Low-Concentration Hypochlorous Acid Drinking Water Alleviates Broiler Gut Microbial Load While Preserving Overall Growth Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Drinking Water Preparation and Dosage Determination
2.3. Sampling
2.4. Cell Count Detection
2.5. Statistical Analysis
3. Results
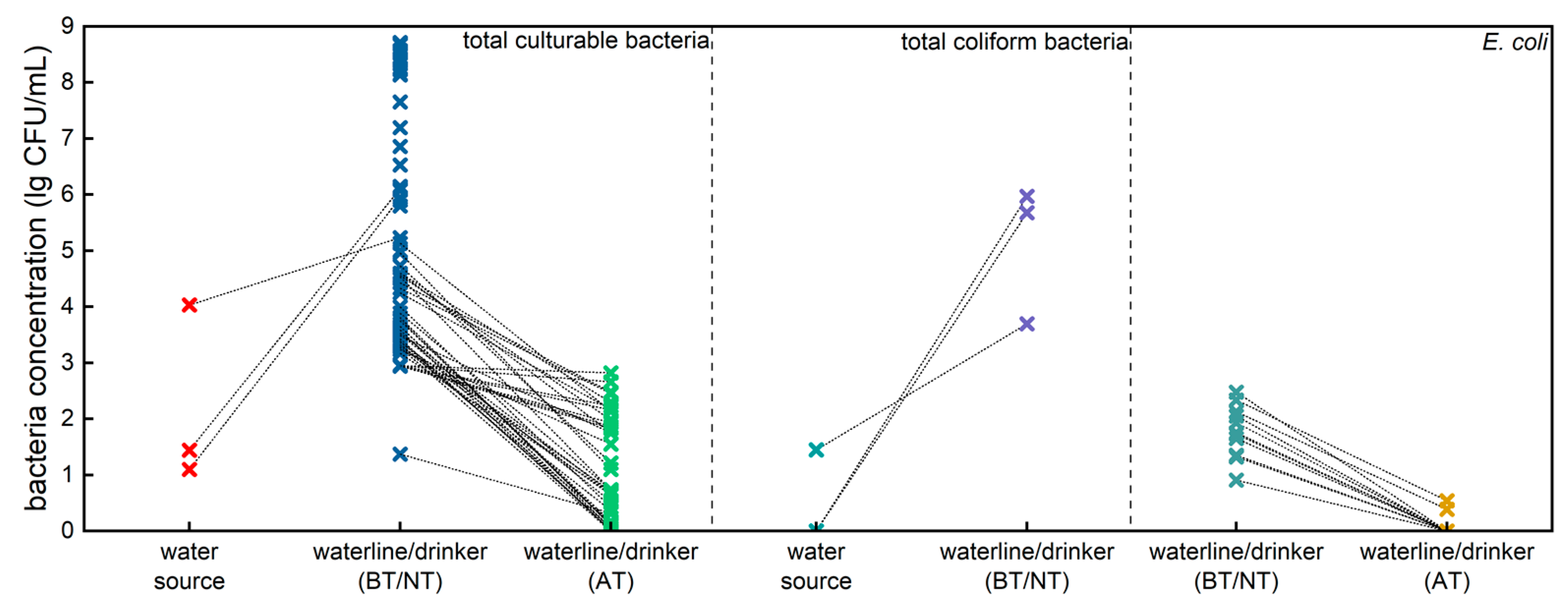
3.1. Bacterial Contamination in Poultry Farm Water System and the Impact of Hypochlorous Acid Addition
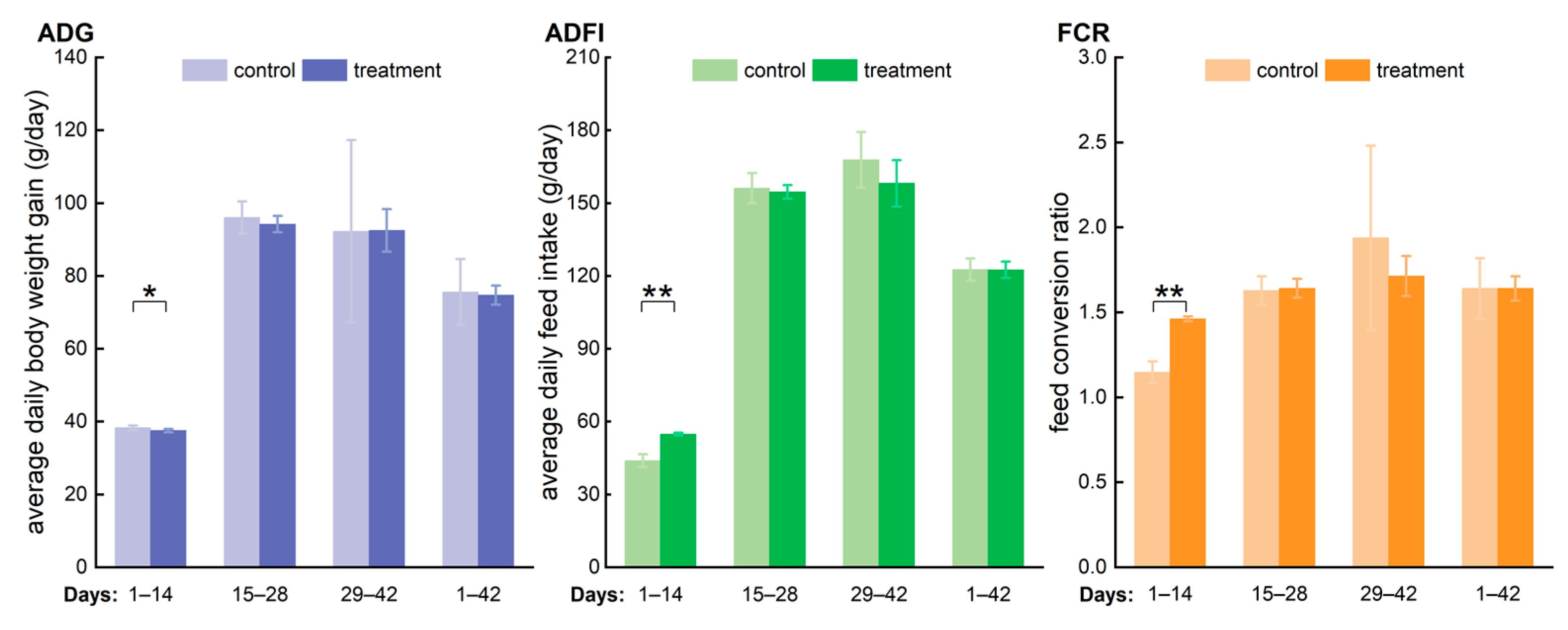
3.2. Effect of Long-Term Consumption of Low-Concentration Hypochlorous Acid Water on Broiler Growth Performance
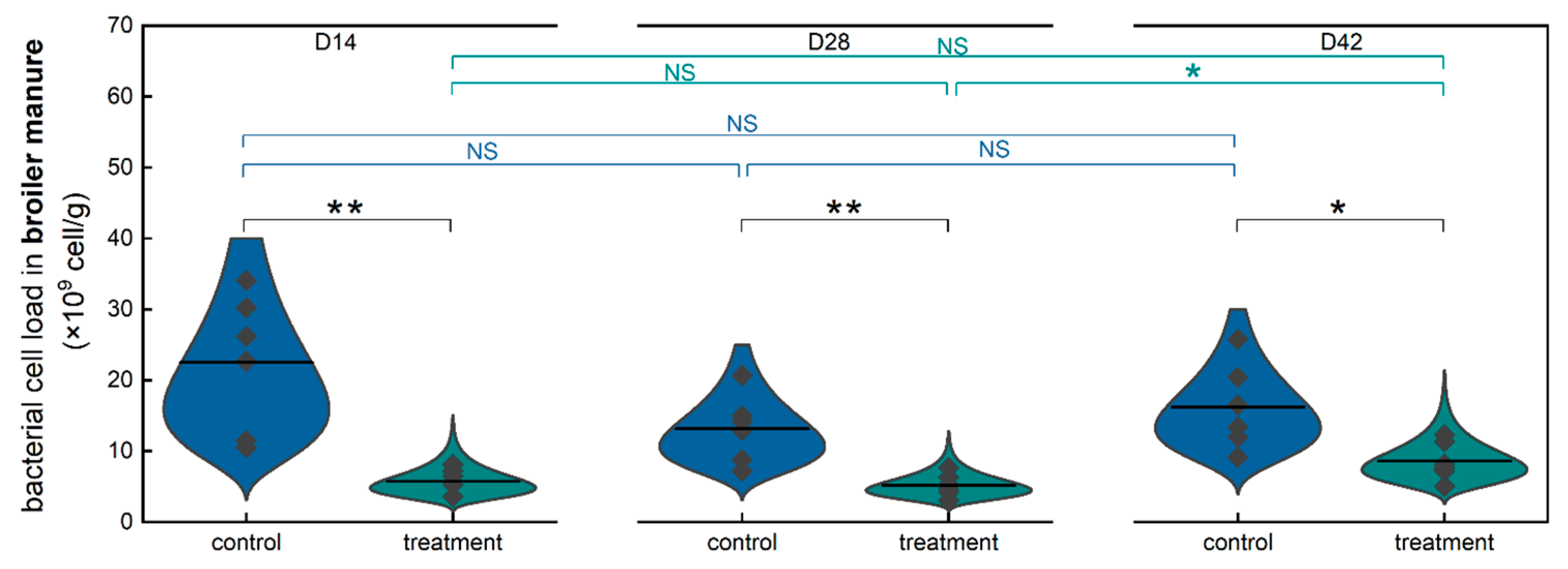
3.3. Effect of Long-Term Consumption of Low-Concentration Hypochlorous Acid Water on the Microbial Load of Fresh Broiler Manure
3.4. Effect of Long-Term Consumption of Low-Concentration Hypochlorous Acid Water on the Microbial Load of Broiler Duodenal Contents and Cecal Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Xu, C.; Zhang, R.; Chen, Y.; Shen, Y.; Hu, F.; Liu, D.; Lu, J.; Guo, Y.; Xia, X.; et al. Changes in colistin resistance and mcr-1 abundance in Escherichia coli of animal and human origins following the ban of colistin-positive additives in China: An epidemiological comparative study. Lancet Infect. Dis. 2020, 20, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Lillehoj, H.; Chuanchuen, R.; Gay, C.G. Alternatives to antibiotics: A symposium on the challenges and solutions for animal health and production. Antibiotics 2021, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Daly, R.; Jordan, E.; Lee, J.; Mathew, A.; Ebner, P. Extension education symposium: The future of biosecurity and antimicrobial use in livestock production in the United States and the role of extension1. J. Anim. Sci. 2012, 90, 2861–2872. [Google Scholar] [CrossRef]
- Subasinghe, R.; Alday-Sanz, V.; Bondad-Reantaso, M.G.; Jie, H.; Shinn, A.P.; Sorgeloos, P. Biosecurity: Reducing the burden of disease. J. World Aquac. Soc. 2023, 54, 397–426. [Google Scholar] [CrossRef]
- Edwards, L.; Drinking Water Quality and Its Impact on the Health and Performance of Pigs. Co-Operative Research Centre for High Integrity Australian Pork. 2018. Available online: https://porkcrc.com.au/wp-content/uploads/2018/08/2A-118-Drinking-Water-Quality-Final-Report.pdf (accessed on 18 August 2022).
- Mustedanagic, A.; Matt, M.; Weyermair, K.; Schrattenecker, A.; Kubitza, I.; Firth, C.L.; Loncaric, I.; Wagner, M.; Stessl, B. Assessment of microbial quality in poultry drinking water on farms in Austria. Front. Vet. Sci. 2023, 10, 1254442. [Google Scholar] [CrossRef]
- Maes, S.; Vackier, T.; Huu, S.N.; Heyndrickx, M.; Steenackers, H.; Sampers, I.; Raes, K.; Verplaetse, A.; De Reu, K. Occurrence and characterisation of biofilms in drinking water systems of broiler houses. BMC Microbiol. 2019, 19, 77. [Google Scholar] [CrossRef]
- Kamal, M.A.; Alhotan, R.A.; Al Sulaiman, A.R.; Hussein, E.O.; Galik, B.; Saleh, A.A. From source to house: Unraveling the seasonal effect of water distribution system on drinking water quality of poultry farms under Egyptian environmental condition. Env. Sci. Pollut. Res. 2024, 31, 12966–12977. [Google Scholar] [CrossRef]
- Mohammed, A.N.; Mohamed, D.A.; Mohamed, M.B.E.; Bably, M.A.E. Assessment of drinking water quality and new disinfectants for water treatment in a small commercial poultry farm. J. Adv. Vet. Res. 2020, 10, 206–212. [Google Scholar]
- Araújo, D.; Silva, A.R.; Fernandes, R.; Serra, P.; Barros, M.M.; Campos, A.M.; Oliveira, R.; Silva, S.; Almeida, C.; Castro, J. Emerging approaches for mitigating biofilm-formation-associated infections in farm, wild, and companion animals. Pathogens 2024, 13, 320. [Google Scholar] [CrossRef]
- Pineda, M.R.; Byrd, J.A.; Genovese, K.J.; Farnell, Y.Z.; Zhao, D.; Wang, X.; Milby, A.C.; Farnell, M.B. Evaluation of sodium bisulfate on reducing salmonella heidelberg biofilm and colonization in broiler crops and ceca. Microorganisms 2021, 9, 2047. [Google Scholar] [CrossRef]
- Zheng, W.; Ni, L.; Li, B. Electrolyzed water and its application in animal houses. Front. Agric. Sci. Eng. 2016, 3, 195–205. [Google Scholar] [CrossRef]
- Bodas, R.; Bartolomé, D.J.; De Paz, M.J.T.; Posado, R.; García, J.J.; Rodríguez, L.; Olmedo, S.; Martín-Diana, A.B. Electrolyzed water as novel technology to improve hygiene of drinking water for dairy ewes. Res. Vet. Sci. 2013, 95, 1169–1170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Hu, X.; Li, B. Experiment on bactericidal efficacy in drinking system using slightly acidic electrolyzed water in large-scale poultry houses. Trans. Chin. Soc. Agric. Eng. 2017, 33, 230–236. [Google Scholar]
- Block, M.S.; Rowan, B.G. Hypochlorous acid: A review. J. Oral Maxillofac. Surg. 2020, 78, 1461–1466. [Google Scholar] [CrossRef]
- Kim, Y.-R.; Nam, S.-H. Comparison of the preventive effects of slightly acidic HOCl mouthwash and CHX mouthwash for oral diseases. Biomed. Res. 2018, 29, 1718–1723. [Google Scholar] [CrossRef]
- Morita, C.; Nishida, T.; Ito, K. Biological toxicity of acid electrolyzed functional water: Effect of oral administration on mouse digestive tract and changes in body weight. Arch. Oral Biol. 2011, 56, 359–366. [Google Scholar] [CrossRef]
- Inagaki, H.; Shibata, Y.; Obata, T.; Kawagoe, M.; Ikeda, K.; Sato, M.; Toida, K.; Kushima, H.; Matsuda, Y. Effects of slightly acidic electrolysed drinking water on mice. Lab Anim. 2011, 45, 283–285. [Google Scholar] [CrossRef]
- Bügener, E.; Kump, A.W.-S.; Casteel, M.; Klein, G. Benefits of neutral electrolyzed oxidizing water as a drinking water additive for broiler chickens. Poult. Sci. 2014, 93, 2320–2326. [Google Scholar] [CrossRef]
- Hao, X.; Xie, D.; Jiang, D.; Zhu, L.; Shen, L.; Gan, M.; Bai, L. Effect of slightly acidic electrolyzed water on growth, diarrhea and intestinal bacteria of newly weaned piglets. Genes 2023, 14, 1398. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zheng, W.; Xia, T.; Kong, X.; Yuan, Z.; Niu, B.; Wei, G.; Li, B. Comprehensive evaluation of treating drinking water for laying hens using slightly acidic electrolyzed water. Poult. Sci. 2024, 103, 103176. [Google Scholar] [CrossRef]
- Goulet, O. Potential role of the intestinal microbiota in programming health and disease. Nutr. Rev. 2015, 73, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013, 92, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Kogut, M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci. Technol. 2019, 250, 32–40. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Vandeputte, D.; Kathagen, G.; D’hoe, K.; Vieira-Silva, S.; Valles-Colomer, M.; Sabino, J.; Wang, J.; Tito, R.Y.; De Commer, L.; Darzi, Y.; et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 2017, 551, 507–511. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Valles-Colomer, M.; Darzi, Y.; Vieira-Silva, S.; Falony, G.; Raes, J.; Joossens, M. Meta-omics in inflammatory bowel disease research: Applications, challenges, and guidelines. J. Crohn’s Colitis 2016, 10, 735–746. [Google Scholar] [CrossRef]
- Widder, S.; Allen, R.J.; Pfeiffer, T.; Curtis, T.P.; Wiuf, C.; Sloan, W.T.; Cordero, O.X.; Brown, S.P.; Momeni, B.; Shou, W.; et al. Soyer, Challenges in microbial ecology: Building predictive understanding of community function and dynamics. ISME J. 2016, 10, 2557–2568. [Google Scholar] [CrossRef]
- Morton, J.T.; Sanders, J.; Quinn, R.A.; McDonald, D.; Gonzalez, A.; Vázquez-Baeza, Y.; Navas-Molina, J.A.; Song, S.J.; Metcalf, J.L.; Hyde, E.R.; et al. Balance trees reveal microbial niche differentiation. mSystems 2017, 2, e00162–16. [Google Scholar] [CrossRef]
- Props, R.; Kerckhof, F.-M.; Rubbens, P.; De Vrieze, J.; Sanabria, E.H.; Waegeman, W.; Monsieurs, P.; Hammes, F.; Boon, N. Absolute quantification of microbial taxon abundances. ISME J. 2017, 11, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Hui, X.; Shi, Z.; Xi, L.; Ning, L. Effects of drinking slightly acidic electrolyzed water on intestinal microbial and immune function of broilers. J. Northwest Univ. Nat. Sci. Ed. 2020, 48, 27–32. [Google Scholar]
- Wang, Y.; Zhang, J.; Li, B. Study on the effects of slightly acidic electrolyzed water on the intestinal microflora of laying hens. J. China Agric. Univ. 2018, 1, 113–119. [Google Scholar] [CrossRef]
- Wang, X.; Howe, S.; Deng, F.; Zhao, J. Current applications of absolute bacterial quantification in microbiome studies and decision-making regarding different biological questions. Microorganisms 2021, 9, 1797. [Google Scholar] [CrossRef]
- Folorunso, O.R.; Kayode, S.; Onibon, V.O. Poultry farm hygiene: Microbiological quality assessment of drinking water used in layer chickens managed under the battery cage and deep litter systems at three poultry farms in southwestern Nigeria. Pak. J. Biol. Sci. 2014, 17, 74–79. [Google Scholar] [CrossRef]
- Sarkingobir, Y.; Sambo, S.; Tukur, U. Bacterial quality assessment of drinking water for layer chicken managed under battery cage and deep litter systems from Sokoto Metropolis, Nigeria. J. Appl. Sci. Environ. Manag. 2020, 24, 97–103. [Google Scholar] [CrossRef]
- Arbor Acres Group. Arbor Acres Broiler Management Handbook; Arbor Acres: Winston-Salem, NC, USA, 2018; Available online: https://aviagen.com/assets/Tech_Center/AA_Broiler/AA-BroilerHandbook2018-EN.pdf (accessed on 20 October 2022).
- Gao, P.; Ma, C.; Sun, Z.; Wang, L.; Huang, S.; Su, X.; Xu, J.; Zhang, H. Feed-additive probiotics accelerate yet antibiotics delay intestinal microbiota maturation in broiler chicken. Microbiome 2017, 5, 91. [Google Scholar] [CrossRef]
- Goto, K.; Kuwayama, E.; Nozu, R.; Ueno, M.; Hayashimoto, N. Effect of hypochlorous acid solution on the eradication and prevention of Pseudomonas aeruginosa infection, serum biochemical variables, and cecum microbiota in rats. Exp. Anim. 2015, 64, 191–197. [Google Scholar] [CrossRef]
- Lucio, A.J.; Macpherson, J.V. Combined voltammetric measurement of pH and free chlorine speciation using a micro-spot sp2 bonded carbon–boron doped diamond electrode. Anal. Chem. 2020, 92, 16072–16078. [Google Scholar] [CrossRef]
- Prest, E.I.; Hammes, F.; Kötzsch, S.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Monitoring microbiological changes in drinking water systems using a fast and reproducible flow cytometric method. Water Res. 2013, 47, 7131–7142. [Google Scholar] [CrossRef]
- Hammes, F.; Berney, M.; Wang, Y.; Vital, M.; Köster, O.; Egli, T. Flow-cytometric total bacterial cell counts as a descriptive microbiological parameter for drinking water treatment processes. Water Res. 2008, 42, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hoefel, D.; Grooby, W.L.; Monis, P.T.; Andrews, S.; Saint, C.P. Enumeration of water-borne bacteria using viability assays and flow cytometry: A comparison to culture-based techniques. J. Microbiol. Methods 2003, 55, 585–597. [Google Scholar] [CrossRef] [PubMed]
- van de Velde, C.C.; Joseph, C.; Biclot, A.; Huys, G.R.B.; Pinheiro, V.B.; Bernaerts, K.; Raes, J.; Faust, K. Fast quantification of gut bacterial species in cocultures using flow cytometry and supervised classification. ISME Commun. 2022, 2, 40. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Dignum, M.; Magic-Knezev, A.; Ross, P.; Rietveld, L.; Hammes, F. Flow cytometry and adenosine tri-phosphate analysis: Alternative possibilities to evaluate major bacteriological changes in drinking water treatment and distribution systems. Water Res. 2012, 46, 4665–4676. [Google Scholar] [CrossRef]
- Higashimura, Y.; Baba, Y.; Inoue, R.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Hirai, Y.; Ushiroda, C.; Tanaka, Y.; Naito, Y. Effects of molecular hydrogen-dissolved alkaline electrolyzed water on intestinal environment in mice. Med. Gas Res. 2018, 8, 6. [Google Scholar] [CrossRef]
- Meng, W.S.; Zou, Q.; Xiao, Y.; Ma, W.; Zhang, J.; Wang, T.; Li, D. Growth performance and cecal microbiota of broiler chicks as affected by drinking water disinfection and/or herbal extract blend supplementation. Poult. Sci. 2023, 102, 102707. [Google Scholar] [CrossRef]
- Meng, W.S.; Sui, X.; Xiao, Y.; Zou, Q.; Cui, Y.; Wang, T.; Chen, Z.; Li, D. Regulating effects of chlorinated drinking water on cecal microbiota of broiler chicks. Poult. Sci. 2023, 102, 103140. [Google Scholar] [CrossRef]
- Jandova, J.; Schiro, G.; Duca, F.A.; Laubitz, D.; Wondrak, G.T. Exposure to chlorinated drinking water alters the murine fecal microbiota. Sci. Total Environ. 2024, 914, 169933. [Google Scholar] [CrossRef]
- Yasuda, K.; Oh, K.; Ren, B.; Tickle, T.L.; Franzosa, E.A.; Wachtman, L.M.; Miller, A.D.; Westmoreland, S.V.; Mansfield, K.G.; Vallender, E.J.; et al. Biogeography of the intestinal mucosal and lumenal microbiome in the rhesus macaque. Cell Host Microbe 2015, 17, 385–391. [Google Scholar] [CrossRef]
- Jutzi, S. Assessing Quality and Safety of Animal Feeds; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Liu, C.; Shao, D.; Tan, C.; Cao, Y.; Deng, S.; Lim, T.T.; Xu, F. Low-Concentration Hypochlorous Acid Drinking Water Alleviates Broiler Gut Microbial Load While Preserving Overall Growth Performance. Toxics 2025, 13, 48. https://doi.org/10.3390/toxics13010048
Li Z, Liu C, Shao D, Tan C, Cao Y, Deng S, Lim TT, Xu F. Low-Concentration Hypochlorous Acid Drinking Water Alleviates Broiler Gut Microbial Load While Preserving Overall Growth Performance. Toxics. 2025; 13(1):48. https://doi.org/10.3390/toxics13010048
Chicago/Turabian StyleLi, Zonggang, Chang Liu, Dongyan Shao, Chune Tan, Yingqi Cao, Senzhong Deng, Teng Teeh Lim, and Fei Xu. 2025. "Low-Concentration Hypochlorous Acid Drinking Water Alleviates Broiler Gut Microbial Load While Preserving Overall Growth Performance" Toxics 13, no. 1: 48. https://doi.org/10.3390/toxics13010048
APA StyleLi, Z., Liu, C., Shao, D., Tan, C., Cao, Y., Deng, S., Lim, T. T., & Xu, F. (2025). Low-Concentration Hypochlorous Acid Drinking Water Alleviates Broiler Gut Microbial Load While Preserving Overall Growth Performance. Toxics, 13(1), 48. https://doi.org/10.3390/toxics13010048




