Evaluating the Risks of Heated Tobacco Products: Toxicological Effects on Two Selected Respiratory Bacteria and Human Lung Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain
2.2. Smoke and Aerosol Generation and Aqueous Extracts (AqExs) Production
2.3. Agar Diffusion Test
2.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
- CAMHB bubbled with tobacco cigarette smoke;
- CAMHB bubbled with IQOS vapors.
2.5. Cell Cultures
2.6. Cell Viability Assay
2.7. Mutagenicity: AMES Screen
2.8. Statistical Analysis
3. Results
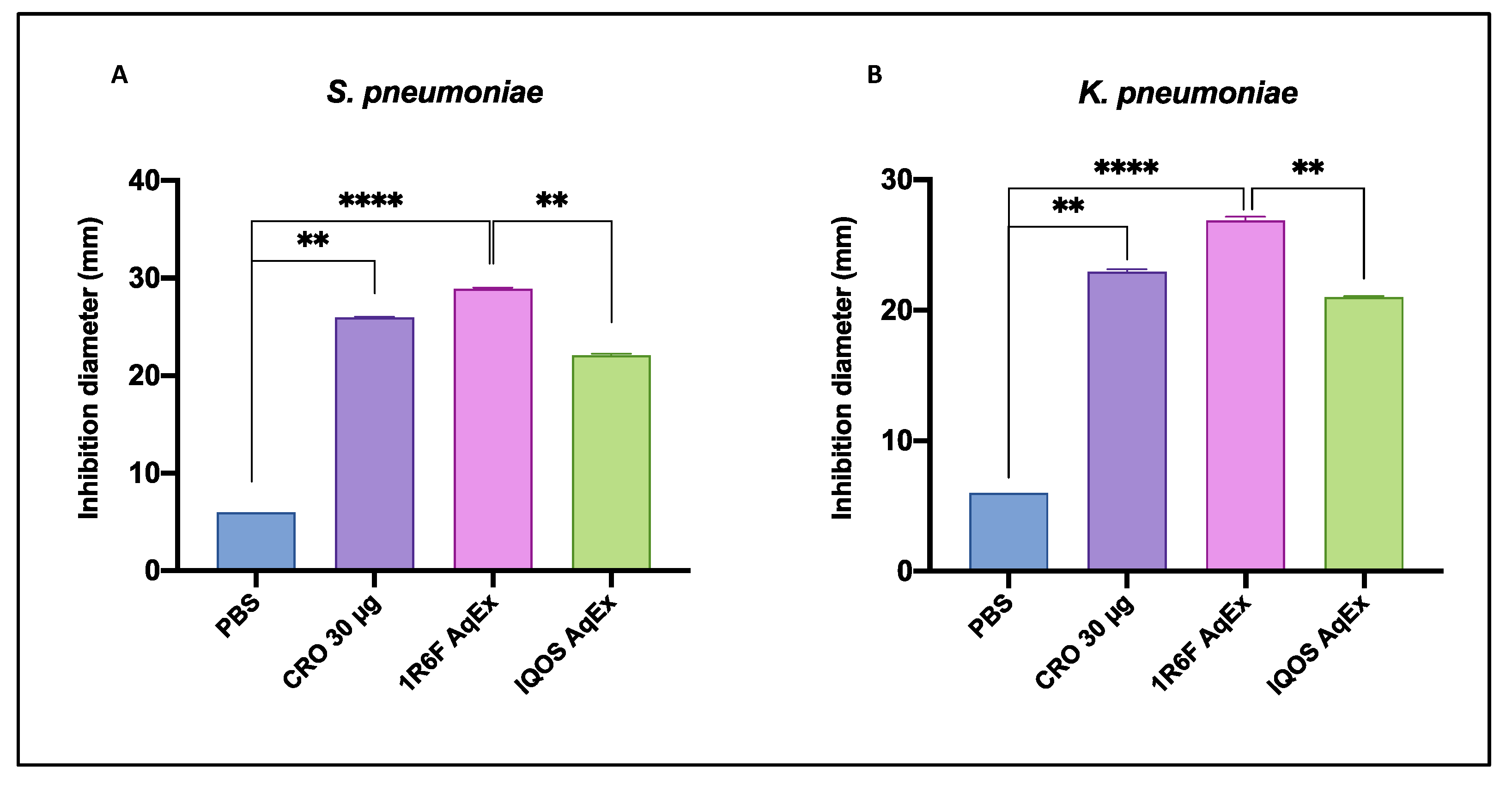
3.1. Antibacterial Activity
3.1.1. Comparative Efficacy of AqExs in Agar Diffusion Test
3.1.2. Bactericidal and Bacteriostatic Effects Against S. pneumoniae and K. pneumoniae
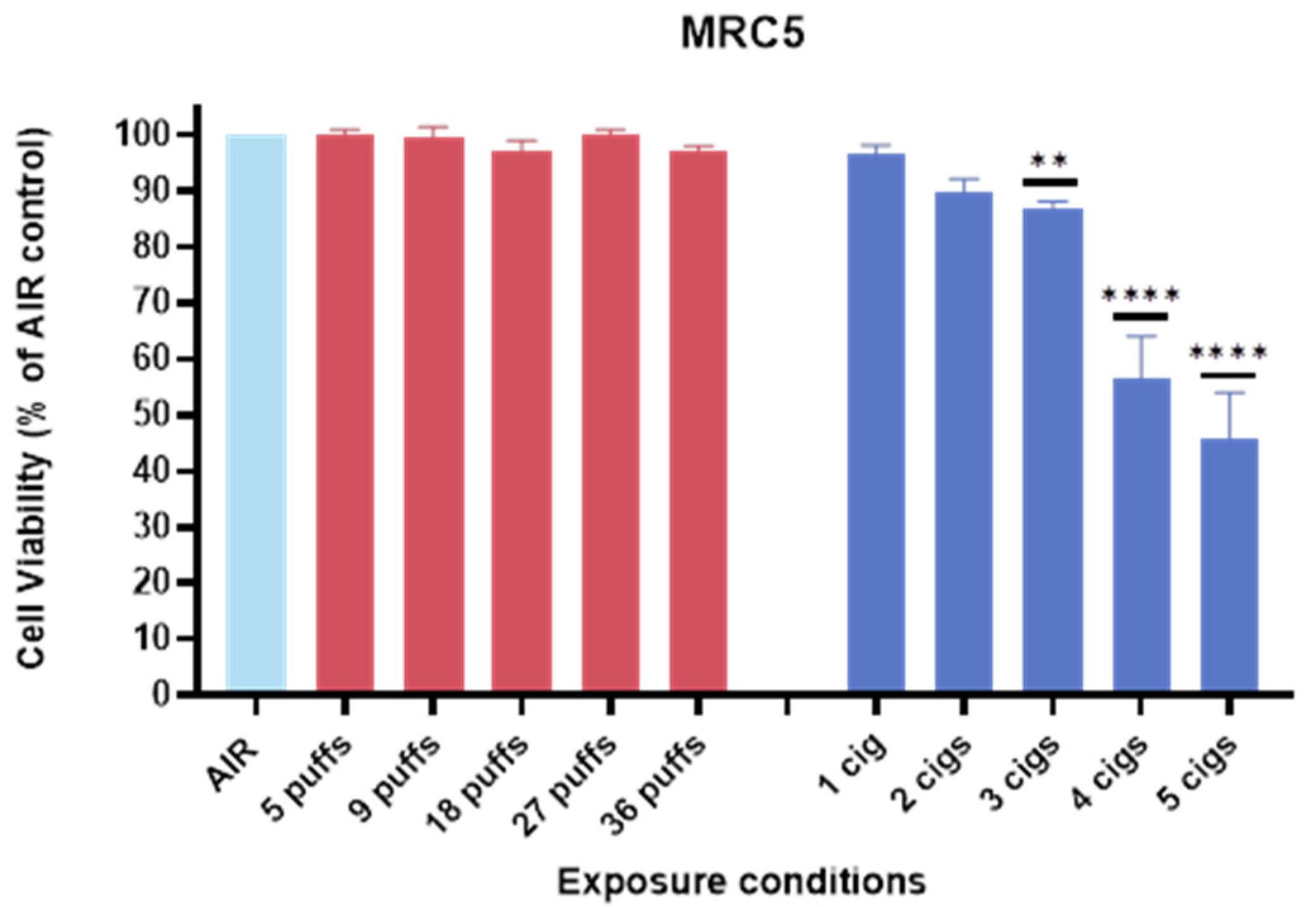
3.2. Cell Viability of MRC-5 Against IQOS vs. 1R6F
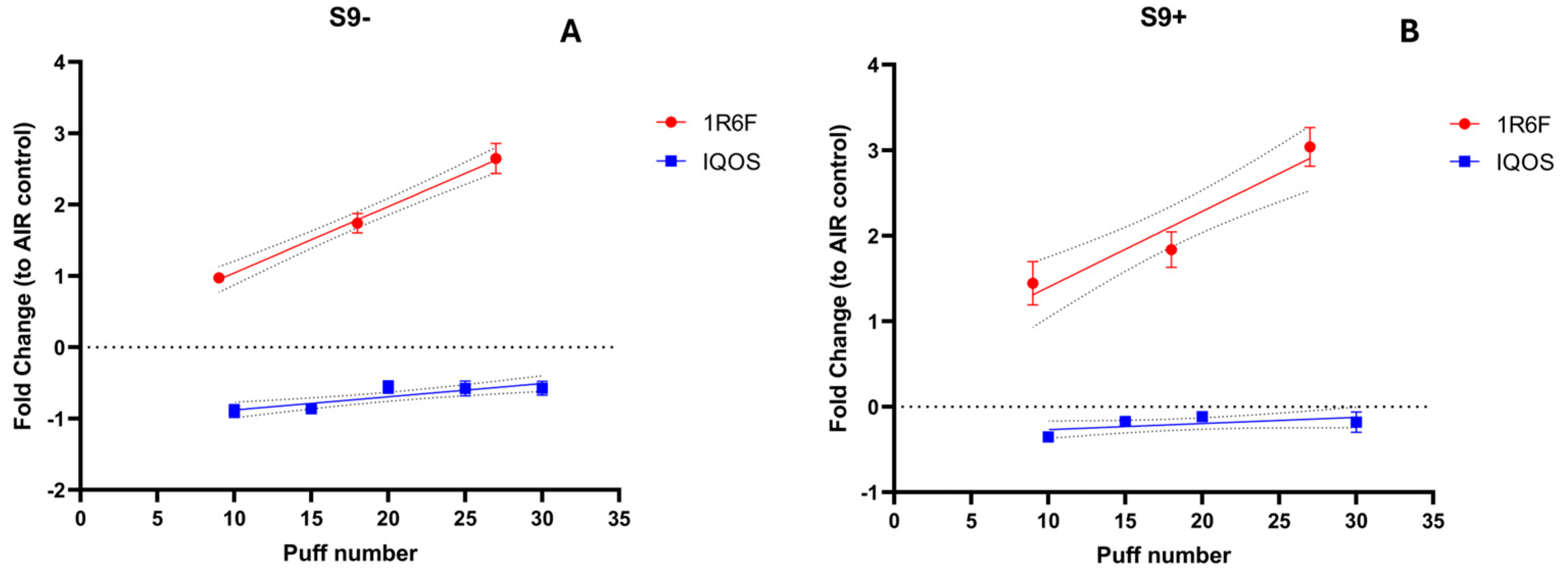
3.3. Mutagenicity Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| ALI | air–liquid interface |
| AqExs | aqueous extracts |
| BAqEx | Bacterial aqueous extracts |
| COPD | chronic obstructive pulmonary disease |
| CRO | Ceftriaxone |
| MBC | Minimal bactericidal concentration |
| MIC | Minimal inhibitory concentration |
| THPs | tobacco heating products |
References
- Boué, S.; Goedertier, D.; Hoeng, J.; Kuczaj, A.; Majeed, S.; Mathis, C.; May, A.; Phillips, B.; Peitsch, M.C.; Radtke, F. State-of-the-art methods and devices for the generation, exposure, and collection of aerosols from heat-not-burn tobacco products. Toxicol. Res. Appl. 2020, 4, 2397847319897869. [Google Scholar] [CrossRef]
- Emma, R.; Caruso, M.; Campagna, D.; Pulvirenti, R.; Li Volti, G. The impact of tobacco cigarettes, vaping products and tobacco heating products on oxidative stress. Antioxidants 2022, 11, 1829. [Google Scholar] [CrossRef]
- Goodall, S.; Gale, N.; Thorne, D.; Hadley, S.; Prasad, K.; Gilmour, I.; Miazzi, F.; Proctor, C. Evaluation of behavioural, chemical, toxicological and clinical studies of a tobacco heated product glo™ and the potential for bridging from a foundational dataset to new product iterations. Toxicol. Rep. 2022, 9, 1426–1442. [Google Scholar] [CrossRef]
- Kanobe, M.N.; Dull, G.M.; Darnell, J.; Jin, T.; Brown, B.; Coffield, J.; Keyser, B.M.; Fearon, I.M.; Makena, P.; Baxter, S.A. Evaluation of environmental emissions from glo heated tobacco products and combustible Cigarettes. Environ. Adv. 2024, 17, 100580. [Google Scholar] [CrossRef]
- Gunduz, I.; Nordlund, M.; King, J.; Gustin, B.; Cudazzo, G.; Nesovic, M.; Butin, Y.; Stura, E.; Alriquet, M.; Chauhan, M. A comparative assessment of HPHC yields and in vitro toxicity for 1R6F reference cigarette smoke versus aerosol generated by Tobacco Heating System 3.0. Aerosol Sci. Technol. 2024, 59, 146–162. [Google Scholar] [CrossRef]
- Tsolakos, N.; Haswell, L.E.; Miazzi, F.; Bishop, E.; Antoranz, A.; Pliaka, V.; Minia, A.; Alexopoulos, L.G.; Gaca, M.; Breheny, D. Comparative toxicological assessment of cigarettes and new category products via an in vitro multiplex proteomics platform. Toxicol. Rep. 2024, 12, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Mahlich, J.; Kamae, I. Switching from Cigarettes to Heated Tobacco Products in Japan—Potential Impact on Health Outcomes and Associated Health Care Costs. Healthcare 2024, 12, 1937. [Google Scholar] [CrossRef] [PubMed]
- Hickman, E.; Roca, C.; Zorn, B.T.; Rebuli, M.E.; Robinette, C.; Wolfgang, M.C.; Jaspers, I. E-Cigarette Use, Cigarette Smoking, and Sex Are Associated With Nasal Microbiome Dysbiosis. Nicotine Tob. Res. 2024, 27, 114–124. [Google Scholar] [CrossRef]
- Tattan-Birch, H.; Hartmann-Boyce, J.; Kock, L.; Simonavicius, E.; Brose, L.; Jackson, S.; Shahab, L.; Brown, J. Heated tobacco products for smoking cessation and reducing smoking prevalence. Cochrane Database Syst. Rev. 2022, 1, CD013790. [Google Scholar]
- Jankowski, M.; Brożek, G.M.; Lawson, J.; Skoczyński, S.; Majek, P.; Zejda, J.E. New ideas, old problems? Heated tobacco products–a systematic review. Int. J. Occup. Med. Environ. Health 2019, 32, 595–634. [Google Scholar] [CrossRef]
- Sawa, M.; Ushiyama, A.; Inaba, Y.; Hattori, K. Increased oxidative stress and effects on inflammatory cytokine secretion by heated tobacco products aerosol exposure to mice. Biochem. Biophys. Res. Commun. 2022, 610, 43–48. [Google Scholar] [CrossRef]
- Forster, M.; Fiebelkorn, S.; Yurteri, C.; Mariner, D.; Liu, C.; Wright, C.; McAdam, K.; Murphy, J.; Proctor, C. Assessment of novel tobacco heating product THP1. 0. Part 3: Comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul. Toxicol. Pharmacol. 2018, 93, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cobas, A.E.; Rodriguez-Beltran, J.; Baquero, F.; Coque, T.M. Ecology of the respiratory tract microbiome. Trends Microbiol. 2023, 31, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Kitsios, G.D.; Nguyen, V.D.; Sayed, K.; Al-Yousif, N.; Schaefer, C.; Shah, F.A.; Bain, W.; Yang, H.; Fitch, A.; Li, K.; et al. The upper and lower respiratory tract microbiome in severe aspiration pneumonia. iScience 2023, 26, 106832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, M.; Zhou, H.; Yang, Y.; Shen, S.; You, Y.; Xue, Z. The role of gut microbiome in the complex relationship between respiratory tract infection and asthma. Front. Microbiol. 2023, 14, 1219942. [Google Scholar] [CrossRef] [PubMed]
- Cicchinelli, S.; Rosa, F.; Manca, F.; Zanza, C.; Ojetti, V.; Covino, M.; Candelli, M.; Gasbarrini, A.; Franceschi, F.; Piccioni, A. The impact of smoking on microbiota: A narrative review. Biomedicines 2023, 11, 1144. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Mantero, M.; Santus, P.; Tarsia, P. Understanding the burden of pneumococcal disease in adults. Clin. Microbiol. Infect. 2012, 18 (Suppl. S5), 7–14. [Google Scholar] [CrossRef]
- Bogaert, D.; De Groot, R.; Hermans, P.W. Streptococcus pneumoniae colonisation: The key to pneumococcal disease. Lancet Infect. Dis. 2004, 4, 144–154. [Google Scholar] [CrossRef]
- Thevaranjan, N.; Whelan, F.J.; Puchta, A.; Ashu, E.; Rossi, L.; Surette, M.G.; Bowdish, D.M. Streptococcus pneumoniae colonization disrupts the microbial community within the upper respiratory tract of aging mice. Infect. Immun. 2016, 84, 906–916. [Google Scholar] [CrossRef]
- Novick, S.; Shagan, M.; Blau, K.; Lifshitz, S.; Givon-Lavi, N.; Grossman, N.; Bodner, L.; Dagan, R.; Mizrachi Nebenzahl, Y. Adhesion and invasion of Streptococcus pneumoniae to primary and secondary respiratory epithelial cells. Mol. Med. Rep. 2017, 15, 65–74. [Google Scholar] [CrossRef]
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Piperaki, E.T.; Syrogiannopoulos, G.A.; Tzouvelekis, L.S.; Daikos, G.L. Klebsiella pneumoniae: Virulence, Biofilm and Antimicrobial Resistance. Pediatr. Infect. Dis. J. 2017, 36, 1002–1005. [Google Scholar] [CrossRef] [PubMed]
- Sharan, H. Aerobic Bacteriological Study of Acute Exacerbations of Chronic Obstructive Pulmonary Disease. J. Clin. Diagn. Res. 2015, 9, DC10–DC12. [Google Scholar] [CrossRef] [PubMed]
- Budisan, L.; Zanoaga, O.; Braicu, C.; Pirlog, R.; Covaliu, B.; Esanu, V.; Korban, S.S.; Berindan-Neagoe, I. Links between Infections, Lung Cancer, and the Immune System. Int. J. Mol. Sci. 2021, 22, 9394. [Google Scholar] [CrossRef]
- Shen, P.; Morissette, M.C.; Vanderstocken, G.; Gao, Y.; Hassan, M.; Roos, A.; Thayaparan, D.; Merlano, M.; Dorrington, M.G.; Nikota, J.K.; et al. Cigarette Smoke Attenuates the Nasal Host Response to Streptococcus pneumoniae and Predisposes to Invasive Pneumococcal Disease in Mice. Infect. Immun. 2016, 84, 1536–1547. [Google Scholar] [CrossRef]
- Mallock, N.; Pieper, E.; Hutzler, C.; Henkler-Stephani, F.; Luch, A. Heated tobacco products: A review of current knowledge and initial assessments. Front. Public Health 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed]
- Fuochi, V.; Caruso, M.; Emma, R.; Stivala, A.; Polosa, R.; Distefano, A.; Furneri, P.M. Investigation on the antibacterial activity of electronic cigarette liquids (ECLs): A proof of concept study. Curr. Pharm. Biotechnol. 2020, 22, 983–994. [Google Scholar] [CrossRef] [PubMed]
- CLSI. M100: Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2022. [Google Scholar]
- Emma, R.; Fuochi, V.; Distefano, A.; Partsinevelos, K.; Rust, S.; Zadjali, F.; Al Tobi, M.; Zadjali, R.; Alharthi, Z.; Pulvirenti, R.; et al. Cytotoxicity, mutagenicity and genotoxicity of electronic cigarettes emission aerosols compared to cigarette smoke: The REPLICA project. Sci. Rep. 2024, 14, 10018. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-R.; Lin, S.-N.; Wu, X.-N.; Chou, S.-H.; Wang, F.-D.; Lin, Y.-T. Clinical and microbiological characteristics of bacteremic pneumonia caused by Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2022, 12, 903682. [Google Scholar] [CrossRef]
- Hakansson, A.; Orihuela, C.; Bogaert, D. Bacterial-host interactions: Physiology and pathophysiology of respiratory infection. Physiol. Rev. 2018, 98, 781–811. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, W.J.; Gupta, K.; Itani, K.M.F. Association of Postoperative Infection with Risk of Long-term Infection and Mortality. JAMA Surg. 2020, 155, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Itani, R.; Khojah, H.M.J.; Kibrit, R.; Raychouni, H.; Shuhaiber, P.; Dib, C.; Hassan, M.; Mukattash, T.L.; El-Lakany, A. Risk factors associated with multidrug-resistant Klebsiella pneumoniae infections: A multicenter observational study in Lebanese hospitals. BMC Public Health 2024, 24, 2958. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Gutierrez, O.A.; Falfan-Valencia, R.; Ramirez-Venegas, A.; Sansores, R.H.; Ponciano-Rodriguez, G.; Perez-Rubio, G. Lung Damage Caused by Heated Tobacco Products and Electronic Nicotine Delivery Systems: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 4079. [Google Scholar] [CrossRef] [PubMed]
- Polosa, R.; Morjaria, J.B.; Prosperini, U.; Busa, B.; Pennisi, A.; Gussoni, G.; Rust, S.; Maglia, M.; Caponnetto, P. Health outcomes in COPD smokers using heated tobacco products: A 3-year follow-up. Intern. Emerg. Med. 2021, 16, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Tsou, H.H.; Wang, P.H.; Ting, T.H.; Ping, Y.H.; Liu, T.Y.; Cheng, H.W.; Wang, H.T. Effect of heated tobacco products and traditional cigarettes on pulmonary toxicity and SARS-CoV-2-induced lung injury. Toxicology 2022, 479, 153318. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. Host-pathogen interactions: Basic concepts of microbial commensalism, colonization, infection, and disease. Infect. Immun. 2000, 68, 6511–6518. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.F.; Fitzgerald, J.R. Mechanisms of host adaptation by bacterial pathogens. FEMS Microbiol. Rev. 2024, 48, fuae019. [Google Scholar] [CrossRef]
- Brugger, S.D.; Bomar, L.; Lemon, K.P. Commensal-Pathogen Interactions along the Human Nasal Passages. PLoS Pathog. 2016, 12, e1005633. [Google Scholar] [CrossRef]
- Distefano, A.; Orlando, L.; Partsinevelos, K.; Longhitano, L.; Emma, R.; Caruso, M.; Vicario, N.; Denaro, S.; Sun, A.; Giordano, A.; et al. Comparative evaluation of cigarette smoke and a heated tobacco product on microglial toxicity, oxidative stress and inflammatory response. J. Transl. Med. 2024, 22, 876. [Google Scholar] [CrossRef] [PubMed]
- Pavia, C.S.; Plummer, M.M. Clinical implications of nicotine as an antimicrobial agent and immune modulator. Biomed. Pharmacother. 2020, 129, 110404. [Google Scholar] [CrossRef] [PubMed]
| Assay Mix | Components to Add into Melted Agar | |||
|---|---|---|---|---|
| BAqEx * | Air Control | Solvent Control | Control Chem | |
| S9− | 200 μL of BAqEx | 200 μL of BAqEx air control | 100 μL of PBS + 100 μL of the untreated PBS bacterial suspension | 100 μL of controlchem solution + 100 μL of untreated PBS bacterial suspension |
| S9+ | 500 μL S9+ mix and 50 μL of BAqEx | 500 μL S9+ mix and 50 μL of BAqEx air control | 500 μL S9+ mix + 50 μL of the untreated PBS bacterial suspension | 500 μL S9+ mix + 100 μL of controlchem solution + 50 μL of the untreated PBS bacterial suspension |
| Strain | IQOS AqEx | 1R6F AqEx | PBS | CRO 30 µg | p Value |
|---|---|---|---|---|---|
| S. pneumoniae | 22 ± 0.2 | 29 ± 0.2 | ≤6 * | 26 ± 0.1 | <0.0001 |
| K. pneumoniae | 21 ± 0.1 | 27 ± 0.3 | ≤6 * | 23 ± 0.2 | <0.0001 |
| Strain | IQOS AqEx * | 1R6F AqEx * | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| S. pneumoniae | 6.25 | 12.5 | 6.25 | 6.25 |
| K. pneumoniae | 6.25 | 12.5 | 6.25 | 6.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furnari, S.; Emma, R.; Caruso, M.; Furneri, P.M.; Fuochi, V. Evaluating the Risks of Heated Tobacco Products: Toxicological Effects on Two Selected Respiratory Bacteria and Human Lung Cells. Toxics 2025, 13, 70. https://doi.org/10.3390/toxics13020070
Furnari S, Emma R, Caruso M, Furneri PM, Fuochi V. Evaluating the Risks of Heated Tobacco Products: Toxicological Effects on Two Selected Respiratory Bacteria and Human Lung Cells. Toxics. 2025; 13(2):70. https://doi.org/10.3390/toxics13020070
Chicago/Turabian StyleFurnari, Salvatore, Rosalia Emma, Massimo Caruso, Pio Maria Furneri, and Virginia Fuochi. 2025. "Evaluating the Risks of Heated Tobacco Products: Toxicological Effects on Two Selected Respiratory Bacteria and Human Lung Cells" Toxics 13, no. 2: 70. https://doi.org/10.3390/toxics13020070
APA StyleFurnari, S., Emma, R., Caruso, M., Furneri, P. M., & Fuochi, V. (2025). Evaluating the Risks of Heated Tobacco Products: Toxicological Effects on Two Selected Respiratory Bacteria and Human Lung Cells. Toxics, 13(2), 70. https://doi.org/10.3390/toxics13020070










