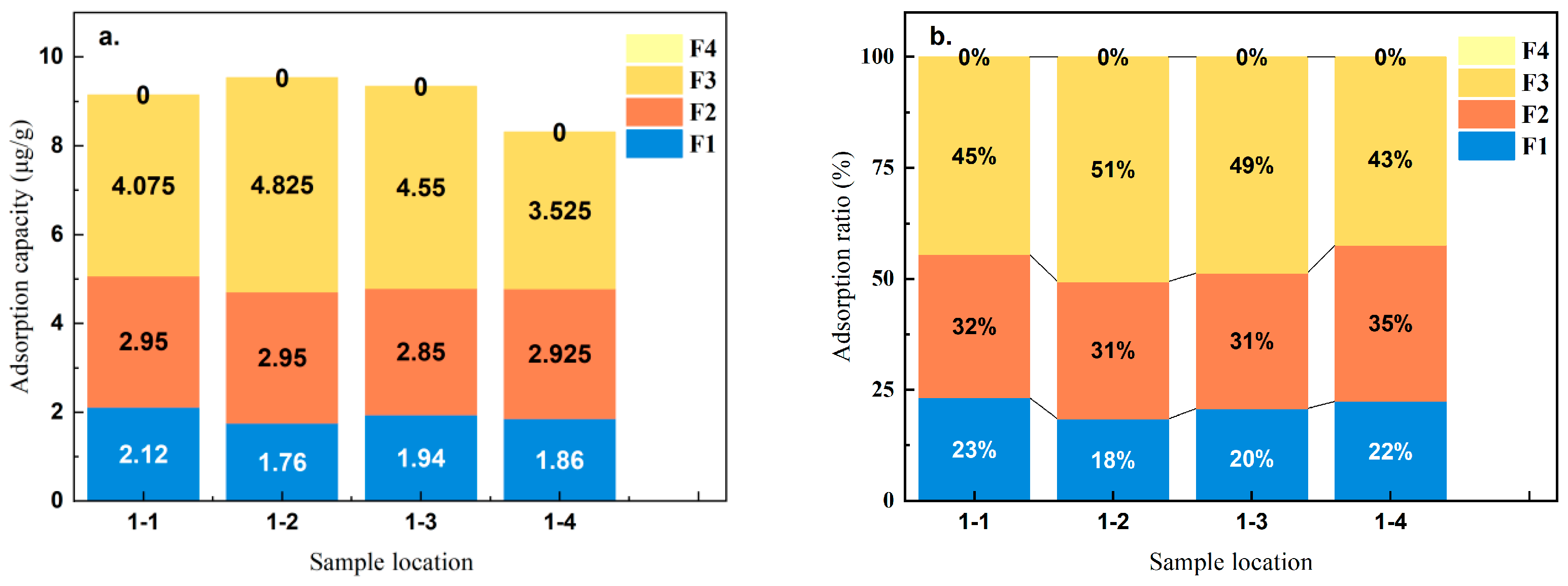

| Chemical Form | Extraction Method | Water–Sand Ratio (mL:g) |
|---|---|---|
| Nonspecifically adsorbed state (F1) | 0.05 mol/L (NH4)2SO4, 25 °C, shaking 4 h | 25:1 |
| Specifically adsorbed state (F2) | 0.05 mol/L NH4H2PO4, 25 °C, shaking 16 h | 25:1 |
| Amorphous iron-aluminum oxide-bound state (F3) | First time: 0.2 mol/L ammonium oxalate buffer (pH = 3.5), 25 °C, shaking 4 h Second time: 0.2 mol/L ammonium oxalate buffer (pH = 3.5), 25 °C, shaking 10 min | First time 25:1 Second time 12.5:1 |
| Crystalline iron-aluminum oxide-bound state (F4) | First time: ammonium oxalate (0.2 mol/L) + ascorbic acid (0.1 mol/L), pH = 3.5, 96 °C, shaking 30 min Second time: ammonium oxalate (0.2 mol/L) + ascorbic acid (0.1 mol/L), pH = 3.5, 96 °C, shaking 10 min | First time 25:1 Second time 12.5:1 |
| Condition | C | O | Si | Al | Fe | As |
|---|---|---|---|---|---|---|
| Before reaction | 4.47 | 52.14 | 42.45 | 0.21 | 0.73 | 0 |
| 1-1 | 5.97 | 41.66 | 52.13 | 0.23 | 0 | 0 |
| 1-2 | 4.81 | 44.49 | 50.05 | 0.04 | 0.08 | 0.01 |
| 1-3 | 5.07 | 38.89 | 55.63 | 0.07 | 0.31 | 0.02 |
| 1-4 | 6.38 | 43.30 | 50.25 | 0.07 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Shan, H.; Rong, W.; Zhao, Z.; Ma, K.; Peng, S.; Wei, S. Characteristics and Mechanism of Hematite Dissolution and Release on Arsenic Migration in Heterogeneous Materials. Toxics 2024, 12, 687. https://doi.org/10.3390/toxics12090687
Li Z, Shan H, Rong W, Zhao Z, Ma K, Peng S, Wei S. Characteristics and Mechanism of Hematite Dissolution and Release on Arsenic Migration in Heterogeneous Materials. Toxics. 2024; 12(9):687. https://doi.org/10.3390/toxics12090687
Chicago/Turabian StyleLi, Zheying, Huimei Shan, Wanyue Rong, Zhicheng Zhao, Kexin Ma, Sanxi Peng, and Song Wei. 2024. "Characteristics and Mechanism of Hematite Dissolution and Release on Arsenic Migration in Heterogeneous Materials" Toxics 12, no. 9: 687. https://doi.org/10.3390/toxics12090687
APA StyleLi, Z., Shan, H., Rong, W., Zhao, Z., Ma, K., Peng, S., & Wei, S. (2024). Characteristics and Mechanism of Hematite Dissolution and Release on Arsenic Migration in Heterogeneous Materials. Toxics, 12(9), 687. https://doi.org/10.3390/toxics12090687








