Red Mud as an Adsorbent for Hazardous Metal Ions: Trends in Utilization
Abstract
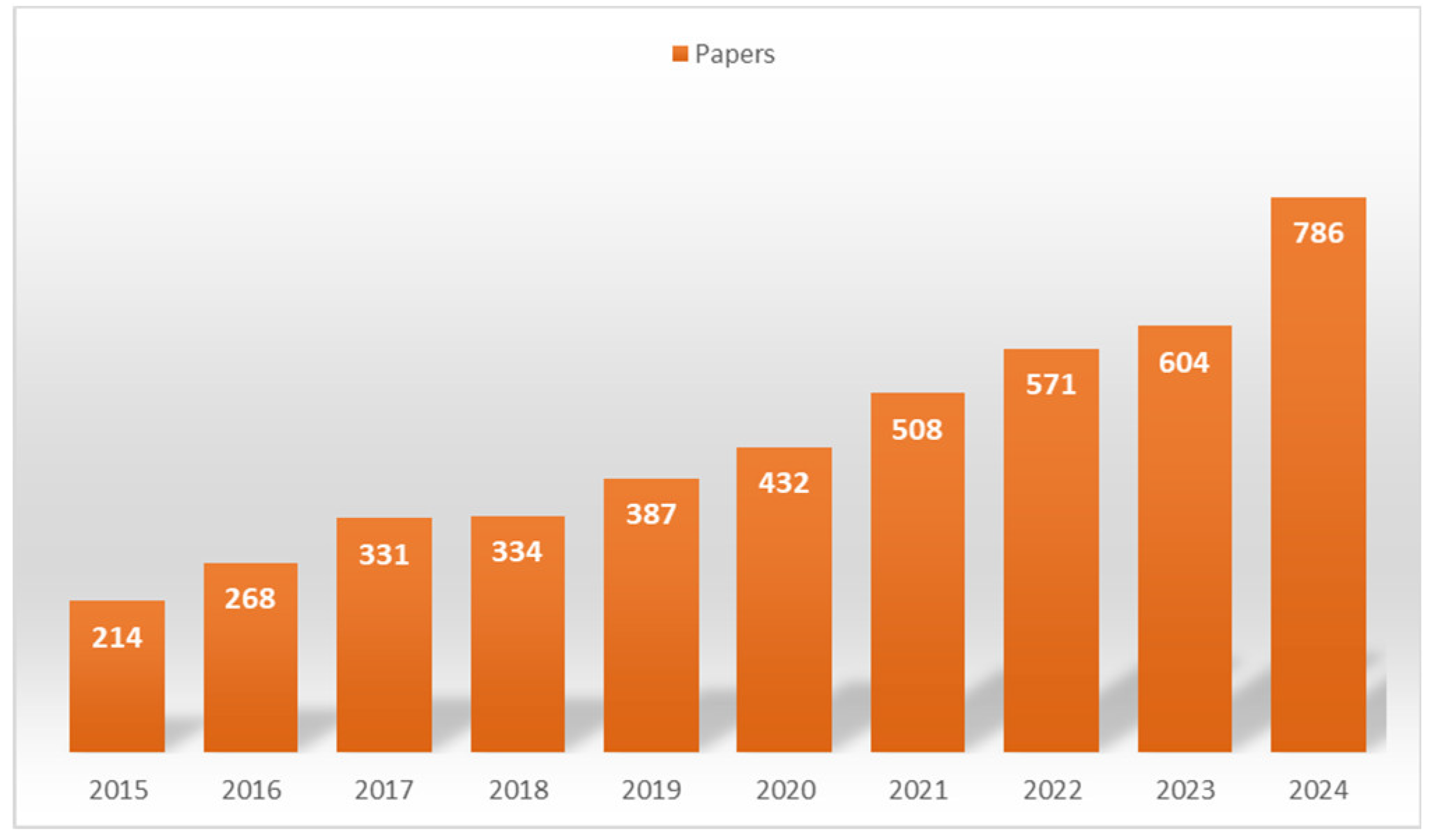
1. Introduction
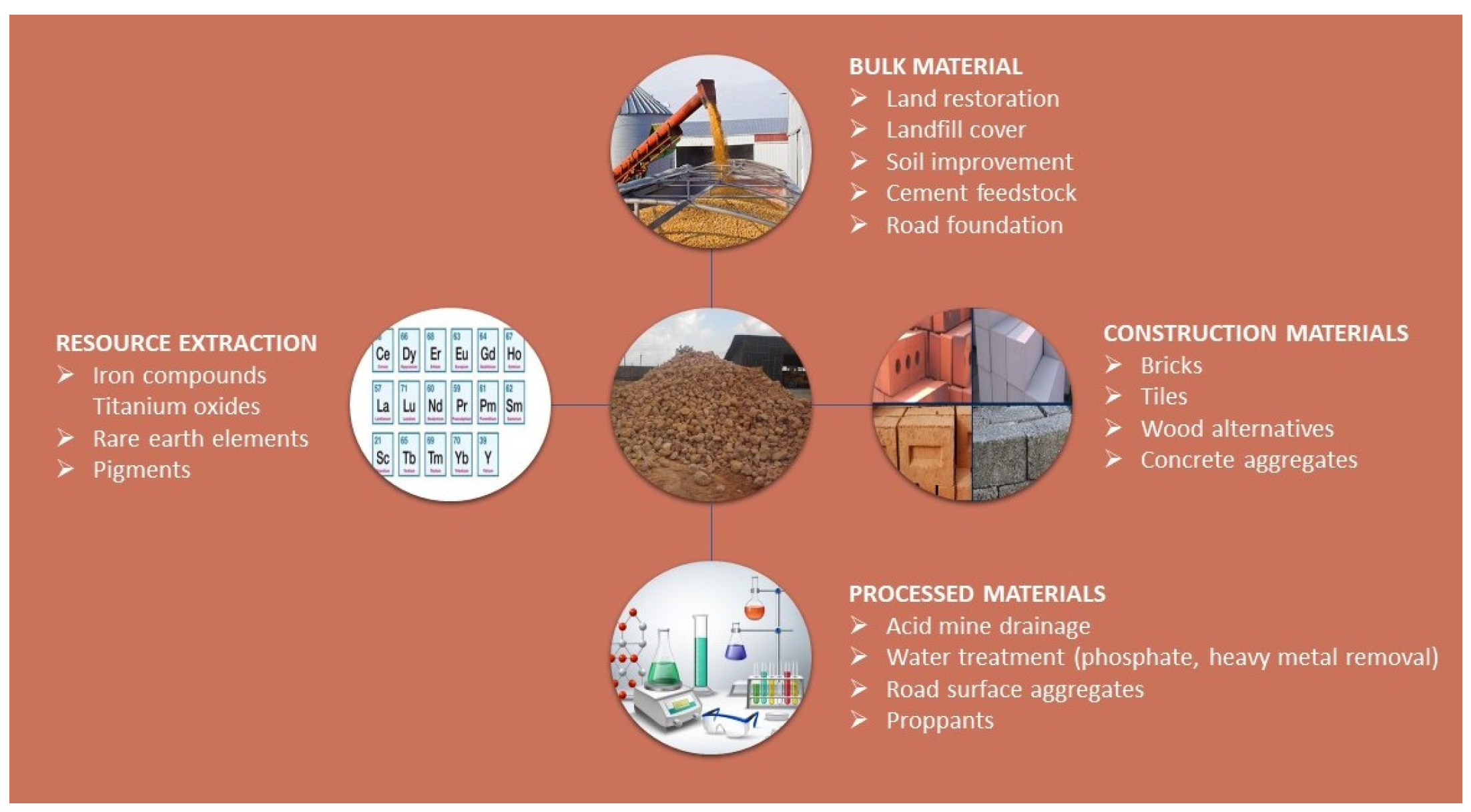
2. Properties and Adsorption Potential of Red Mud
2.1. Red Mud Characterization
2.2. Adsorbent Properties
3. Literature Overview
| A Sample of Red Mud and the Location | Method of Characterization | Adsorption Mechanism | Adsorbent Dose | Applied Adsorbent Modification Method | Pollutant | Adsorption Capacity | Ref. |
|---|---|---|---|---|---|---|---|
| “Birač” Alumina Factory, Bosnia and Herzegovina | ICP-AES | Inner-sphere complex and/or precipitation/co-precipitation | 0.1 g | Dried, powdered raw red mud (without pretreatment) | Co2+ (laboratory-prepared solution) | 0.52 mmol/g | [46] |
| Electrostatic interactions and the ion exchange mechanism | Sr2+ (laboratory-prepared solution) | 0.31 mmol/g | |||||
| Specific cation adsorption | |||||||
| AAS | Inner-sphere complex and/or precipitation/co-precipitation | 0.1 g | Rinsed red mud | Co2+ (laboratory-prepared solution) | 0.203 mmol/g | [47] | |
| Specific cation adsorption | Sr2+ (laboratory-prepared solution) | 0.117 mmol/g | |||||
| TGA/DT and AAS | Precipitation of Ni(OH)2 | 5 g/L | Rinsed, heat-treated red mud at | Ni2+ (laboratory-prepared solution) | 27.54 mg/g | [48] | |
| XRD and AAS | Precipitation of Ni(OH)2 | 5 g/L | Rinsed, heat-treated red mud at 600 °C | Ni2+ (laboratory-prepared solution) | 0.372 mmol/g | [49] | |
| AAS, XRD, and FT-IR | Precipitation/co-precipitation | 5 g/L | Raw red mud powder | Ni(II) and citrate ions (laboratory-prepared solutions) | 27.4 mg/g (1:0) * | [50] | |
| 25 mg/g (1:0.25) * | |||||||
| 21 mg/g (1:0.5) * | |||||||
| 7.6 mg/g (1:1) * | |||||||
| 2.5 mg/g (1:2) * | |||||||
| XRD, ATR-FT-IR, and AAS | Precipitation of | 5 g/L | Acid-treated red mud | Ni(II) (laboratory-prepared solution) | 11.8 mg/g (0.05 mol/L HCl) | [51] | |
| 9.6 mg/g (0.1 mol/L HCl) | |||||||
| 1.1 mg/g (0.25 mol/L HCl) | |||||||
| 1.5 mg/g (1 mol/L HCl) | |||||||
| ICP-OES | Co2+ inner-sphere complexes and/or the surface precipitates | 0.1 g | Rinsed red mud | Co2+, Sr2+, and Cs+ (simulated wastes; laboratory-prepared solutions) | Co2+ 0.16–0.44 mmol/g | [52] | |
| Sr2+-specific, with the contribution of ion exchange | Sr2+ 0.029–0.19 mmol/g | ||||||
| Cs+ irreversible fixation and partly ion exchange | Cs+ 0.017 mmol/g | ||||||
| Vedanta Aluminum Industries, Langigarh, Odisha, India | XRD, SEM, EDX, BET, and FT-IR | Metal ion exchange | 0.4 g | Thermally activated acid-neutralized red mud () | Pb(II) (synthetic sample) | 6.0273 mg/g | [53] |
| Alumina factory, north China | XRD, FT-IR, BET, CEC, XPS, and sequential extraction | Metal–metal ion exchange and the specific adsorption (inner-sphere complex formation) | 10 g | Heat-treated red mud at | Cd(II) (synthetic sample) | 42.64 mg/g | [54] |
| Alumina production plant, southwest China (disposal landfill) | ICP-OES, FT-IR, SEM, and TEM | Physical adsorption and chemical co-precipitation | 40 g/L | Non-treated | (wastewater from non-ferrous smelter) | 101.5 mg/g | [55] |
| Pingguo Aluminum Co.Ltd., Baise city, Guangxi province, China | FT-IR, SEM, and XRD | Physical adsorption | 3.0 g/L | Nitric acid-neutralized red mud | (standard solution) | 29.42 mg/g | [56] |
| Endothermic chemical adsorption | Ferric nitrate-modified red mud | 32.92 mg/g | |||||
| Physical adsorption | Aluminum nitrate-modified red mud | 30.64 mg/g | |||||
| XRD, DTG, SEM, FT-IR, and ICP-MS | Surface electrostatic attraction, physical adsorption by porous structure, and chemical adsorption | 3.0 g/L | Carbon-calcined red mud at | (laboratory-treated natural sample) | 48.85 mg/g | [57] | |
| Iron and carbon-combined calcined red mud at | 59.45 mg/g | ||||||
| Shandong Aluminum Industry Company, China | XRF, XRD, BET, XPS, TEM, SEM, TGA, and ICP-AES | Specific adsorption (involves the formation of supported nano zero-valent iron) | 0.20 g | Carbothermally treated mixed red mud–coal powder (previous acid-neutralized red mud) | (synthetic sample) | Not specified | [58] |
3.1. Literature Finding Discussion
3.2. Red Mud Adsorption Features vs. Other Waste Materials
| Adsorbent | Target Pollutant | Adsorption Capacity, mmol/g | Main Findings | Ref. |
|---|---|---|---|---|
| Raw red mud | Co2⁺ | 0.520 | Potential in the efficient removal of Co2⁺ and Sr2⁺ simultaneously. | [46] |
| Sr2⁺ | 0.310 | |||
| Cd2⁺ | 0.286 | The adsorption rate of untreated red mud was approximately four times slower compared to that of red mud subjected to thermal treatment at 500 °C. | [54] | |
| Rinsed red mud | Co2⁺ | 0.510 | An increase in the initial pH and metal concentration resulted in higher sorption capacities. For the most diluted solution with an initial pH of 5, equilibrium for both cations was achieved almost immediately. | [47] |
| Sr2⁺ | 0.205 | |||
| Ni2⁺ | 0.372 | Prove to be an economical, composite adsorbent for aqueous Ni2+ ions. | [49] | |
| Co2⁺ | 0.160–0.440 | The dominant interaction between the cations resulted in diminished Sr2⁺ sorption in the presence of Co2⁺. | [52] | |
| Sr⁺ | 0.029–0.190 | |||
| Cs⁺ | 0.017 | |||
| Thermally treated red mud | Ni2⁺ | 0.470 | Improvement by annealing raw red mud powder at the optimum heating temperature of 600 °C, leading to improved adsorption efficiency. | [49] |
| Cd2⁺ | 0.379 | Thermal treatment at 500 °C yielded the highest sorption capacity, as this temperature notably enhanced both the specific surface area and the maximum sorption capacity. | [54] | |
| Acid-treated red mud | Ni2⁺ | 0.019–0.201 | Findings revealed that acid treatment significantly altered red mud’s mineralogical composition and surface characteristics (higher acid concentrations led to reduced adsorption efficiency). | [54] |
| Pb2⁺ | ≈0.030 | The removal efficiency gradually increased as the pH decreased, with the highest removal observed at pH 4. | [53] | |
| Hydrothermally modified red mud | Pb2⁺ | 2.662 | The modification using colloidal silica and NaOH led to the formation of a zeolite structure, resulting in a significant enhancement of adsorption capacity, increasing it several times. | [59] |
| Adsorbent | Target Pollutant | Adsorption Capacity, mmol/g | Main Findings | Ref. |
|---|---|---|---|---|
| Calcined bovine bones | Co2⁺ | 0.140–0.460 | Co2+ adsorbed adequately in the presence of Sr2+ and Cs+ ions; removal of Sr2+ was more suppressed in the presence of Co2+ than Cs+ ions; for Cs+ ions, the assessment of the influence of coexisting cations is impractical. | [52] |
| Sr⁺ | 0.070–0.310 | |||
| Cs⁺ | 0–0.030 | |||
| Waste concretes | Sr⁺ | 0.250 | The results revealed that C&DW has the potential to separate metal pollutants from aqueous solutions. Findings highlight the potential of cement-based materials in treating and conditioning radioactive waste due to their high adsorption capacity. | [68,69] |
| Co2 | 0.270–0.320 | |||
| Ni2⁺ | 0.130–0.540 | |||
| Waste bricks | Sr⁺ | 0.010–0.050 | ||
| Co2 | 0.030–0.060 | |||
| Ni2⁺ | 0.130–0.170 | |||
| Facade material | Sr⁺ | 0.250 | ||
| Co2⁺ | 0.120 | |||
| Ni2⁺ | 0.300 | |||
| Waste asphalt | Sr⁺ | 0.020 | ||
| Co2⁺ | 0.060 | |||
| Ni2⁺ | 0.130 | |||
| Ceramic tiles | Sr⁺ | 0.030 | Based on the predicted adsorption capacities of metals from a multi-component solution, and considering the generated amounts of this waste, it is assumed that it can be used in the treatment of wastewater. | [72] |
| Co2⁺ | 0.170 | |||
| Ni2⁺ | 0.120 | |||
| Roof tiles | Sr⁺ | 0.030 | Similar potential for wastewater treatment is assumed for roof tiles. | [72] |
| Co2⁺ | 0.060 | |||
| Ni2⁺ | 0.100 | |||
| Zeolite | Co2⁺ | 0.013–0.056 | Co2+ removal was suppressed in the presence of other ions in multi-component mixtures; least selective towards Sr2+ and best adsorbent for Cs+ ions. | [55] |
| Sr2+ | 0.049–0.140 | |||
| Cs+ | 0.140–0.680 | |||
| Copper slag flotation tailings (CSFT) | Cd(II) | 0.081 | Limited effectiveness in removing metal ions from aqueous solutions (sorption capacities decreased in the sequence Cd(II) > Pb(II) > Zn(II) > Mn(II) > Ni(II) > Co(II)); potential as a low-cost and easily accessible sorbent. | [73] |
| Pb(II) | 0.035 | |||
| Zn(II) | 0.032 | |||
| Mn(II) | 0.029 | |||
| Ni(II) | 0.022 | |||
| Co(II) | 0.012 |
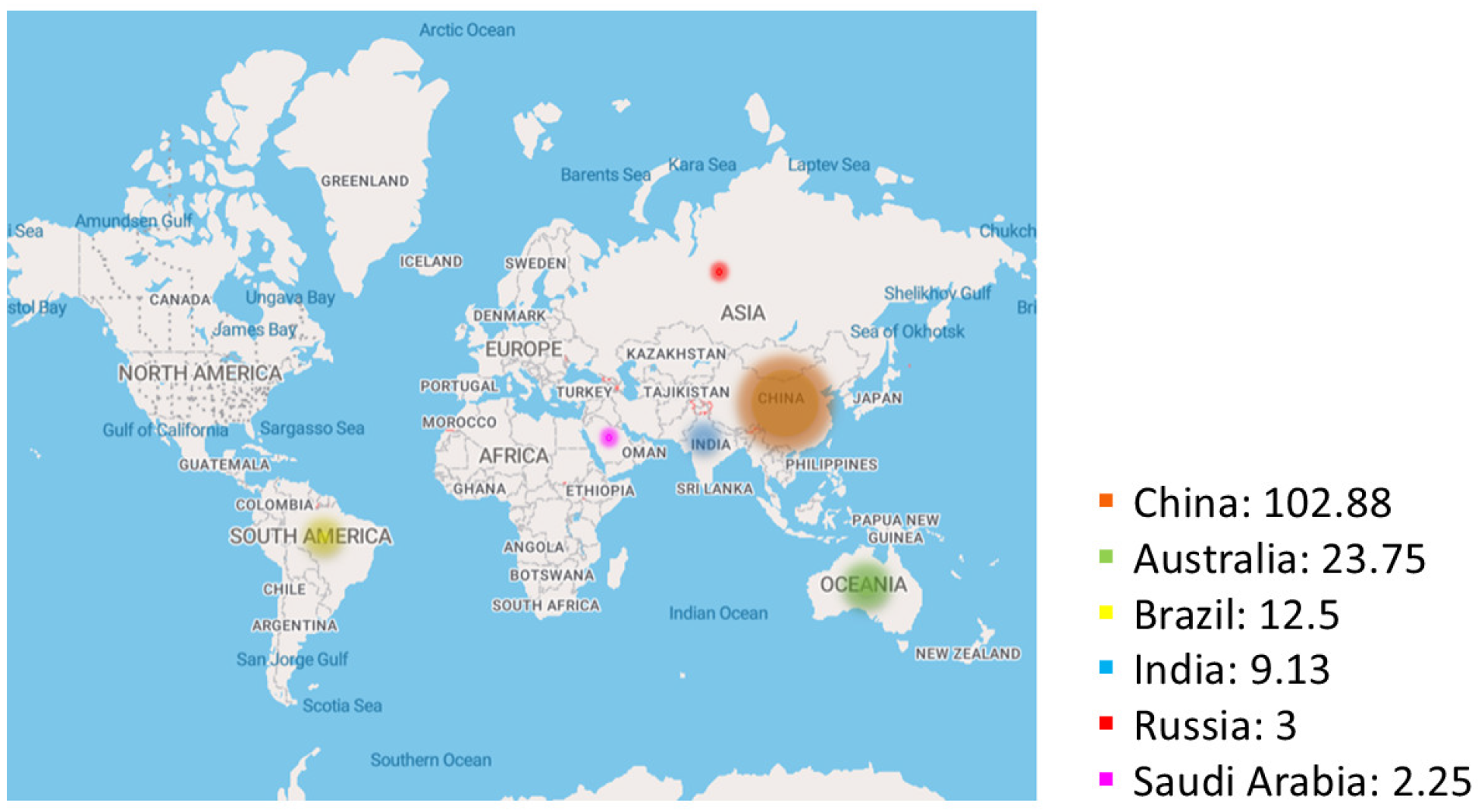
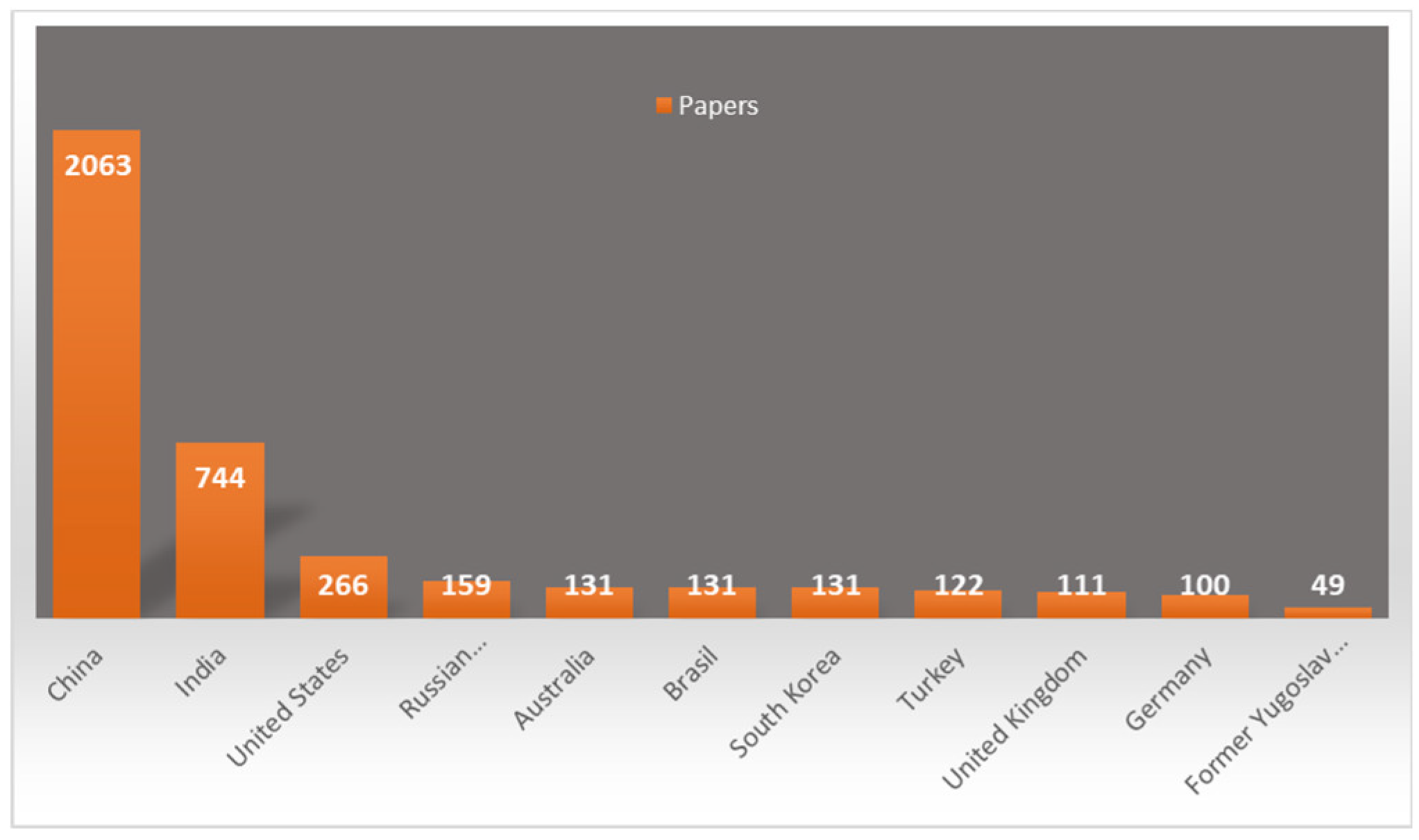
3.3. Geographical Spread
4. Bauxite Residue Applications in Wastewater Treatment
4.1. Recent Trends
4.2. The Drawbacks and Advantages
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Nies, D.; Silver, S. Molecular Microbiology of Heavy Metals; Springer: Berlin, Germany, 2007; p. 451. [Google Scholar]
- Nieboer, E.; Richardson, D.H. The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environ. Pollut. Ser. B Chem. Phys. 1980, 1, 2–26. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X. Applications of red mud as an environmental remediation material: A review. J. Hazard. Mater. 2021, 408, 124420. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Huang, M.R.; Wang, H.Y.; Peng, Q.Y. Strong Adsorbents for Heavy and Noble-Metal Ions. In Milestones in Powerful Adsorbents of Heavy-Metal Ions, 1st ed.; Li, X.G., Huang, M.R., Eds.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2024; pp. 2–53. [Google Scholar]
- Yamashita, K.; Kurita, K.; Ohara, K.; Tamura, K.; Nango, M.; Tsuda, K. Syntheses of thiacrown ethers polymers and their application for heavy metal ion adsorbents. React. Funct. Polym. 1996, 31, 47–55. [Google Scholar] [CrossRef]
- Smičiklas, I.; Jović, M.; Janković, M.; Smiljanić, S.; Onjia, A. Environmental safety aspects of solid residues resulting from acid mine drainage neutralization with fresh and aged red mud. Water Air Soil. Pollut. 2021, 232, 490. [Google Scholar] [CrossRef]
- Mi, H.; Yi, L.; Wu, Q.; Xia, J.; Zhang, B. A review of comprehensive utilization of red mud. Waste Manag. Res. 2022, 40, 1594–1607. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ang, H.M.; Tadé, M.O. Novel applications of red mud as coagulant, adsorbent and catalyst for environmentally benign processes. Chemosphere 2008, 72, 1621–1635. [Google Scholar] [CrossRef] [PubMed]
- Sutar, H.; Chandra Mishra, S.; Kumar Sahoo, S.; Prasad Chakraverty, A.; Sekhar Maharana, H. Progress of Red Mud Utilization: An Overview. Chem. Sci. Int. J. 2014, 4, 255–279. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R.; Ming, H. Red mud as an amendment for pollutants in solid and liquid phases. Geoderma 2011, 163, 1–12. [Google Scholar] [CrossRef]
- Altenpohl, D.G. Materials in World Perspective; Springer: Berlin, Germany, 1980; p. 217. [Google Scholar]
- Askeland, D.R. Polymers. In The Science and Engineering of Materials, 6th ed.; Askeland, D.R., Fulay, P.P., Wright, W.J., Eds.; Springer: Boston, MA, USA, 1996; pp. 488–548. [Google Scholar]
- Archambo, M.S.; Kawatra, S.K. Utilization of Bauxite Residue: Recovering Iron Values Using the Iron Nugget Process. Miner. Process. Extr. Metall. Rev. 2020, 42, 222–230. [Google Scholar] [CrossRef]
- Li, X.-F.; Zhang, T.-A.; Lv, G.-Z.; Wang, K.; Wang, S. Summary of Research Progress on Metallurgical Utilization Technology of Red Mud. Minerals 2023, 13, 737. [Google Scholar] [CrossRef]
- Akcil, A.; Swami, K.R.; Gardas, R.L.; Hazrati, E.; Dembele, S. Overview on Hydrometallurgical Recovery of Rare-Earth Metals from Red Mud. Minerals 2024, 14, 587. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater. Environ. Technol. 2011, 32, 231–249. [Google Scholar] [CrossRef]
- Vuković, J.; Perušić, M.; Stopić, S.; Kostić, D.; Smiljanić, S.; Filipović, R.; Damjanović, V. A review of the red mud utilization possibilities. Ovidius Univ. Ann. Chem. 2024, 35, 165–173. [Google Scholar] [CrossRef]
- Samal, S. Utilization of Red Mud as a Source for Metal Ions—A Review. Materials 2021, 14, 2211. [Google Scholar] [CrossRef] [PubMed]
- Taneez, M.; Hurel, C. A review on the potential uses of red mud as amendment for pollution control in environmental media. Environ. Sci. Pollut. Res. 2019, 26, 22106–22125. [Google Scholar] [CrossRef]
- Saveliev, S.G.; Yarosh, T.P.; Kondratenko, M.M.; Babaievska, O.V.; Baboshko, D.Y. Current state and prospects of red mud utilisation: A review. Earth Environ. Sci. 2024, 1415, 012021. [Google Scholar] [CrossRef]
- Cui, W.; Cui, Q.; Dong, X.; Liu, J.; Song, K.; Xie, M.; Yao, X. Current research status and emerging trends in utilization of red mud resources: A study based on bibliometric network analysis. Constr. Build. Mater. 2024, 442, 137605. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Z.; Wang, K.; Wang, F.; Jiang, H.; Liang, M.; Wei, J.; Airey, G. Sustainable utilization of bauxite residue (Red Mud) as a road material in pavements: A critical review. Constr. Build. Mater. 2021, 270, 121419. [Google Scholar] [CrossRef]
- Hind, A.R.; Bhargava, S.K.; Grocott, S.C. The surface chemistry of Bayer process solids: A review. Colloids Surf. A Physicochem. Eng. Asp. 1999, 146, 359–374. [Google Scholar] [CrossRef]
- Shanghai Metals Market (SMM)—European Waste Catalogue. Available online: https://news.metal.com/newscontent/103082883/Red-mud-generation-trend-across-major-countries,-2018-to-2023/ (accessed on 14 January 2025).
- International Aluminium Institute—Primary Aluminium Production. Available online: https://international-aluminium.org/statistics/primary-aluminium-production (accessed on 14 January 2025).
- Your Dsposal—European Waste Catalogue. Available online: https://dsposal.uk/ewc-codes/01/01-03/01-03-10star (accessed on 25 June 2024).
- Mintaș, O.S.; Simeanu, C.; Berchez, O.; Marele, D.C.; Osiceanu, A.G.; Rusu, T. Impact of Red Sludge Dumps, Originating from Industrial Activity, on the Soil and Underground Water. Water 2023, 15, 898. [Google Scholar] [CrossRef]
- Wang, S.; Jin, H.; Deng, Y.; Xiao, Y. Comprehensive utilization status of red mud in China: A critical review. J. Clean. Prod. 2021, 289, 125136. [Google Scholar] [CrossRef]
- Dimović, S.; Šljivić-Ivanović, M.; Jelić, I. Utilization of waste materials in heavy metals and radionuclides imobilization by sorption. Tehnika 2019, 74, 337–344. [Google Scholar] [CrossRef]
- Smičiklas, I.; Jović, M.; Šljivić-Ivanović, M.; Milenković, A.; Smiljanić, S. Metals speciation in bauxite residue with implications to its use as an immobilisation agent. In Proceedings of the Bauxite Residue Valorisation and Best Practices Conference, Leuven, Belgium, 5–7 October 2015; pp. 241–247. [Google Scholar]
- Chai, W.S.; Cheun, J.Y.; Kumar, P.S.; Mubashir, M.; Majeed, Z.; Banat, F.; Ho, S.H.; Show, P.L. A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application. J. Clean. Prod. 2021, 296, 126589. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D.Y. Physical and Chemical Properties of Sintering Red Mud and Bayer Red Mud and the Implications for Beneficial Utilization. Materials 2012, 5, 1800–1810. [Google Scholar] [CrossRef]
- Wang, L.; Sun, N.; Tang, H.; Sun, W. A Review on Comprehensive Utilization of Red Mud and Prospect Analysis. Minerals 2019, 9, 362. [Google Scholar] [CrossRef]
- Silveira, N.C.G.; Martins, M.L.F.; Bezerra, A.C.S.; Araújo, F.G.S. Red Mud from the Aluminium Industry: Production, Characteristics, and Alternative Applications in Construction Materials—A Review. Sustainability 2021, 13, 12741. [Google Scholar] [CrossRef]
- Milenković, A.; Smičiklas, I.; Bundaleski, N.; Teodoro, O.M.; Veljović, Đ.; Vukelić, N. The role of different minerals from red mud assemblage in Co(II) sorption mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 8–20. [Google Scholar] [CrossRef]
- Paramguru, R.K.; Rath, P.C.; Misra, V.N. Trends in red mud utilization—A review. Min. Process Extr. Met. Rev. 2005, 26, 1–29. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): Recovery of metals. Waste Manag. 2013, 33, 2662–2673. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Lu, J.; Zhou, Y. Novel cyclodextrin-based adsorbents for removing pollutants from wastewater: A critical review. Chemosphere 2020, 241, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Anil, I.; Gunday, S.T.; Bozkurt, A.; Alagha, O. Design of crosslinked hydrogels comprising poly (vinylphosphonic acid) and bis [2-(methacryloyloxy) ethyl] phosphate as an efficient adsorbent for wastewater dye removal. Nanomaterials 2020, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, B. Guidelines for general adsorption kinetics modeling. Hem. Ind. 2020, 74, 65–70. [Google Scholar] [CrossRef]
- Qi, Y. The neutralization and recycling of red mud—A review. J. Phys. Conf. Ser. 2021, 1759, 012004. [Google Scholar] [CrossRef]
- Collin, G.J.; Yun Hin, T.Y.; Vigneswar, K.; Gianluca, L.P. Application of modified red mud in environmentally-benign applications: A review. Environ. Eng. Res. 2020, 25, 795–806. [Google Scholar] [CrossRef]
- Hu, Z.P.; Gao, Z.M.; Liu, X.; Yuan, Z.Y. High-surface-area activated red mud for efficient removal of methylene blue from wastewater. Adsorpt. Sci. Technol. 2018, 36, 62–79. [Google Scholar] [CrossRef]
- Bai, Y.; Pang, Y.; Wu, Z.; Li, X.; Jing, J.; Wang, H.; Zhou, Z. Adsorption of Lead from Water Using MnO2-Modified Red Mud: Performance, Mechanism, and Environmental Risk. Water 2023, 15, 4314. [Google Scholar] [CrossRef]
- Kyrii, S.; Maletskyi, Z.; Klymenko, N.; Ratnaweera, H.; Mitchenko, T.; Dontsova, T.; Kosogina, I. Impact of modification by red mud components on the sorption properties of activated carbon. Appl. Surf. Sci. Adv. 2023, 16, 100412. [Google Scholar] [CrossRef]
- Milenković, A.S.; Smičiklas, I.D.; Šljivić-Ivanović, M.Z.; Živković, L.S.; Vukelić, N.S. Effect of experimental variables onto Co2⁺ and Sr2⁺ sorption behavior in red mud-water suspensions. J. Environ. Sci. Health A. 2016, 51, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Smiljanic, S.; Smiciklas, I.; Peric-Grujic, A.; Sljivic, M.; Ðukic, B.; Loncar, B. Study of factors affecting Ni²⁺ immobilization efficiency by temperature activated red mud. Chem. Eng. J. 2011, 168, 610–619. [Google Scholar] [CrossRef]
- Smiljanic, S.; Smiciklas, I.; Peric-Grujic, A.; Loncar, B.; Mitric, M. Rinsed and thermally treated red mud sorbents for aqueous Ni²⁺ ions. Chem. Eng. J. 2010, 162, 75–83. [Google Scholar] [CrossRef]
- Smičiklas, I.; Smiljanić, S.; Perić-Grujić, A.; Šljivić-Ivanović, M.; Antonović, D. The influence of citrate anion on Ni(II) removal by raw red mud from aluminium industry. Chem. Eng. J. 2013, 214, 327–335. [Google Scholar] [CrossRef]
- Smičiklas, I.; Smiljanić, S.; Perić-Grujić, A.; Šljivić-Ivanović, M.; Mitrić, M.; Antonović, D. Effect of acid treatment on red mud properties with implications on Ni(II) sorption and stability. Chem. Eng. J. 2014, 242, 27–35. [Google Scholar] [CrossRef]
- Šljivić-Ivanović, M.; Smičiklas, I.; Dimović, S.D.; Jović, M.; Dojčinović, B. Study of Simultaneous Radionuclide Sorption by Mixture Design Methodology. Ind. Eng. Chem. Res. 2015, 54, 11212–11221. [Google Scholar] [CrossRef]
- Sahu, M.K.; Mandal, S.; Dash, S.S.; Badhai, P.; Patel, R.K. Removal of Pb(II) from aqueous solution by acid activated red mud. J. Environ. Chem. Eng. 2013, 1, 1315–1324. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Y.; Sheng, L.; He, C.; Sun, W.; He, Q. Enhancing Cd(II) sorption by red mud with heat treatment: Performance and mechanisms of sorption. J. Environ. Manag. 2020, 255, 109870. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Qi, X.; Zhu, X.; Li, X.; Li, K.; Wang, H. Highly effective remediation of high-arsenic wastewater using red mud through formation of AlAsO4@silicate precipitate. Environ. Pollut. 2021, 287, 117484. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, D.; Li, J.; Su, M.; Chen, N. Enhanced adsorption of uranium by modified red muds: Adsorption behavior study. Environ. Sci. Pollut. Res. Int. 2018, 25, 18096–18108. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, Z.; Huang, Y.; Li, J.; Chen, D.; Chen, N.; Su, M. Red mud for the efficient adsorption of U(VI) from aqueous solution: Influence of calcination on performance and mechanism. J. Hazard. Mater. 2021, 409, 124925. [Google Scholar] [CrossRef]
- Li, C.; Yu, J.; Li, W.; He, Y.; Qiu, Y.; Li, P.; Wang, C.; Huang, F.; Wang, D.; Gao, S. Immobilization, enrichment and recycling of Cr(VI) from wastewater using a red mud/carbon material to produce the valuable chromite (FeCr2O4). Chem. Eng. J. 2018, 350, 1103–1113. [Google Scholar] [CrossRef]
- Yang, T.; Sheng, L.; Wang, Y.; Wyckoff, K.N.; He, C.; He, Q. Characteristics of Cadmium Sorption by Heat-Activated Red Mud in Aqueous Solution. Sci. Rep. 2018, 8, 13558. [Google Scholar] [CrossRef]
- Lyu, F.; Niu, S.; Wang, L.; Liu, R.; Sun, W.; He, D. Efficient removal of Pb(II) ions from aqueous solution by modified red mud. J. Hazard. Mater. 2021, 406, 124678. [Google Scholar] [CrossRef] [PubMed]
- Shabani, E.; Salimi, F.; Jahangiri, A. Removal of arsenic and copper from water solution using magnetic iron/bentonite nanoparticles (Fe3O4/bentonite). Silicon 2019, 11, 961–971. [Google Scholar] [CrossRef]
- Šljivić-Ivanović, M.; Smičiklas, I.; Pejanović, S. Analysis and comparison of mass transfer phenomena related to Cu2+ sorption by hydroxyapatite and zeolite. Chem. Eng. J. 2013, 223, 833–843. [Google Scholar] [CrossRef]
- Milenković, A.; Smičiklas, I.; Marković, J.P.; Vukelić, N. Immobilization of 60Co and 90Sr ions using red mud from aluminium industry. Nucl. Technol. Radiat. Prot. 2014, 29, 79–87. [Google Scholar] [CrossRef]
- Smičiklas, I.; Coha, I.; Jović, M.; Nodilo, M.; Šljivić Ivanović, M.; Smiljanić, S.; Grahek, Ž. Efficient separation of strontium radionuclides from high-salinity wastewater by zeolite 4A synthesized from Bayer process liquids. Sci. Rep. 2021, 11, 1738. [Google Scholar] [CrossRef]
- Šljivić-Ivanović, M.Z.; Smičiklas, I.D.; Marković, J.P.; Milenković, A. Analysis of factors influencing Cu(II) sorption by clinoptilolite. Hem. Ind. 2013, 67, 739–745. [Google Scholar] [CrossRef]
- Smičiklas, I.; Dimović, S.; Šljivić, M.; Lončar, B.; Mitrić, M. Resource recovery of animal bones: Study on sorptive properties and mechanism for Sr2+ ions. J. Nucl. Mater. 2010, 400, 15–24. [Google Scholar] [CrossRef]
- Šljivić-Ivanović, M.; Milenković, A.; Jović, M.; Dimović, S.; Mraković, A.; Smičiklas, I. Ni(II) immobilization by bio-apatite materials: Appraisal of chemical, thermal, and combined treatments. Chem. Ind. Chem. Eng. Q. 2016, 22, 117–126. [Google Scholar] [CrossRef]
- Šljivić-Ivanović, M.; Jelić, I.; Dimović, S.; Antonijević, D.; Jović, M.; Mraković, A.; Smičiklas, I. Exploring innovative solutions for aged concrete utilization: Treatment of liquid radioactive waste. Clean. Technol. Environ. Policy 2018, 20, 1273–1284. [Google Scholar] [CrossRef]
- Jelic, I.; Sljivic-Ivanovic, M.; Dimovic, S.; Antonijevic, D.; Jovic, M.; Mirkovic, M.; Smiciklas, I. The Applicability of Construction and Demolition Waste Components for Radionuclide Sorption. J. Clean. Prod. 2018, 171, 322–332. [Google Scholar] [CrossRef]
- Jelić, I.; Antonijević, D.; Šljivić-Ivanović, M.; Dimović, S. Application of composite construction and demolition debris in heavy metals removal from industrial wastewater. Therm. Sci. 2023, 27, 1–10. [Google Scholar] [CrossRef]
- Jelić, I.; Šljivić-Ivanović, M.; Dimović, S.; Antonijević, D.; Jović, M.; Vujović, Z.; Smičiklas, I. Radionuclide Immobilization by Sorption onto Waste Concrete and Bricks—Experimental Design Methodology. Water Air Soil. Pollut. 2019, 230, 242. [Google Scholar] [CrossRef]
- Jelić, I.; Šljivić-Ivanović, M.; Dimović, S.; Antonijević, D.; Jović, M.; Serović, R.; Smičiklas, I. Utilization of waste ceramics and roof tiles for radionuclide sorption. Process Saf. Environ. Prot. 2017, 105, 348–360. [Google Scholar] [CrossRef]
- Dimović, S.; Jelić, I.; Šljivić-Ivanović, M.; Štirbanović, Z.; Gardić, V.; Marković, R.; Savić, A.; Zakic, D. Application of Copper Mining Waste in Radionuclide and Heavy Metal Immobilization. Clean. -Soil Air Water 2022, 50, 2000419. [Google Scholar] [CrossRef]
- Sokić, K.; Milojković, N.; Dapčević, A.; Jevtić, S.; Gasik, M. Novel micro- and nano-composite materials for water purification. Hem. Ind. 2024, 78, 69. [Google Scholar]
- Ma, Y.; Lin, C.; Jiang, Y.; Lu, W.; Si, C.; Liu, Y. Competitive removal of water-borne copper, zinc and cadmium by a CaCO3-dominated red mud. J. Hazard. Mater. 2009, 172, 1288–1296. [Google Scholar] [CrossRef]
- Eid, A.; Abdel-Aleem, G. A systematic approach for design of distributed wastewater treatment systems. Hem. Ind. 2024, 78, 75–85. [Google Scholar] [CrossRef]
- Reddy, P.S.; Reddy, N.G.; Serjun, V.; Mohanty, B.; Das, S.K.; Reddy, K.R.; Rao, B.H. Properties and Assessment of Applications of Red Mud (Bauxite Residue): Current Status and Research Needs. Waste Biomass Valor. 2021, 12, 1185–1217. [Google Scholar] [CrossRef]
- Zeng, H.; Lyu, F.; Sun, W.; Zhang, H.; Wang, L.; Wang, Y. Progress on the Industrial Applications of Red Mud with a Focus on China. Minerals 2020, 10, 773. [Google Scholar] [CrossRef]
- Gauthier, A.; Omana, B.; Amin, F.; Le Coustumer, P. Waste Bauxite Residue Valorization as Trace Metal Sorbent: Application to Acid Mine Drainage Remediation. Water 2024, 16, 3255. [Google Scholar] [CrossRef]
- Forghani Tehrani, G.; Rubinos, D.A.; Rahimi-Nia, A.; Bagherian, G.; Goudarzi, N. Lead(II) removal from aqueous solutions and battery industry wastewater by sorption using seawater-neutralized red mud. Int. J. Environ. Sci. Technol. 2023, 20, 3713–3732. [Google Scholar] [CrossRef]
- Chen, Z.; Su, M.; Chen, N.; Liang, D.; Chen, D. Effectiveness and mechanism of uranium adsorption on size-graded red mud. Environ. Res. 2022, 212, 113491. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Bai, F.; Li, X.; Nie, Q.; Jia, X.; Wu, H. The remediation efficiency of heavy metal pollutants in water by industrial red mud particle waste. Environ. Technol. Innov. 2022, 28, 102944. [Google Scholar] [CrossRef]
- Zhao, D.; Deng, H.; Wang, W.; Hu, L.; Ye, S.; Fu, J.; Zhang, S. Synthesis, characterization and adsorption of Pb(II), Cd(II) and Cu(II) by red mud/polyacrylic acid/sodium carboxymethyl cellulose hydrogel. Arab. J. Chem. 2025, 18, 106067. [Google Scholar] [CrossRef]
- Kumar, R.; Laskar, M.A.; Hewaidy, I.F.; Barakat, M.A. Modified Adsorbents for Removal of Heavy Metals from Aqueous Environment: A Review. Earth Syst. Environ. 2019, 3, 83–93. [Google Scholar] [CrossRef]
- Wang, L.; Hu, G.; Lyu, F.; Yue, T.; Tang, H.; Han, H.; Yang, Y.; Liu, R.; Sun, W. Application of Red Mud in Wastewater Treatment. Minerals 2019, 9, 281. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Fischel, M.; Lin, X.; Zhou, S.; Zhang, L.; Wang, L.; Yan, J. Applying Red Mud in Cadmium Contamination Remediation: A Scoping Review. Toxics. 2024, 12, 347. [Google Scholar] [CrossRef]
- Niu, A.; Lin, C. Trends in research on characterization, treatment and valorization of hazardous red mud: A systematic review. J. Environ. Manag. 2024, 351, 119660. [Google Scholar] [CrossRef]

| Component | Content, wt.% |
|---|---|
| Fe2O3 | 20–60 |
| Al2O3 | 10–30 |
| SiO2 | 2–20 |
| CaO | 2–8 |
| TiO2 | Trace–28 |
| Na2O | 2–10 |
| Keywords | Main Findings | Reference |
|---|---|---|
| industrial by-products; composite; adsorption; heavy metal; wastewater purification | Explores industrial wastes (slag, sludge, red mud, lignin, and fly ash) as economical and effective adsorbents for heavy metal removal from wastewater. | [84] |
| red mud; environmental remediation; polluted water; waste gas; soil | Covers the background, properties, and applications of red mud as an adsorbent, systematically comparing methods for removing metal and non-metal elements from wastewater, with a focus on surface modification. | [85] |
| adsorption; wastewater treatment; waste gas purification; soil remediation; RM-ERMs | Reviews methods utilizing red mud for environmental remediation, focusing on its application in treating waste streams, including wastewater treatment and heavy metal adsorption. | [4] |
| red mud; cadmium; contaminant immobilization; environmental remediation; heavy metal sorption | Assesses the potential of red mud for cadmium removal from soil and water, suggesting its prospective use in engineered wastewater treatment systems. | [86] |
| alumina refining; caustic material; waste treatment; waste valorization; research trend | Analysis of the red mud literature highlights its potential for remediation, focusing on harmfulness minimization and wastewater treatment, with key findings on characterization, treatment methods, metal recovery, environmental applications, and construction uses. | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajković, M.; Jelić, I.; Janković, M.; Antonijević, D.; Šljivić-Ivanović, M. Red Mud as an Adsorbent for Hazardous Metal Ions: Trends in Utilization. Toxics 2025, 13, 107. https://doi.org/10.3390/toxics13020107
Rajković M, Jelić I, Janković M, Antonijević D, Šljivić-Ivanović M. Red Mud as an Adsorbent for Hazardous Metal Ions: Trends in Utilization. Toxics. 2025; 13(2):107. https://doi.org/10.3390/toxics13020107
Chicago/Turabian StyleRajković, Maja, Ivana Jelić, Marija Janković, Dragi Antonijević, and Marija Šljivić-Ivanović. 2025. "Red Mud as an Adsorbent for Hazardous Metal Ions: Trends in Utilization" Toxics 13, no. 2: 107. https://doi.org/10.3390/toxics13020107
APA StyleRajković, M., Jelić, I., Janković, M., Antonijević, D., & Šljivić-Ivanović, M. (2025). Red Mud as an Adsorbent for Hazardous Metal Ions: Trends in Utilization. Toxics, 13(2), 107. https://doi.org/10.3390/toxics13020107








