Comprehensive Analysis of Unsymmetrical Dimethylhydrazine: Adsorption Behavior, Environmental Fate, and Toxicity Across Contrasting Soil Matrices
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection and Physicochemical Characteristics
2.2. Methods
2.2.1. Adsorption Kinetics Experiment
2.2.2. Isothermal Adsorption Experiment
2.2.3. Phytotoxicity Assessment
2.3. Model Calculation Method and Statistical Analysis
2.3.1. Adsorption Kinetics Calculation
2.3.2. Environmental Toxicity Simulation Calculation Method
2.3.3. Statistical Analysis
3. Results and Discussion
3.1. Characteristics and Mechanisms of Adsorption for UDMH
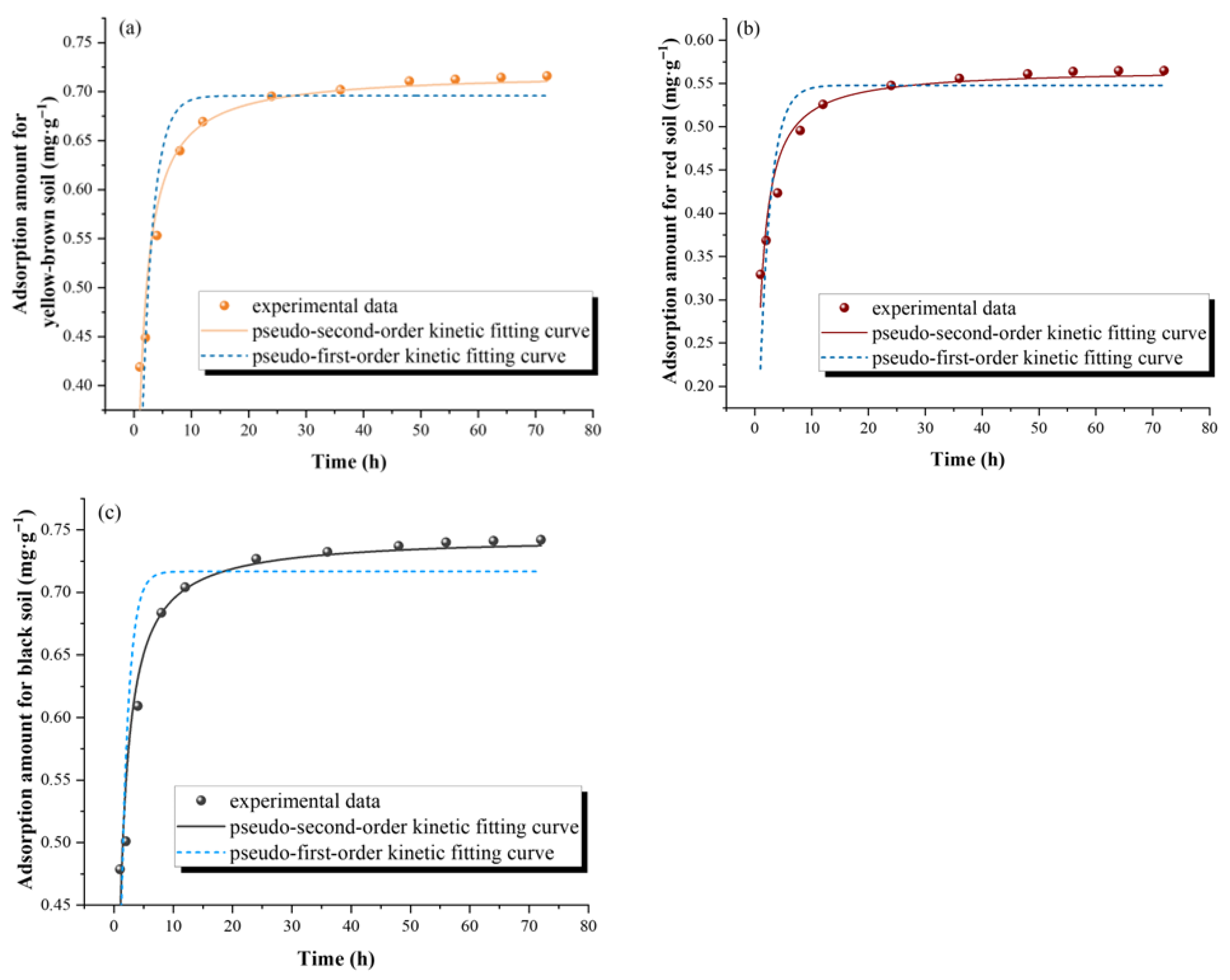
3.1.1. Adsorption Kinetics
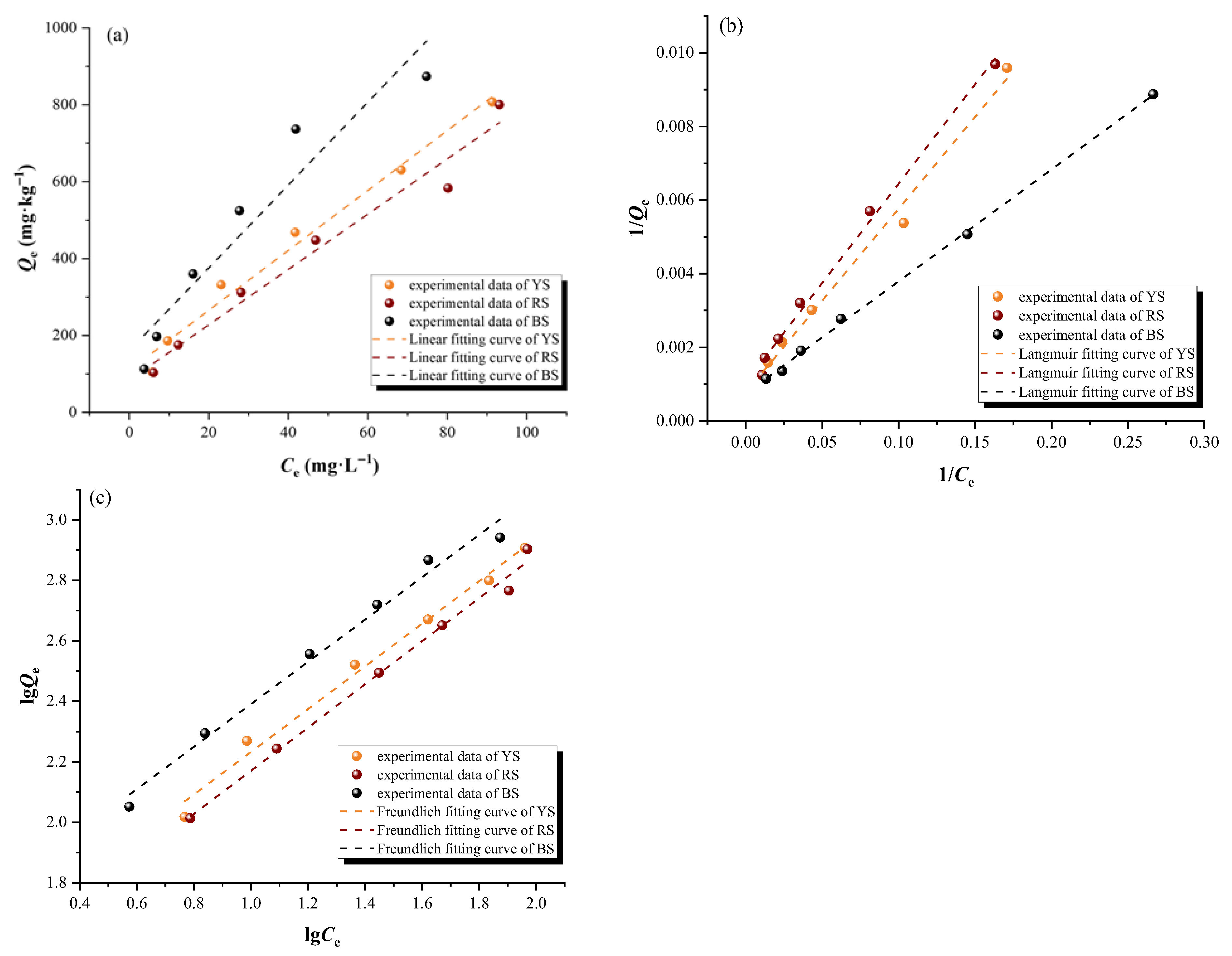
3.1.2. Adsorption Thermodynamic Characteristics
3.2. Correlation Analysis of Soil Characteristics and UDMH Adsorption Behavior
3.3. Environmental Fate and Toxicity of UDMH
3.3.1. Environmental Fate and Toxicity Simulation Calculation
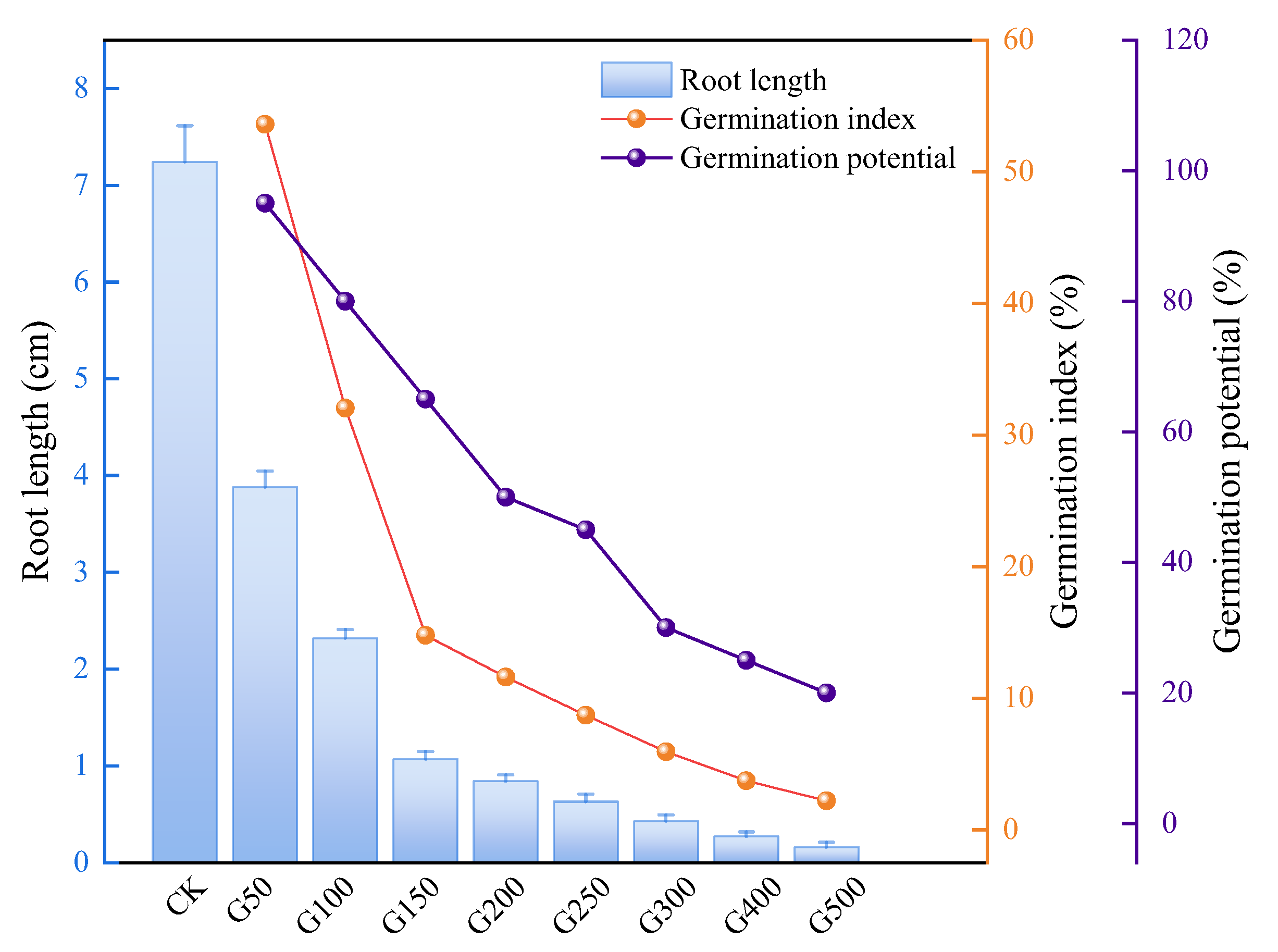
3.3.2. Phytotoxicity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Efremov, S.; Nechipurenko, S.; Tokmurzin, D.; Kaiaidarova, A.K.; Kalugin, S.; Tassibekov, K.S. Remediation of soil contaminated by toxic rocket fuel components using modified carbon-mineral adsorbing material produced from shungite rock modified with Mn4+ and Fe3+. Environ. Technol. Innov. 2021, 24, 101962. [Google Scholar] [CrossRef]
- Popov, M.S.; Ul’yanovskii, N.V.; Kosyakov, D.S. Direct quantification of 1,1-dimethylhydrazine transformation products in sandy soil by thermal desorption gas chromatography—Tandemmass spectrometry. Microchem. J. 2024, 197, 109833. [Google Scholar] [CrossRef]
- Ul’yanovskii, N.V.; Kosyakov, D.S.; Pikovskoi, I.I.; Popov, M.S. Study of the products of oxidation of 1,1-dimethylhydrazine by nitrogen dioxide in an aqueous solution by high-resolution mass spectrometry. J. Anal. Chem. 2019, 73, 1223–1228. [Google Scholar] [CrossRef]
- Wu, G.Q.; Wang, Z.Y.; Yang, C.L.; Wang, H.L.; Nie, W.Z. Experimental study of the evaporation characteristics of unsymmetrical dimethylhydrazine in a sub-supercritical environment. Phys. Fluids 2024, 36, 023321. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Z.R.; Sun, P.F.; Liu, X.Y.; Luo, Z.; Li, J.Y.; Zhang, D.X.; Xu, X.Y. Removal of unsymmetrical dimethylhydrazine: A critical review with particular focus on photocatalytic oxidation. Sep. Purif. Technol. 2023, 312, 123425. [Google Scholar] [CrossRef]
- Chai, Y.; Chen, X.; Wang, Y.; Guo, X.; Zhang, R.; Wei, H.; Jin, H.; Li, Z.; Ma, L. Environmental and economic assessment of advanced oxidation for the treatment of unsymmetrical dimethylhydrazine wastewater from a life cycle perspective. Sci. Total Environ. 2023, 873, 162264. [Google Scholar] [CrossRef]
- Koroleva, T.V.; Semenkov, I.N.; Sharapova, A.V.; Krechetov, P.P.; Lednev, S.A. Ecological consequences of space rocket accidents in Kazakhstan between 1999 and 2018. Environ. Pollut. 2021, 268, 115711. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Chenoweth, J.A.; Bebarta, V.S.; Albertson, T.E.; Nowadly, C.D. The toxicity, pathophysiology, and treatment of acute hydrazine propellant exposure: A systematic review. Mil. Med. 2021, 186, 319–326. [Google Scholar] [CrossRef]
- Su, J.; Jia, Y.; Shi, M.L.; Shen, K.K.; Zhang, J.Q. Highly efficient unsymmetrical dimethylhydrazine removal from wastewater using MIL-53(Al)-derived carbons: Adsorption performance and mechanisms exploration. J. Environ. Chem. Eng. 2022, 10, 108975. [Google Scholar] [CrossRef]
- Popov, M.S.; Ul’yanovskii, N.V.; Kosyakov, D.S. Gas chromatography-mass spectrometry quantification of 1,1-dimethylhydrazine transformation products in aqueous solutions: Accelerated water sample preparation. Molecules 2021, 26, 5673. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Y.; Zhou, Y.; Liu, Z.F.; Feng, X.S. Unsymmetrical dimethylhydrazine and related compounds in the environment: Recent updates on pretreatment, analysis, and removal techniques. J. Hazard. Mater. 2022, 432, 128708. [Google Scholar] [CrossRef]
- Karnaeva, A.E.; Milyushkin, A.L.; Yarykin, D.I. Uptake and leaching of toxic heterocyclic UDMH transformation products in water-plant system. J. Food Compos. Anal. 2025, 141, 107345. [Google Scholar] [CrossRef]
- Milyushkin, A.L.; Karnaeva, A.E. Unsymmetrical dimethylhydrazine transformation products: A review. Sci. Total Environ. 2023, 891, 164367. [Google Scholar] [CrossRef] [PubMed]
- Orazbayeva, D.; Kenessov, B.; Zhakupbekova, A. Quantification of transformation products of unsymmetrical dimethylhydrazine in aqueous extracts from soil based on vacuum-assisted headspace solid-phase microextraction. Chem. Bull. Kazakh Natl. Univ. 2018, 2, 4–11. [Google Scholar] [CrossRef]
- Tabares-Mosquera, O.E.; Ju’arez-Díaz, J.A.; Camacho-Carranza, R.; Ramos-Morales, P. Transgenerational reproductive and developmental toxicity induced by N-nitrosodimethylamine and its metabolite formaldehyde in drosophila melanogaster. J. Appl. Toxicol. 2025, 45, 841–857. [Google Scholar] [CrossRef]
- Su, J.; Jia, Y.; Shi, M.L.; Wang, H.Q.; Wang, Q.R.; Huang, Y.Z.; Shen, K.K.; Zhang, J.Q.; Zhu, X.Y. Highly efficient unsymmetrical dimethylhydrazine adsorption using alginate microspheres encapsulated with ZIF-67-derived carbons. Chem. Eng. J. 2025, 506, 159378. [Google Scholar] [CrossRef]
- Ul’yanovskii, N.V.; Kosyakov, D.S.; Popov, M.S.; Pikovskoi, I.I.; Khoroshev, O.Y. Using a stationary phase based on porous graphitized carbon for the determination of 1,1-dimethylhydrazine transformation products by liquid chromatography-mass spectrometry. J. Anal. Chem. 2020, 75, 510–518. [Google Scholar] [CrossRef]
- Ul’yanovskii, N.V.; Lakhmanov, D.E.; Pikovskoi, I.I.; Falev, D.I.; Popov, M.S.; Yu, A.; Kozhevnikov; Kosyakov, D.S. Migration and transformation of 1,1-dimethylhydrazine in peat bog soil of rocket stage fall site in Russian North. Sci. Total Environ. 2020, 726, 138483. [Google Scholar] [CrossRef]
- Wang, H.Y.; Jia, Y. Escape inhibition of unsymmetrical dimethylhydrazine in aqueous solution by carboxyl-rich graphene oxide loaded with Fe3O4. J. Mol. Liq. 2023, 386, 122240. [Google Scholar] [CrossRef]
- Kenessov, B.; Alimzhanova, M.; Sailaukhanuly, Y.; Baimatova, N.; Abilev, M.; Batyrbekova, S.; Carlsen, L.; Tulegenov, A.; Nauryzbayev, M. Transformation products of 1,1-dimethylhydrazine and their distribution in soils of fall places of rocket carriers in Central Kazakhstan. Sci. Total Environ. 2012, 427–428, 78–85. [Google Scholar] [CrossRef]
- Rodin, I.A.; Smirnov, R.S.; Smolenkov, A.D.; Krechetov, P.P.; Shpigun, O.A. Transformation of unsymmetrical dimethylhydrazine in soils. Eurasia Soil Sci. 2012, 45, 386–391. [Google Scholar] [CrossRef]
- Krechetov, P.P.; Kasimov, N.S.; Koroleva, T.V. Soil-geochemical factors of rocket fuel migration in the landscape. Dokl. Earth Sci. 2015, 464, 1080–1082. [Google Scholar] [CrossRef]
- Koroleva, T.V.; Krechetov, P.P.; Semenkov, I.N.; Sharapova, A.V.; Lednev, S.A.; Karpachevskiy, A.M.; Kondratyev, A.D.; Kasimov, N.S. The environmental impact of space transport. Transp. Res. Part D Transp. Environ. 2018, 58, 54–69. [Google Scholar] [CrossRef]
- Kosyakov, D.S.; Ul’yanovskii, N.V.; Pikovskoi, I.I.; Kenessov, B.N.; Bakaikina, V.; Zhubatov, Z.; Lebedev, A.T. Effects of oxidant and catalyst on the transformation products of rocket fuel 1,1-dimethylhydrazine in water and soil. Chemosphere 2019, 228, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Ul’yanovskii, N.V.; Kosyakov, D.S.; Pikovskoi, I.I.; Khabarov, Y.G. Characterisation of oxidation products of 1,1-dimethylhydrazine by high-resolution orbitrap mass spectrometry. Chemosphere 2017, 174, 66–75. [Google Scholar] [CrossRef] [PubMed]
- USEPA Method 9045D; Soil and Waste pH. U.S. Environmental Protection Agency: Washington, DC, USA, 2004.
- ASTM D2974-13; Standard Test Methods for Moisture, Ash, and Organic Matter of Peat and Other Organic Soils. ASTM International: West Conshohocken, PA, USA, 2014.
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis, Part 2; Soil Science Society America: Madison, WI, USA, 1982; pp. 99–100. [Google Scholar]
- GB/T 14376-93; Water Quality-Determination of Asymmetrical Dimethyl Hydrazine-Amino Ferrocyanide Sodium Spectrophotometric Method. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 1993.
- OECD. Guideline for the Testing of Chemicals; Method 106, Adsorption-Desorption Using Batch Equilibrium Method; OECD: Paris, France, 2000. [Google Scholar]
- Gasco, G.; Cely, P.; Paz-Ferreiro, J.; Plaza, C.; Mendez, A. Relation between biochar properties and effects on seed germination and plant development. Biol. Agric. Hortic. 2016, 32, 237–247. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Li, Y.; Ok, Y.S.; Shen, C.; Xue, S. Biochar provides a safe and value-added solution for hyperaccumulating plant disposal: A case study of Phytolacca acinosa Roxb. (Phytolaccaceae). Chemosphere 2017, 178, 59–64. [Google Scholar] [CrossRef]
- Chen, Y.J.; Li, J.N.; Wang, F.H.; Yang, H.; Liu, L. Adsorption of tetracyclines onto polyethylene microplastics: A combined study of experiment and molecular dynamics simulation. Chemosphere 2021, 265, 129133. [Google Scholar] [CrossRef]
- Liang, X.R.; Liu, L.L.; Jiang, Y.F.; Nan, Z.J.; Deng, X.R.; Ma, F.F.; Wang, G.; Wu, Y.Q. Study of the sorption/desorption behavior of chlortetracycline on sediments in the upper reaches of the Yellow River. Chem. Eng. J. 2022, 428, 131958. [Google Scholar] [CrossRef]
- Jiang, Y.F.; Liang, X.R.; Yuan, L.M.; Nan, Z.J.; Deng, X.R.; Wu, Y.Q.; Ma, F.F.; Diao, J.R. Effect of livestock manure on chlortetracycline sorption behavior and mechanism in agricultural soil in Northwest China. Chem. Eng. J. 2021, 415, 129020. [Google Scholar] [CrossRef]
- Liu, Z.C.; Ren, Y.N.; Zhou, W.J. Effects of kinetics and pH on adsorption of levofloxacin on iron oxide surfaces. J. Agro-Environ. Sci. 2023, 42, 362–372. [Google Scholar]
- Sousa, D.N.R.; Insa, S.; Mozeto, A.A.; Petrovic, M.; Chaves, T.F.; Fadini, P.S. Equilibrium and kinetic studies of the adsorption of antibiotics from aqueous solutions onto powdered zeolites. Chemosphere 2018, 205, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, G.C.; Moodley, B. Kinetic and thermodynamic studies on partitioning of polychlorinated biphenyls (PCBs) between aqueous solution and modeled individual soil particle grain sizes. J. Environ. Sci. 2019, 76, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Liu, L.C.; Chen, W.R. Adsorption of sulfamethoxazole and sulfapyridine antibiotics in high organic content soils. Environ. Pollut. 2017, 231, 1163–1171. [Google Scholar] [CrossRef]
- Yu, Y.; Zhuang, Y.; Wang, Z. Adsorption of water-soluble dyes onto modified resin. Chemosphere 2004, 54, 425–430. [Google Scholar] [CrossRef]
- Rath, S.; Fostier, A.H.; Pereira, L.A.; Dioniso, A.C.; Ferreira, F.O.; Doretto, K.M.; Peruchi, L.M.; Viera, A.; Neto, O.F.O.; Bosco, S.M.; et al. Sorption behaviors of antimicrobial and antiparasitic veterinary drugs on subtropical soils. Chemosphere 2019, 214, 111–122. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sarmah, A.K.; Manley-Harris, M. Sorption of selected veterinary antibiotics onto dairy farming soils of contrasting nature. Sci. Total Environ. 2014, 472, 695–703. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.L.; Liu, D.Q.; Ning, Y.; Yang, S.; Yang, Z. Adsorption of benzene vapor on natural silicate clay minerals under different moisture contents and binary mineral mixtures. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124072. [Google Scholar] [CrossRef]
- Carlsen, L.; Kenesova, O.A.; Batyrbekova, S.E. A preliminary assessment of the potential environmental and human health impact of unsymmetrical dimethylhydrazine as a result of space activities. Chemosphere 2007, 67, 1108–1116. [Google Scholar] [CrossRef]
- Francesca, G.; Viviana, C.; Sara, V.; Marco, V.; Roberto, T. QSAR models for bioconcentration: Is the increase in the complexity justified by more accurate predictions? Chemosphere 2015, 127, 171–179. [Google Scholar] [CrossRef]
- Carlsen, L.; Walker, J.D. QSARs for prioritizing PBT substances to promote pollution prevention. QSAR Comb. Sci. 2003, 22, 49–57. [Google Scholar] [CrossRef]
- Emino, E.R.; Warman, P.R. Biological assay for compost quality. Compost. Sci. Util. 2004, 12, 342–348. [Google Scholar] [CrossRef]

| Items | pH | EC (mS m−1) | CEC (mmol∙kg−1) | OM (g∙kg−1) | K (g∙kg−1) | AK (mg∙kg−1) | P (g∙kg−1) |
|---|---|---|---|---|---|---|---|
| YS | 7.93 | 17.7 | 164.0 | 22.0 | 21.3 | 258 | 0.705 |
| RS | 5.2 | 5.7 | 186.4 | 8.86 | 19.3 | 52 | 0.379 |
| BS | 7.45 | 33.8 | 233.6 | 24.1 | 22.0 | 345 | 0.785 |
| Items | AP (mg∙kg−1) | TN (mg∙kg−1) | NH4-N (mg∙kg−1) | NO3-N (mg∙kg−1) | MBC (mg∙kg−1) | MBN (mg∙kg−1) | MBP (mg∙kg−1) |
| YS | 7.6 | 1328 | 3.07 | 80.9 | 57.1 | 22.2 | 0.646 |
| RS | 1.1 | 938.6 | 1.59 | 42.4 | 17.3 | 11.9 | 0.507 |
| BS | 72.6 | 1552 | 3.91 | 260 | 110 | 92.0 | 0.782 |
| Items | (2.0~0.2 mm), % | (0.2~0.02 mm), % | (0.02~0.002 mm), % | (<0.002 mm), % | Density (g∙cm−3) | ||
| YS | 6.26 | 38.92 | 39.14 | 15.68 | 2.6 | ||
| RS | 15.92 | 46.37 | 36.22 | 1.49 | 2.6 | ||
| BS | 15.01 | 40.20 | 35.66 | 9.13 | 2.6 | ||
| Samples | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|
| k1 (h−1) | Qe (mg·g−1) | R2 | k2 (g·mg−1·h−1) | Qe (mg·g−1) | R2 | |
| YS | 0.511 | 0.696 | 0.842 | 1.459 | 0.720 | 0.959 |
| RS | 0.514 | 0.548 | 0.841 | 1.868 | 0.567 | 0.957 |
| BS | 0.781 | 0.717 | 0.774 | 1.858 | 0.745 | 0.950 |
| Samples | Linear Model | Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|---|---|
| Kd (L·kg−1) | R2 | KL (L·mg−1) | Qm (mg·g−1) | R2 | KF | 1/n | R2 | |
| YS | 7.792 | 0.979 | 0.016 | 1.259 | 0.991 | 33.729 | 0.705 | 0.989 |
| RS | 7.191 | 0.963 | 0.020 | 1.000 | 0.994 | 28.721 | 0.713 | 0.992 |
| BS | 10.771 | 0.894 | 0.014 | 1.428 | 0.998 | 48.955 | 0.700 | 0.986 |
| Compounds | Molecular Formula | CAS No. | SW (mg·L−1) | H (atm·m3·mol−1) | P (kPa) | logKOW | logKOC | logBCF |
|---|---|---|---|---|---|---|---|---|
| 1,1-Dimethylhydrazine | C2H8N2 | 57-14-7 | 1.0 × 106 | 4.409 × 10−5 | 21.7 | −1.19 | 1.077 | 0.5 |
| Tetramethylhydrazine | C4N2H12 | 6415-12-9 | 3.467 × 105 | 4.383 × 10−5 | 17.1 | −0.52 | 1.127 | 0.5 |
| Formaldehyde dimethylhydrazone | C3N2H8 | 2035-89-4 | 3.525 × 104 | 8.883 × 10−4 | 43.7 | 0.68 | 1.358 | 0.5 |
| Acetaldehyde dimethylhydrazone | C4N2H10 | 7422-90-4 | 5.799 × 104 | 1.569 × 10−4 | 10.3 | 0.4 | 1.618 | 0.5 |
| Formaldehyde hydrazone | CN2H4 | 6629-91-0 | 1.305 × 105 | 2.026 × 10−4 | 60.5 | 0.004 | 1.337 | 0.5 |
| Formic acid | CH2O2 | 64-18-6 | 1.0 × 106 | 2.276 × 10−6 | 5.68 | −0.46 | 0 | 0.5 |
| 2-Butanone | C4H8O | 78-93-3 | 2.11 × 105 | 1.223 × 10−4 | 12.1 | 0.256 | 0.654 | 0.5 |
| Dimethylaminoacetonitrile | C4N2H8 | 926-64-7 | 1.0 × 106 | 7.881 × 10−7 | 0.875 | −0.4338 | 0.738 | 0.5 |
| N-Nitrosodimethylamine | C2N2H6O | 62-75-9 | 1.0 × 106 | 1.116 × 10−6 | 0.36 | −0.639 | 1.358 | 0.5 |
| Dimethylamine | C2NH7 | 124-40-3 | 1.63 × 106 | 1.77 × 10−5 | 203 | −0.173 | 0.912 | 0.5 |
| Formamide | CNH3O | 75-12-7 | 1.0 × 106 | 7.763 × 10−9 | 0.00813 | −1.61 | 0 | 0.5 |
| N, N-Dimethylformamide | C3NH7O | 68-12-2 | 1.0 × 106 | 3.462 × 10−7 | 0.516 | −0.934 | 0 | 0.5 |
| Dimethylcyanamide | C3N2H6 | 1467-79-4 | 1.818 × 105 | 1.081 × 10−6 | 0.259 | −0.1336 | 0.69 | 0.5 |
| 1,1,4,4-Tetramethyl-2-tetrazene | C4N4H12 | 6130-87-6 | 2.647 × 104 | 1.23 × 10−4 | 2.67 | 0.694 | 0.992 | 0.5 |
| Compounds | Model-Predicted Soil Half-Life (h) | Model-Predicted Sediment Half-Life (h) | BDP3 | Model-Predicted Biodegradation Half-Life | Is It Easily Biodegradable? |
|---|---|---|---|---|---|
| 1,1-Dimethylhydrazine | 720 | 3240 | 3.0664 | weeks | NO |
| Tetramethylhydrazine | 720 | 3240 | 3.0044 | weeks | YES |
| Formaldehyde dimethylhydrazone | 720 | 3240 | 3.0398 | weeks | YES |
| Acetaldehyde dimethylhydrazone | 720 | 3240 | 3.0088 | weeks | NO |
| Formaldehyde hydrazone | 720 | 3240 | 3.1018 | weeks | YES |
| Formic acid | 416 | 1870 | 3.4621 | days–weeks | YES |
| 2-Butanone | 720 | 3240 | 3.0173 | weeks | YES |
| Dimethylaminoacetonitrile | 1800 | 8100 | 2.6761 | weeks–months | NO |
| N-Nitrosodimethylamine | 1800 | 8100 | 2.6503 | weeks–months | NO |
| Dimethylamine | 720 | 3240 | 3.1240 | weeks | YES |
| Formamide | 720 | 3240 | 3.0454 | weeks | YES |
| N,N-Dimethylformamide | 720 | 3240 | 2.9834 | weeks | YES |
| Dimethylcyanamide | 720 | 3240 | 3.0443 | weeks | YES |
| 1,1,4,4-Tetramethyl-2-tetrazene | 720 | 3240 | 2.9425 | weeks | YES |
| Compounds | LC50 (mg∙L−1) | EC50 (mg∙L−1) | ChV (mg∙L−1) | LC50 (mg∙kg−1∙dw−1) | |||
|---|---|---|---|---|---|---|---|
| Fish (96 h) Acute a /Baseline b | Daphnid (48 h) Acute a /Baseline b | Green Algae (96 h) Acute a /Baseline b | Fish Chronic c/ Baseline b | Daphnid Chronic c/ Baseline b | Green Algae Chronic c/Baseline b | Earthworm | |
| 1,1-Dimethylhydrazine | 8.056 a 36,392.77 b | 79.28/15,387.91 | 1.574/3387.64 | 1.148/2512.41 | 9.98/660.62 | 0.319/460.12 | _ |
| Tetramethylhydrazine | 6.757/13,160.76 | 56.37/5923.76 | 1.411/1688.80 | 0.788/978.10 | 6.227/302.65 | 0.312/263.64 | _ |
| Formaldehyde dimethylhydrazone | 2.06/908.01 | 12.83/456.42 | 0.484/205.40 | 0.168/76.87 | 1.126/31.71 | 0.125/41.0 | _ |
| Acetaldehyde dimethylhydrazone | 3.103/1939.33 | 20.70/949.84 | 0.709/383.98 | 0.276/159.23 | 1.917/61.39 | 0.177/72.35 | _ |
| Formaldehyde hydrazone | 2.198/2243.61 | 16.151/1059.55 | 0.483/368.41 | 0.220/176.46 | 1.614/61.87 | 0.114/64.01 | _ |
| Formic acid | 6128.83 b | 2772.75 b | 807.35 b | 458.24 b | 143.69 b | 127.48 b | 144.0 |
| 2-Butanone | 2181.62 b | 1054.51 b | 403.66 b | 176.36 b | 65.70 b | 73.86 b | 190.12 |
| Dimethylaminoacetonitrile | 645.62/10,685.8 | 57.97/4844.57 | 83.79/1422.93 | 89.17/800.95 | 3.615/252.53 | 22.68/225.74 | _ |
| N-Nitrosodimethylamine | 770.51/14,257.56 | 67.58/6345.08 | 102.31/1726.07 | 114.28/1045.54 | 4.12/314.10 | 27.23/262.74 | _ |
| Dimethylamine | 231.70/3310.12 | 21.45/1537.88 | 29.18/499.81 | 29.13/255.38 | 1.379/85.81 | 0.074/83.73 | _ |
| Formamide | 5140.35/64,724.62 | 29,875.42/26,331.54 | 74.18/4942.03 | 1.03/4269.52 | 154.53/1015.34 | 15.19/615.97 | _ |
| N,N-Dimethylformamide | 2735.77/25,896.8 | 11,638.91/11,215.14 | 45.69/2725.82 | 0.82/1839.0 | 94.13/514.64 | 12.45/390.5 | _ |
| Dimethylcyanamide | 4767.25 b | 222.44 b | 732.57 b | 369.28 b | 125.20 b | 123.66 b | 202.93 |
| 1,1,4,4-Tetramethyl-2-tetrazene | 1421.32 b | 715.35 b | 323.64 b | 120.51 b | 49.87 b | 64.79 b | 275.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, J.; Ren, X.; Zeng, Y.; Zhang, L.; Shi, J.; Yang, S. Comprehensive Analysis of Unsymmetrical Dimethylhydrazine: Adsorption Behavior, Environmental Fate, and Toxicity Across Contrasting Soil Matrices. Toxics 2025, 13, 859. https://doi.org/10.3390/toxics13100859
Du J, Ren X, Zeng Y, Zhang L, Shi J, Yang S. Comprehensive Analysis of Unsymmetrical Dimethylhydrazine: Adsorption Behavior, Environmental Fate, and Toxicity Across Contrasting Soil Matrices. Toxics. 2025; 13(10):859. https://doi.org/10.3390/toxics13100859
Chicago/Turabian StyleDu, Juan, Xianghong Ren, Yizhi Zeng, Lei Zhang, Jinfeng Shi, and Shuai Yang. 2025. "Comprehensive Analysis of Unsymmetrical Dimethylhydrazine: Adsorption Behavior, Environmental Fate, and Toxicity Across Contrasting Soil Matrices" Toxics 13, no. 10: 859. https://doi.org/10.3390/toxics13100859
APA StyleDu, J., Ren, X., Zeng, Y., Zhang, L., Shi, J., & Yang, S. (2025). Comprehensive Analysis of Unsymmetrical Dimethylhydrazine: Adsorption Behavior, Environmental Fate, and Toxicity Across Contrasting Soil Matrices. Toxics, 13(10), 859. https://doi.org/10.3390/toxics13100859







