Fluoride-Mediated Immune Damage Through Cytokine Network Regulation of Tregs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selection of Study Area and Population
2.2. Construction of a Rat Model of Fluorosis via Drinking Water
2.3. Preparation of Peripheral Blood Mononuclear Cells (PBMCs)
2.4. Preparation of Thymic Single-Cell Suspension
2.5. Preparation of Spleen Single-Cell Suspension
2.6. Measurement of Urinary Fluoride
2.7. Measurement of the Proportion of Tregs in the Samples
2.8. Measurement of Cytokine Expression in the Samples
2.9. Statistical Analysis
3. Results
3.1. The Cytokine Network Mediates the Increase of Tregs in the Peripheral Blood of Fluoride-Exposed Populations
3.1.1. Basic Characteristics of the Study Participants and Their Fluoride Accumulation Levels
3.1.2. Long-Term Fluoride Exposure Alters the Immune Microenvironment
3.1.3. IL-2 and IFN-γ Mediated the Regulation of Tregs by Fluoride
3.2. Effects of Fluoride Exposure on the Immune Microenvironment in Rats
3.2.1. Fluoride Exposure Has Long-Term Effects on Urinary Fluoride Levels in Rats
3.2.2. Fluoride-Induced Changes in the Proportion of Tregs in Peripheral Blood and Tissues
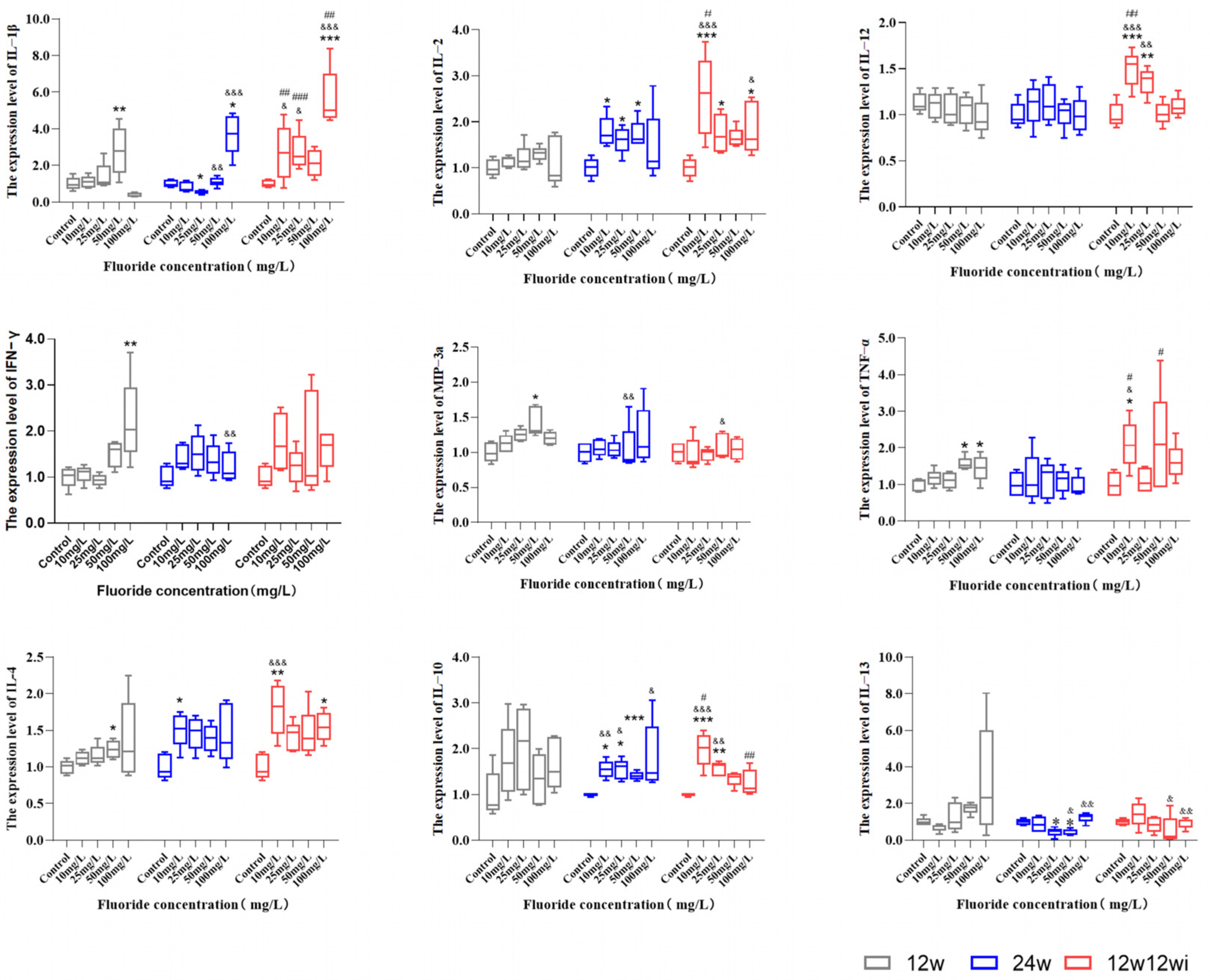
3.2.3. The Effects of Fluoride Exposure Duration and Dosage on the Expression of Cytokines
3.2.4. The Relationship Between Changes in Tregs Numbers in Peripheral Blood and Immune Organs and the Expression of Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dharmaratne, R.W. Exploring the role of excess fluoride in chronic kidney disease: A review. Hum. Exp. Toxicol. 2019, 38, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Iqbal, M.; Mehmood, K.; Li, Y.; Tang, Z.; Zhang, H. Challenges of fluoride pollution in environment: Mechanisms and pathological significance of toxicity—A review. Environ. Pollut. 2022, 304, 119241. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D. Environmental anthropology of Fluoride: A comprehensive anthropological study of Fluoride mining, production, and contamination impacts. Fluoride 2024, 57, 1. [Google Scholar]
- Bukhari, S.; Ahmed, S.; Alia, Z.; Sardar, R.; Hassan, S. Fluoride contamination in foods and drinking warter: A review on its toxic effects and mitigation strategles. Fluoride 2023, 56, 671–690. [Google Scholar]
- Wei, W.; Pang, S.; Sun, D. The pathogenesis of endemic fluorosis: Research progress in the last 5 years. J. Cell Mol. Med. 2019, 23, 2333–2342. [Google Scholar] [CrossRef]
- Abduweli Uyghurturk, D.; Goin, D.E.; Martinez-Mier, E.A.; Woodruff, T.J.; DenBesten, P.K. Maternal and fetal exposures to fluoride during mid-gestation among pregnant women in northern California. Environ. Health 2020, 19, 38. [Google Scholar] [CrossRef]
- Danziger, J.; Dodge, L.E.; Hu, H. Role of renal function in the association of drinking water fluoride and plasma fluoride among adolescents in the United States: NHANES, 2013–2016. Environ. Res. 2022, 213, 113603. [Google Scholar] [CrossRef]
- Dong, H.; Yang, X.; Zhang, S.; Wang, X.; Guo, C.; Zhang, X.; Ma, J.; Niu, P.; Chen, T. Associations of low level of fluoride exposure with dental fluorosis among U.S. children and adolescents, NHANES 2015–2016. Ecotoxicol. Environ. Saf. 2021, 221, 112439. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Z.; Li, M.; Sun, H.; Wei, W.; Gao, L.; Zhao, Q.; Liu, Y.; Ji, X.; Li, C.; et al. Spatial-Temporal Analysis of Drinking Water Type of Endemic Fluorosis-China, 2009–2022. China CDC Wkly 2024, 6, 25–29. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, D.; Wei, W. Necessity to Pay Attention to the Effects of Low Fluoride on Human Health: An Overview of Skeletal and Non-skeletal Damages in Epidemiologic Investigations and Laboratory Studies. Biol. Trace Elem. Res. 2023, 201, 1627–1638. [Google Scholar] [CrossRef]
- Arab, N.; Derakhshani, R.; Sayadi, M.H. Approaches for the Efficient Removal of Fluoride from Groundwater: A Comprehensive Review. Toxics 2024, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- (CAS) CAoS. Groundwater Sciences; Science Press: Beijing, China, 2018. [Google Scholar]
- WHO. A Global Overview of National Regulations and Standards for Drinking-Water Quality; WHO: Geneva, Switzerland, 2018; p. 13. [Google Scholar]
- Jin, T.; Huang, T.; Zhang, T.; Li, Q.; Yan, C.; Wang, Q.; Chen, X.; Zhou, J.; Sun, Y.; Bo, W.; et al. A bayesian benchmark concentration analysis for urinary fluoride and intelligence in adults in Guizhou, China. Sci. Total Environ. 2024, 925, 171326. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhu, X.; Peng, C.; Xu, W.; Li, D.; Wang, Y.; Fang, S.; Li, Y.; Hu, S.; Wan, X. Critical factors determining fluoride concentration in tea leaves produced from Anhui province, China. Ecotoxicol. Environ. Saf. 2016, 131, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, D. Endemic fluorosis. In Endemiology Disease in China; People’s Health Publishing House: Beijing, China, 2017; pp. 61–96. [Google Scholar]
- Zhu, S.; Wei, W. Progress in research on the role of fluoride in immune damage. Front. Immunol. 2024, 15, 1394161. [Google Scholar] [CrossRef]
- Rocha-Amador, D.O.; Calderón, J.; Carrizales, L.; Costilla-Salazar, R.; Pérez-Maldonado, I.N. Apoptosis of peripheral blood mononuclear cells in children exposed to arsenic and fluoride. Environ. Toxicol. Pharmacol. 2011, 32, 399–405. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, K.; Ren, F.; Wang, J. Developmental fluoride exposure influenced rat’s splenic development and cell cycle via disruption of the ERK signal pathway. Chemosphere 2017, 187, 173–180. [Google Scholar] [CrossRef]
- Li, Y.; Du, X.; Zhao, Y.; Wang, J.; Wang, J. Fluoride Can Damage the Spleen of Mice by Perturbing Th1/Th2 Cell Balance. Biol. Trace Elem. Res. 2021, 199, 1493–1500. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Chen, H.; Shu, Y.; Peng, W.; Lai, C.; Kong, R.; Lan, R.; Huang, L.; Xin, J.; et al. Effects of prolonged fluoride exposure on innate immunity, intestinal mechanical, and immune barriers in mice. Res. Vet. Sci. 2023, 164, 105019. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, B.; Fu, G.; Yang, L.; Wei, D.; Zhang, L.; Zhang, Q.; Gao, Y.; Sun, D.; Wei, W. PKC-θ is an important driver of fluoride-induced immune imbalance of regulatory T cells/effector T cells. Sci. Total Environ. 2024, 934, 173081. [Google Scholar] [CrossRef]
- Singh, R.; Hussain, M.A.; Kumar, J.; Kumar, M.; Kumari, U.; Mazumder, S. Chronic fluoride exposure exacerbates headkidney pathology and causes immune commotion in Clarias gariepinus. Aquat. Toxicol. 2017, 192, 30–39. [Google Scholar] [CrossRef]
- Shi, Z.; Zhan, Y.; Zhao, J.; Wang, J.; Ma, H. Effects of Fluoride on the Expression of p38MAPK Signaling Pathway-Related Genes and Proteins in Spleen Lymphocytes of Mice. Biol. Trace Elem. Res. 2016, 173, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Pang, S.; Zhu, S.; Wei, W.; Sun, D. Potective effect of anthocyanins from blueberry on fluoride-induced immune system injury in Wistars rats. Fluoride 2023, 56, 217–243. [Google Scholar]
- Gutowska, I.; Baranowska-Bosiacka, I.; Safranow, K.; Jakubowska, K.; Olszewska, M.; Telesiński, A.; Siennicka, A.; Droździk, M.; Chlubek, D.; Stachowska, E. Fluoride in low concentration modifies expression and activity of 15 lipoxygenase in human PBMC differentiated monocyte/macrophage. Toxicology 2012, 295, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Mehany, H.M.; El-Shafai, N.M.; Attia, A.M.; Ibrahim, M.M.; El-Mehasseb, I.M. Potential of chitosan nanoparticle/fluoride nanocomposite for reducing the toxicity of fluoride an in-vivo study on the rat heart functions: Hematopoietic and immune systems. Int. J. Biol. Macromol. 2022, 216, 251–262. [Google Scholar] [CrossRef]
- Wei, W.; Pang, S.; Fu, X.; Tan, S.; Wang, Q.; Wang, S.; Sun, D. The role of PERK and IRE1 signaling pathways in excessive fluoride mediated impairment of lymphocytes in rats’ spleen in vivo and in vitro. Chemosphere 2019, 223, 1–11. [Google Scholar] [CrossRef]
- Wu, P.; Yang, K.; Sun, Z.; Zhao, Y.; Manthari, R.K.; Wang, J.; Cao, J. Interleukin-17A knockout or self-recovery alleviated autoimmune reaction induced by fluoride in mouse testis. Sci. Total Environ. 2023, 884, 163616. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, J.; Yuan, Y.; Ji, D.; Sun, Y.; Liu, Y.; Li, S.; Zhu, X.; Wu, X.; Hu, J.; et al. Proximity-enabled covalent binding of IL-2 to IL-2Rα selectively activates regulatory T cells and suppresses autoimmunity. Signal Transduct. Target. Ther. 2023, 8, 28. [Google Scholar] [CrossRef]
- Proto, J.D.; Doran, A.C.; Gusarova, G.; Yurdagul, A., Jr.; Sozen, E.; Subramanian, M.; Islam, M.N.; Rymond, C.C.; Du, J.; Hook, J.; et al. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity 2018, 49, 666–677.e666. [Google Scholar] [CrossRef]
- Arpaia, N.; Green, J.A.; Moltedo, B.; Arvey, A.; Hemmers, S.; Yuan, S.; Treuting, P.M.; Rudensky, A.Y. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 2015, 162, 1078–1089. [Google Scholar] [CrossRef]
- Pal, P.; Jha, N.K.; Pal, D.; Jha, S.K.; Anand, U.; Gopalakrishnan, A.V.; Dey, A.; Mukhopadhyay, P.K. Molecular basis of fluoride toxicities: Beyond benefits and implications in human disorders. Genes. Dis. 2023, 10, 1470–1493. [Google Scholar] [CrossRef]
- Fu, R.; Niu, R.; Zhao, F.; Wang, J.; Cao, Q.; Yu, Y.; Liu, C.; Zhang, D.; Sun, Z. Exercise alleviated intestinal damage and microbial disturbances in mice exposed to fluoride. Chemosphere 2022, 288, 132658. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.Y.; Xiang, J.; Zeng, X.X.; He, W.W.; Dong, Y.T.; Yu, W.F.; Qi, X.L.; Xiao, Y.; Cao, K.; Zou, J.; et al. The influence of NQO2 on the dysfunctional autophagy and oxidative stress induced in the hippocampus of rats and in SH-SY5Y cells by fluoride. CNS Neurosci. Ther. 2023, 29, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, Q.; Wang, S.; Ji, Y.; Ma, X.; Qin, M.; Gao, Y.; Yang, Y. Sirtuin 3-activated superoxide dismutase 2 mediates fluoride-induced osteoblastic differentiation in vitro and in vivo by down-regulating reactive oxygen species. Arch. Toxicol. 2024, 98, 3351–3363. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tian, Z.; Zhou, G.; Niu, Q.; Chen, J.; Li, P.; Dong, L.; Xia, T.; Zhang, S.; Wang, A. Sirt1-dependent mitochondrial biogenesis supports therapeutic effects of resveratrol against neurodevelopment damage by fluoride. Theranostics 2020, 10, 4822–4838. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, L.; Li, H.; Li, Y.; Liu, H.; Hou, C.; Zeng, Q.; Li, P.; Zhao, Q.; Dong, L.; et al. Thyroid function, intelligence, and low-moderate fluoride exposure among Chinese school-age children. Environ. Int. 2020, 134, 105229. [Google Scholar] [CrossRef]
- Kasper, I.R.; Apostolidis, S.A.; Sharabi, A.; Tsokos, G.C. Empowering Regulatory T Cells in Autoimmunity. Trends Mol. Med. 2016, 22, 784–797. [Google Scholar] [CrossRef]
- Estrada Brull, A.; Panetti, C.; Joller, N. Moving to the Outskirts: Interplay Between Regulatory T Cells and Peripheral Tissues. Front. Immunol. 2022, 13, 864628. [Google Scholar] [CrossRef]
- Zong, Y.; Deng, K.; Chong, W.P. Regulation of Treg cells by cytokine signaling and co-stimulatory molecules. Front. Immunol. 2024, 15, 1387975. [Google Scholar] [CrossRef]
- Sehrawat, S.; Rouse, B.T. Tregs and infections: On the potential value of modifying their function. J. Leukoc. Biol. 2011, 90, 1079–1087. [Google Scholar] [CrossRef]
- Cook, K.W.; Letley, D.P.; Ingram, R.J.; Staples, E.; Skjoldmose, H.; Atherton, J.C.; Robinson, K. CCL20/CCR6-mediated migration of regulatory T cells to the Helicobacter pylori-infected human gastric mucosa. Gut 2014, 63, 1550–1559. [Google Scholar] [CrossRef]
- Lai, H.C.; Chen, P.H.; Tang, C.H.; Chen, L.W. IL-10 Enhances the Inhibitory Effect of Adipose-Derived Stromal Cells on Insulin Resistance/Liver Gluconeogenesis by Treg Cell Induction. Int. J. Mol. Sci. 2024, 25, 8088. [Google Scholar] [CrossRef]
- Rubtsov, Y.P.; Rasmussen, J.P.; Chi, E.Y.; Fontenot, J.; Castelli, L.; Ye, X.; Treuting, P.; Siewe, L.; Roers, A.; Henderson, W.R., Jr.; et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 2008, 28, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Rango, T.; Vengosh, A.; Jeuland, M.; Whitford, G.M.; Tekle-Haimanot, R. Biomarkers of chronic fluoride exposure in groundwater in a highly exposed population. Sci. Total Environ. 2017, 596–597, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rango, T.; Vengosh, A.; Jeuland, M.; Tekle-Haimanot, R.; Weinthal, E.; Kravchenko, J.; Paul, C.; McCornick, P. Fluoride exposure from groundwater as reflected by urinary fluoride and children’s dental fluorosis in the Main Ethiopian Rift Valley. Sci. Total Environ. 2014, 496, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Meenakshi; Maheshwari, R.C. Fluoride in drinking water and its removal. J. Hazard. Mater. 2006, 137, 456–463. [Google Scholar] [CrossRef]
- Food and Drug Administration. Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Food and Drug Administration: Rockville, MD, USA, 2005; p. 10.
- Nikolouli, E.; Elfaki, Y.; Herppich, S.; Schelmbauer, C.; Delacher, M.; Falk, C.; Mufazalov, I.A.; Waisman, A.; Feuerer, M.; Huehn, J. Recirculating IL-1R2(+) Tregs fine-tune intrathymic Treg development under inflammatory conditions. Cell. Mol. Immunol. 2021, 18, 182–193. [Google Scholar] [CrossRef]
- Bibi, S.; Habib, R.; Shafiq, S.; Abbas, S.S.; Khan, S.; Eqani, S.; Nepovimova, E.; Khan, M.S.; Kuca, K.; Nurulain, S.M. Influence of the chronic groundwater fluoride consumption on cholinergic enzymes, ACHE and BCHE gene SNPs and pro-inflammatory cytokines: A study with Pakistani population groups. Sci. Total Environ. 2023, 880, 163359. [Google Scholar] [CrossRef]
- Den Besten, P.; Wells, C.R.; Abduweli Uyghurturk, D. Fluoride exposure and blood cell markers of inflammation in children and adolescents in the United States: NHANES, 2013–2016. Environ. Health 2022, 21, 102. [Google Scholar] [CrossRef]
- Parada-Cruz, B.; Aztatzi-Aguilar, O.G.; Ramírez-Martínez, G.; Jacobo-Estrada, T.L.; Cárdenas-González, M.; Escamilla-Rivera, V.; Martínez-Olivas, M.A.; Narváez-Morales, J.; Ávila-Rojas, S.H.; Álvarez-Salas, L.M.; et al. Inflammation- and cancer-related microRNAs in rat renal cortex after subchronic exposure to fluoride. Chem. Biol. Interact. 2023, 379, 110519. [Google Scholar] [CrossRef]
- Tang, H.; Wang, M.; Li, G.; Wang, M.; Luo, C.; Zhou, G.; Zhao, Q.; Dong, L.; Liu, H.; Cui, Y.; et al. Association between dental fluorosis prevalence and inflammation levels in school-aged children with low-to-moderate fluoride exposure. Environ. Pollut. 2023, 320, 120995. [Google Scholar] [CrossRef]
- Luo, Q.; Cui, H.; Peng, X.; Fang, J.; Zuo, Z.; Liu, J.; Wu, B.; Deng, Y. The association between cytokines and intestinal mucosal immunity among broilers fed on diets supplemented with fluorine. Biol. Trace Elem. Res. 2013, 152, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Kuang, P.; Luo, Q.; Cui, H.; Deng, H.; Liu, H.; Lu, Y.; Fang, J.; Zuo, Z.; Deng, J.; et al. Effects of sodium fluoride on blood cellular and humoral immunity in mice. Oncotarget 2017, 8, 85504–85515. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, W.; Jiang, P.; Li, X.; Liu, C.; Chai, C. Role of nitric oxide and vascular endothelial growth factor in fluoride-induced goitrogenesis in rats. Environ. Toxicol. Pharmacol. 2012, 34, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Niu, R.; Zhao, F.; Zhao, Y.; Wang, J.; Wang, J.; Cao, Q.; Fu, R.; Nateghahmadi, M.H.; Sun, Z. Moderate exercise relieves fluoride-induced liver and kidney inflammatory responses through the IKKβ/NFκB pathway. Environ. Sci. Pollut. Res. Int. 2022, 29, 78429–78443. [Google Scholar] [CrossRef]
- He, X.; Sun, Z.; Manthari, R.K.; Wu, P.; Wang, J. Fluoride altered rat’s blood testis barrier by affecting the F-actin via IL-1α. Chemosphere 2018, 211, 826–833. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, Z.; Cao, Q.; Chen, Y.; Gong, J.; Zhang, Q.; Qiang, Y.; Lu, Y.; Cao, G. Reprogramming of Treg cells in the inflammatory microenvironment during immunotherapy: A literature review. Front. Immunol. 2023, 14, 1268188. [Google Scholar] [CrossRef]
- Abbas, A.K.; Trotta, E.; Simeonov, D.R.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3, eaat1482. [Google Scholar] [CrossRef]
- Okamoto, M.; Kuratani, A.; Okuzaki, D.; Kamiyama, N.; Kobayashi, T.; Sasai, M.; Yamamoto, M. IFN-γ-induced Th1-Treg polarization in inflamed brains limits exacerbation of experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2024, 121, e2401692121. [Google Scholar] [CrossRef]
- Nozari, P.; Mokhtari, P.; Nemati, M.; Zainodini, N.; Taghipour, Z.; Asadi, F.; Ayoobi, F.; Jafarzadeh, A. Investigation of the effect of IFN-γ/TNF-α-treated mesenchymal stem cells on Th9- and Treg cell-related parameters in a mouse model of ovalbumin-induced allergic asthma. Immunopharmacol. Immunotoxicol. 2022, 44, 773–785. [Google Scholar] [CrossRef]
- Pol, J.G.; Caudana, P.; Paillet, J.; Piaggio, E.; Kroemer, G. Effects of interleukin-2 in immunostimulation and immunosuppression. J. Exp. Med. 2020, 217, e20191247. [Google Scholar] [CrossRef]
- Graßhoff, H.; Comdühr, S.; Monne, L.R.; Müller, A.; Lamprecht, P.; Riemekasten, G.; Humrich, J.Y. Low-Dose IL-2 Therapy in Autoimmune and Rheumatic Diseases. Front. Immunol. 2021, 12, 648408. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Yan, H.; Xiong, J. Research advances on targeted-Treg therapies on immune-mediated kidney diseases. Autoimmun. Rev. 2023, 22, 103257. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.Y.; Perpiñán, E.; Londoño, M.C.; Miquel, R.; Ruiz, P.; Kurt, A.S.; Kodela, E.; Cross, A.R.; Berlin, C.; Hester, J.; et al. Low dose interleukin-2 selectively expands circulating regulatory T cells but fails to promote liver allograft tolerance in humans. J. Hepatol. 2023, 78, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.Y.; Low, J.S.; Tanimine, N.; Finn, K.K.; Priyadharshini, B.; Germana, S.K.; Kaech, S.M.; Turka, L.A. Differential Roles of IL-2 Signaling in Developing versus Mature Tregs. Cell Rep. 2018, 25, 1204–1213.e1204. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Alvarez, C.A.; Cobb, B.A. Integration of IL-2 and IL-4 signals coordinates divergent regulatory T cell responses and drives therapeutic efficacy. Elife 2021, 10, e57417. [Google Scholar] [CrossRef]
- Li, X.; Liu, M.; Shi, Q.; Fang, Y.; Fu, D.; Shen, Z.X.; Yi, H.; Wang, L.; Zhao, W. Elevated serum IL-13 level is associated with increased Treg cells in tumor microenvironment and disease progression of diffuse large B-cell lymphoma. Hematol. Oncol. 2023, 41, 230–238. [Google Scholar] [CrossRef]
- Field, C.S.; Baixauli, F.; Kyle, R.L.; Puleston, D.J.; Cameron, A.M.; Sanin, D.E.; Hippen, K.L.; Loschi, M.; Thangavelu, G.; Corrado, M.; et al. Mitochondrial Integrity Regulated by Lipid Metabolism Is a Cell-Intrinsic Checkpoint for Treg Suppressive Function. Cell Metab. 2020, 31, 422–437.e425. [Google Scholar] [CrossRef]
- Palomares, O.; Martín-Fontecha, M.; Lauener, R.; Traidl-Hoffmann, C.; Cavkaytar, O.; Akdis, M.; Akdis, C.A. Regulatory T cells and immune regulation of allergic diseases: Roles of IL-10 and TGF-β. Genes. Immun. 2014, 15, 511–520. [Google Scholar] [CrossRef]
- Arellano, G.; Ottum, P.A.; Reyes, L.I.; Burgos, P.I.; Naves, R. Stage-Specific Role of Interferon-Gamma in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis. Front. Immunol. 2015, 6, 492. [Google Scholar] [CrossRef]
- Zhai, N.; Liu, W.; Jin, C.H.; Ding, Y.; Sun, L.; Zhang, D.; Wang, Z.; Tang, Y.; Zhao, W.; LeGuern, C.; et al. Lack of IFN-γ Receptor Signaling Inhibits Graft-versus-Host Disease by Potentiating Regulatory T Cell Expansion and Conversion. J. Immunol. 2023, 211, 885–894. [Google Scholar] [CrossRef]
- Koenecke, C.; Lee, C.W.; Thamm, K.; Föhse, L.; Schafferus, M.; Mittrücker, H.W.; Floess, S.; Huehn, J.; Ganser, A.; Förster, R.; et al. IFN-γ production by allogeneic Foxp3+ regulatory T cells is essential for preventing experimental graft-versus-host disease. J. Immunol. 2012, 189, 2890–2896. [Google Scholar] [CrossRef] [PubMed]
- Page, K.M.; Chaudhary, D.; Goldman, S.J.; Kasaian, M.T. Natural killer cells from protein kinase C theta-/- mice stimulated with interleukin-12 are deficient in production of interferon-gamma. J. Leukoc. Biol. 2008, 83, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Castaño, D.; Wang, S.; Atencio-Garcia, S.; Shields, E.J.; Rico, M.C.; Sharpe, H.; Bustamante, J.; Feng, A.; Le Coz, C.; Romberg, N.; et al. IL-12 drives the differentiation of human T follicular regulatory cells. Sci. Immunol. 2024, 9, eadf2047. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.J.; Pesenacker, A.M.; Wang, A.Y.; Gillies, J.; Mojibian, M.; Morishita, K.; Tan, R.; Kieffer, T.J.; Verchere, C.B.; Panagiotopoulos, C.; et al. T regulatory cell chemokine production mediates pathogenic T cell attraction and suppression. J. Clin. Investig. 2016, 126, 1039–1051. [Google Scholar] [CrossRef]
- Yan, Y.; Huang, L.; Liu, Y.; Yi, M.; Chu, Q.; Jiao, D.; Wu, K. Metabolic profiles of regulatory T cells and their adaptations to the tumor microenvironment: Implications for antitumor immunity. J. Hematol. Oncol. 2022, 15, 104. [Google Scholar] [CrossRef]
- Ouaguia, L.; Moralès, O.; Aoudjehane, L.; Wychowski, C.; Kumar, A.; Dubuisson, J.; Calmus, Y.; Conti, F.; Delhem, N. Hepatitis C Virus Improves Human Tregs Suppressive Function and Promotes Their Recruitment to the Liver. Cells 2019, 8, 1296. [Google Scholar] [CrossRef]
- Ito, M.; Komai, K.; Mise-Omata, S.; Iizuka-Koga, M.; Noguchi, Y.; Kondo, T.; Sakai, R.; Matsuo, K.; Nakayama, T.; Yoshie, O.; et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 2019, 565, 246–250. [Google Scholar] [CrossRef]
- Ouyang, J.; Hu, S.; Zhu, Q.; Li, C.; Kang, T.; Xie, W.; Wang, Y.; Li, Y.; Lu, Y.; Qi, J.; et al. RANKL/RANK signaling recruits Tregs via the CCL20-CCR6 pathway and promotes stemness and metastasis in colorectal cancer. Cell Death Dis. 2024, 15, 437. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Qiao, S.; Zou, H.; Yu, X.J.; Yang, Y.; Li, Z.; Wang, J.; Chen, M.S.; Xu, J.; et al. Lipid droplet accumulation mediates macrophage survival and Treg recruitment via the CCL20/CCR6 axis in human hepatocellular carcinoma. Cell. Mol. Immunol. 2024, 21, 1120–1130. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, X.; Bai, S.; Niu, L.; Zhao, G.; Yao, Y.; Li, B.; Li, H. The effects of TNF-α/TNFR2 in regulatory T cells on the microenvironment and progression of gastric cancer. Int. J. Cancer 2022, 150, 1373–1391. [Google Scholar] [CrossRef]
- Zhou, Y.; Ju, H.; Hu, Y.; Li, T.; Chen, Z.; Si, Y.; Sun, X.; Shi, Y.; Fang, H. Tregs dysfunction aggravates postoperative cognitive impairment in aged mice. J. Neuroinflammation 2023, 20, 75. [Google Scholar] [CrossRef]
- Goldstein, J.D.; Pérol, L.; Zaragoza, B.; Baeyens, A.; Marodon, G.; Piaggio, E. Role of cytokines in thymus- versus peripherally derived-regulatory T cell differentiation and function. Front. Immunol. 2013, 4, 155. [Google Scholar] [CrossRef]
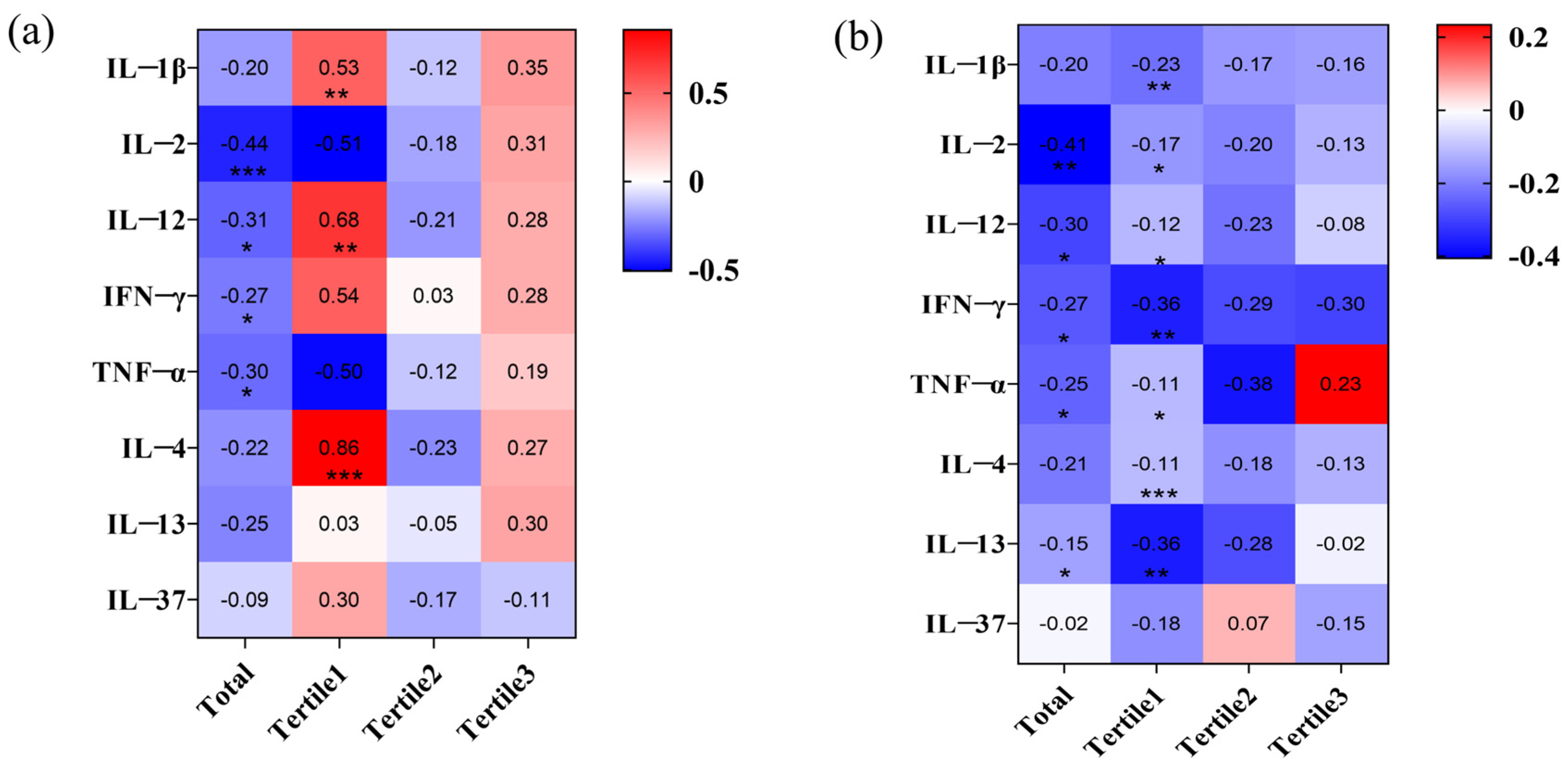
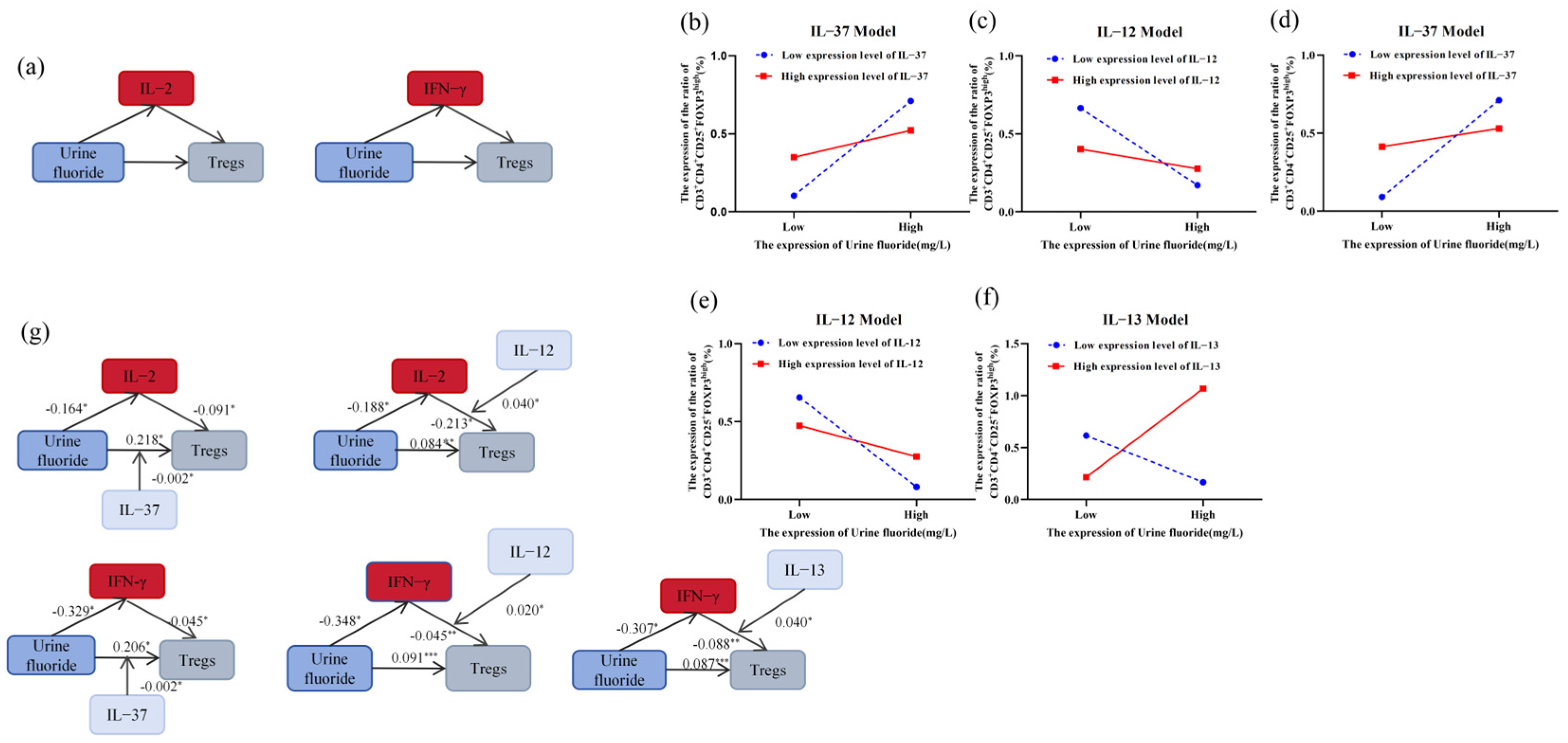
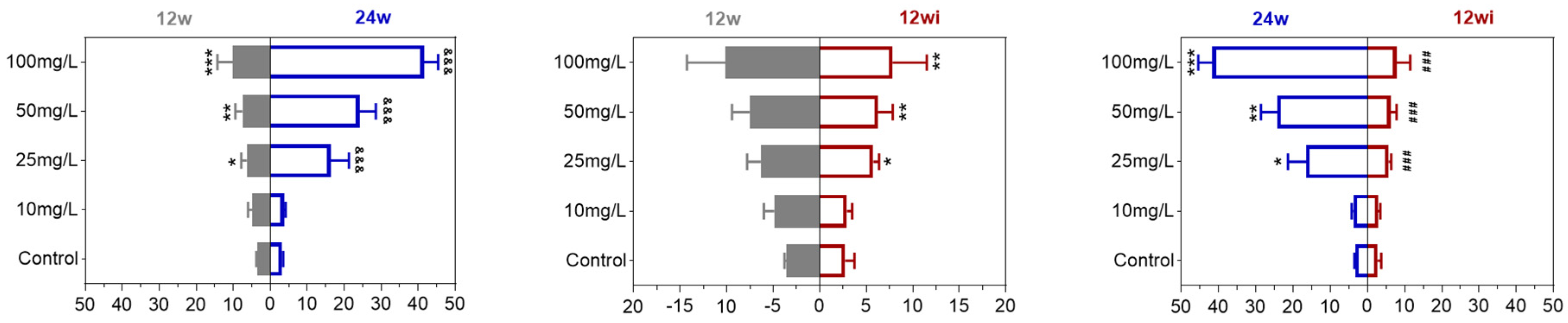
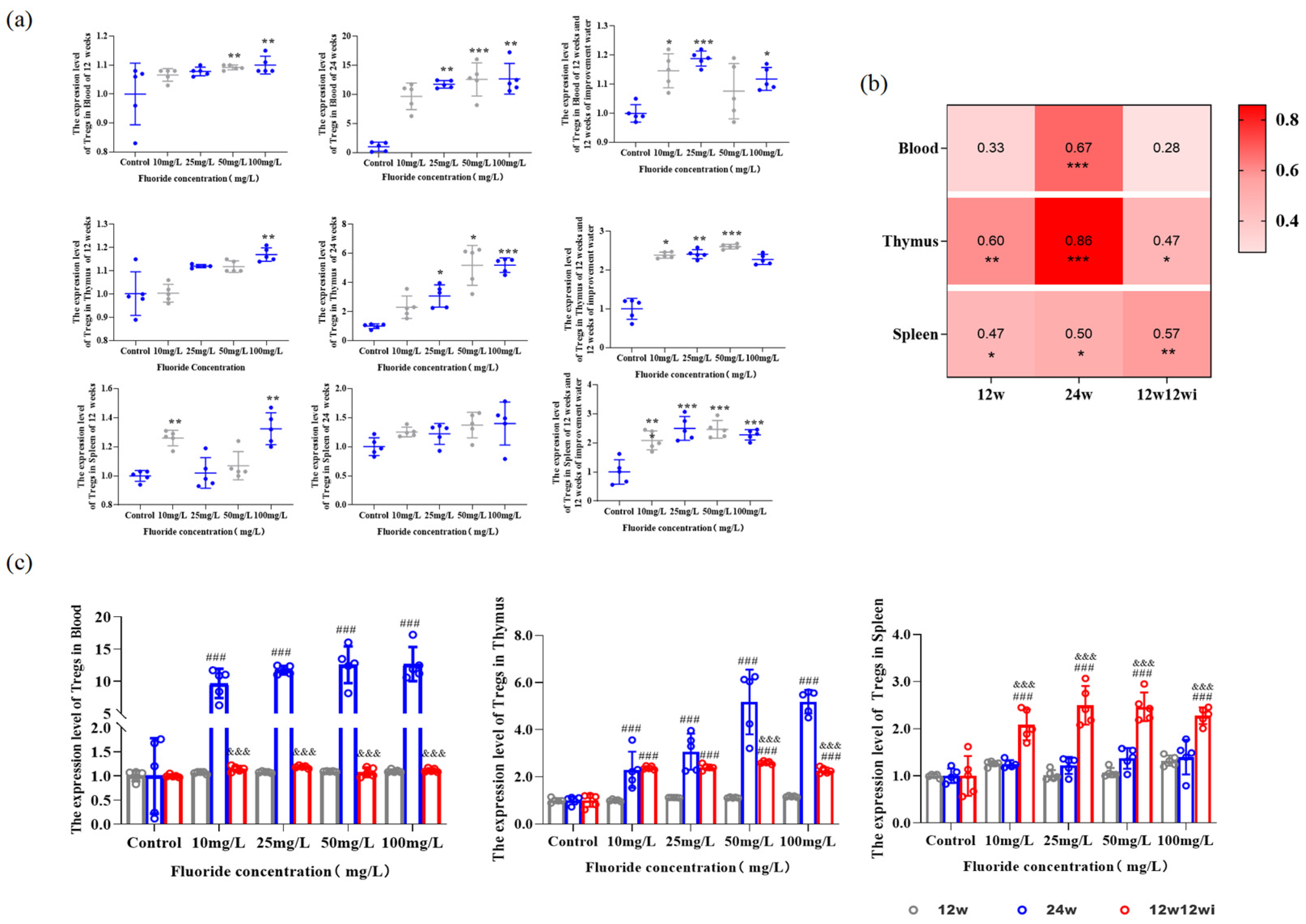

| UF (mg/L) | Crude, β (95% CI) a | p | Adjusted, β (95% CI) a | p |
|---|---|---|---|---|
| IL-2 | ||||
| Tertile1 (≤2.08 mg/L) b | 0.241 (−1.230, 3.394) | 0.336 | 0.284 (−1.370, 3.926) | 0.318 |
| Tertile2 (>2.08–≤3.79 mg/L) c | −0.101 (0.654, −1.503) | 0.654 | −0.155 (−1.732, 0.905) | 0.517 |
| Tertile3 (>3.79 mg/L) b | 0.230 (−0.100, 0.303) | 0.303 | 0.302 (−0.102, 0.370) | 0.247 |
| Total c | −0.303 (−0.317, −0.033) | 0.016 | −0.294 (−0.314, −0.026) | 0.022 |
| IL-12 | ||||
| Tertile1 (≤2.08 mg/L) b | 0.299 (−0.830, 4.544) | 0.165 | 1.367 (−0.981, 4.741) | 0.185 |
| Tertile2 (>2.08–≤3.79 mg/L) c | −0.194 (−2.069, 0.837) | 0.387 | −0.24 (−2.413, 0.887) | 0.343 |
| Tertile3 (>3.79 mg/L) b | 0.243 (−0.109, 0.346) | 0.289 | 0.37 (−0.082, 0.443) | 0.165 |
| Total c | −0.281 (−0.389, −0.031) | 0.022 | −0.291 (−0.402, −0.033) | 0.022 |
| IFN-γ | ||||
| Tertile1 (≤2.08 mg/L) b | 0.140 (−2.772, 5.271) | 0.525 | 0.153 (−2.858, 5.597) | 0.506 |
| Tertile2 (>2.08–≤3.79 mg/L) c | 0.104 (−1.862, 2.979) | 0.636 | 0.049 (−2.396, 2.919) | 0.838 |
| Tertile3 (>3.79 mg/L) b | 0.277 (−0.135, 0.608) | 0.200 | 0.352 (−0.139, 0.739 | 0.169 |
| Total c | −0.263 (−0.570, −0.031) | 0.029 | −0.268 (−0.578, −0.035) | 0.027 |
| TNF-α | ||||
| Tertile1 (≤2.08 mg/L) b | 0.045 (−4.817, 5.932) | 0.832 | 0.028 (−5.251, 5.949) | 0.898 |
| Tertile2 (>2.08–≤3.79 mg/L) c | −0.088 (−3.715, 2.500) | 0.688 | −0.069 (−3.968, 3.024) | 0.780 |
| Tertile3 (>3.79 mg/L) b | 0.240 (−0.292, 0.989) | 0.270 | 0.181 (−0.493, 1.020) | 0.474 |
| Total c | −0.238 (−0.748, −0.008) | 0.046 | −0.248 (−0.772, −0.016) | 0.041 |
| IL-13 | ||||
| Tertile1 (≤2.08 mg/L) b | 0.117 (−0.673, 1.179) | 0.577 | 0.145 (−0.636, 1.264) | 0.500 |
| Tertile2 (>2.08–≤3.79 mg/L) c | −0.025 (−9.130, 8.189) | 0.911 | 0.127 (−6.724, 11.580) | 0.584 |
| Tertile3 (>3.79 mg/L) b | 0.381 (−0.007, 0.147) | 0.073 | 0.351 (−0.028, 0.157) | 0.161 |
| Total c | −0.042 (−0.608, 0.429) | 0.710 | −0.052 (−0.633, 0.409) | 0.668 |
| UF (mg/L) | Tregs | |||||
|---|---|---|---|---|---|---|
| Crude, β (95% CI) a | p d | Adjusted, β (95% CI) a | p d | R | p e | |
| Tertile1 (≤2.08 mg/L) b | −0.040 (−0.111, 0.093) | 0.857 | 0.002 (−0.283, 0.285) | 0.994 | 0.248 | 0.232 |
| Tertile2 (>2.08–≤3.79 mg/L) c | −0.166 (−0.436, 0.200) | 0.448 | −0.311 (−0.600, 0.158) | 0.233 | −0.156 | 0.477 |
| Tertile3 (>3.79 mg/L) b | −0.030 (−0.235, 0.204) | 0.887 | 0.012 (−0.126, 0.150) | 0.855 | 0.091 | 0.681 |
| Total c | 0.547 (0.066, 0.143) | <0.001 | 0.473 (0.048, 0.133) | <0.001 | 0.547 | <0.001 |
| UF (mg/L) | Crude, β (95% CI) a | p | Adjusted, β (95% CI) a | p |
|---|---|---|---|---|
| IL-2 | ||||
| Tertile1 (≤2.08 mg/L) b | −0.082 (−0.078, 0.057) | 0.745 | −0.066 (−0.078, 0.061) | 0.798 |
| Tertile2 (>2.08–≤3.79 mg/L) c | −0.244 (−0.186, 0.056) | 0.274 | −0.338 (−0.226, 0.045) | 0.178 |
| Tertile3 (>3.79 mg/L) b | −0.166 (−0.322, 0.152) | 0.461 | −0.168 (−0.341, 0.169) | 0.486 |
| Total c | −0.369 (−0.201, −0.043) | 0.003 | −0.384 (−0.209, −0.045) | 0.003 |
| IL-12 | ||||
| Tertile1 (≤2.08 mg/L) b | 0.35 (−0.005, 0.051) | 0.101 | −0.091 (−0.246, 0.159) | 0.657 |
| Tertile2 (>2.08–≤3.79 mg/L) c | −0.251 (−0.157, 0.045) | 0.261 | −0.318 (−0.179, 0.037) | 0.183 |
| Tertile3 (>3.79 mg/L) b | −0.128 (−0.269, 0.155) | 0.581 | 0.124 (−0.449, 0.716) | 0.635 |
| Total c | −0.3 (−0.141, −0.016) | 0.014 | −0.315 (−0.145, −0.020) | 0.011 |
| IFN-γ | ||||
| Tertile1 (≤2.08 mg/L) b | 0.165 (−0.016, 0.036) | 0.450 | −0.043 (−0.309, 0.257) | 0.849 |
| Tertile2 (>2.08–≤3.79 mg/L) c | −0.368 (−0.105, 0.007) | 0.084 | −0.137 (−0.427, 0.215) | 0.495 |
| Tertile3 (>3.79 mg/L) b | −0.266 (−0.186, 0.045) | 0.219 | −0.041 (−0.543, 0.458) | 0.871 |
| Total c | −0.370 (−0.100, −0.024) | 0.002 | −0.394 (−0.105, −0.027) | 0.001 |
| TNF-α | ||||
| Tertile1 (≤2.08 mg/L) b | 0.268 (−0.006, 0.028) | 0.194 | 0.242 (−0.008, 0.028) | 0.264 |
| Tertile2 (>2.08–≤3.79 mg/L) c | 0.021 (−0.084, 0.002) | 0.059 | −0.457 (−0.093, −0.002) | 0.043 |
| Tertile3 (>3.79 mg/L) b | 0.234 (−0.032, 0.104) | 0.283 | 0.238 (−0.038, 0.112) | 0.317 |
| Total c | −0.202 (−0.053, 0.004) | 0.091 | −0.214 (−0.055, 0.003) | 0.080 |
| IL-13 | ||||
| Tertile1 (≤2.08 mg/L) b | 0.306 (−0.025, 0.169) | 0.137 | 0.379 (−0.010, 0.189) | 0.076 |
| Tertile2 (>2.08–≤3.79 mg/L) c | −0.092 (−0.020, 0.013) | 0.675 | −0.053 (−0.021, 0.017) | 0.834 |
| Tertile3 (>3.79 mg/L) b | 0.074 (−0.463, 0.645) | 0.737 | 0.066 (−0.522, 0.684) | 0.782 |
| Total c | −0.064 (−0.027, 0.016) | 0.597 | −0.087 (−0.030, 0.014) | 0.487 |
| Variables | Path a | Path b and c′ | Path c | Path a ∗ b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | β | SE | p | β | Boot SE | Boot LLCI | Boot ULCI | |
| Urinary fluoride | −0.294 | 0.072 | 0.022 | 0.423 | 0.022 | <0.001 | 0.499 | 0.022 | <0.001 | 0.076 | 0.039 | 0.010 | 0.162 |
| IL-2 | −0.257 | 0.039 | 0.034 | ||||||||||
| R2 | 0.134 | 0.323 | 0.266 | ||||||||||
| F | 2.207 | 5.338 | 5.158 | ||||||||||
| Urinary fluoride | −0.280 | 0.137 | 0.022 | 0.452 | 0.02 | <0.001 | 0.528 | 0.020 | <0.001 | 0.076 | 0.034 | 0.017 | 0.148 |
| IFN-γ | −0.27 | 0.018 | 0.013 | ||||||||||
| R2 | 0.097 | 0.359 | 0.294 | ||||||||||
| F | 2.330 | 8.973 | 9.002 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zhu, S.; Zhang, Q.; Xie, F.; Wei, D.; Fu, G.; Yang, L.; Gao, Y.; Wei, W. Fluoride-Mediated Immune Damage Through Cytokine Network Regulation of Tregs. Toxics 2025, 13, 95. https://doi.org/10.3390/toxics13020095
Liu B, Zhu S, Zhang Q, Xie F, Wei D, Fu G, Yang L, Gao Y, Wei W. Fluoride-Mediated Immune Damage Through Cytokine Network Regulation of Tregs. Toxics. 2025; 13(2):95. https://doi.org/10.3390/toxics13020095
Chicago/Turabian StyleLiu, Bingshu, Siqi Zhu, Qiong Zhang, Fengyu Xie, Dan Wei, Guiyu Fu, Liu Yang, Yanhui Gao, and Wei Wei. 2025. "Fluoride-Mediated Immune Damage Through Cytokine Network Regulation of Tregs" Toxics 13, no. 2: 95. https://doi.org/10.3390/toxics13020095
APA StyleLiu, B., Zhu, S., Zhang, Q., Xie, F., Wei, D., Fu, G., Yang, L., Gao, Y., & Wei, W. (2025). Fluoride-Mediated Immune Damage Through Cytokine Network Regulation of Tregs. Toxics, 13(2), 95. https://doi.org/10.3390/toxics13020095







