Effects of Exposure to Different Types of Microplastics on the Growth and Development of Rana zhenhaiensis Tadpoles
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Measurement of Morphological Indicators
2.3. Liver Histopathology
2.4. Intestinal Microbiota
2.5. Liver Transcriptome
2.6. Statistical Analysis
3. Results
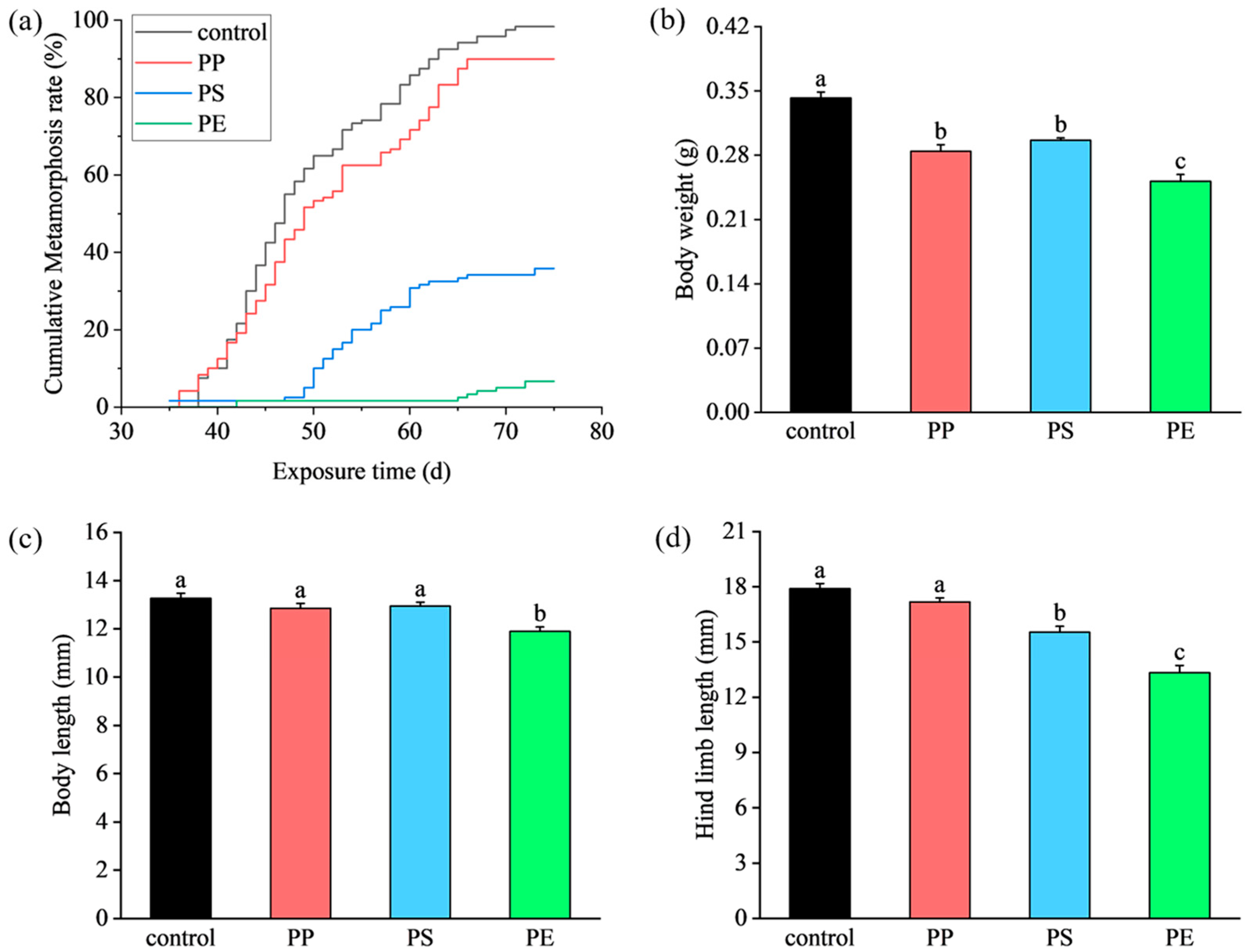
3.1. Effects of MP Exposure on Mortality, Metamorphosis, and Morphology of Tadpoles
3.2. Effects of MP Exposure on Liver Histology
3.3. Effects of MP Exposure on Intestinal Microbiota
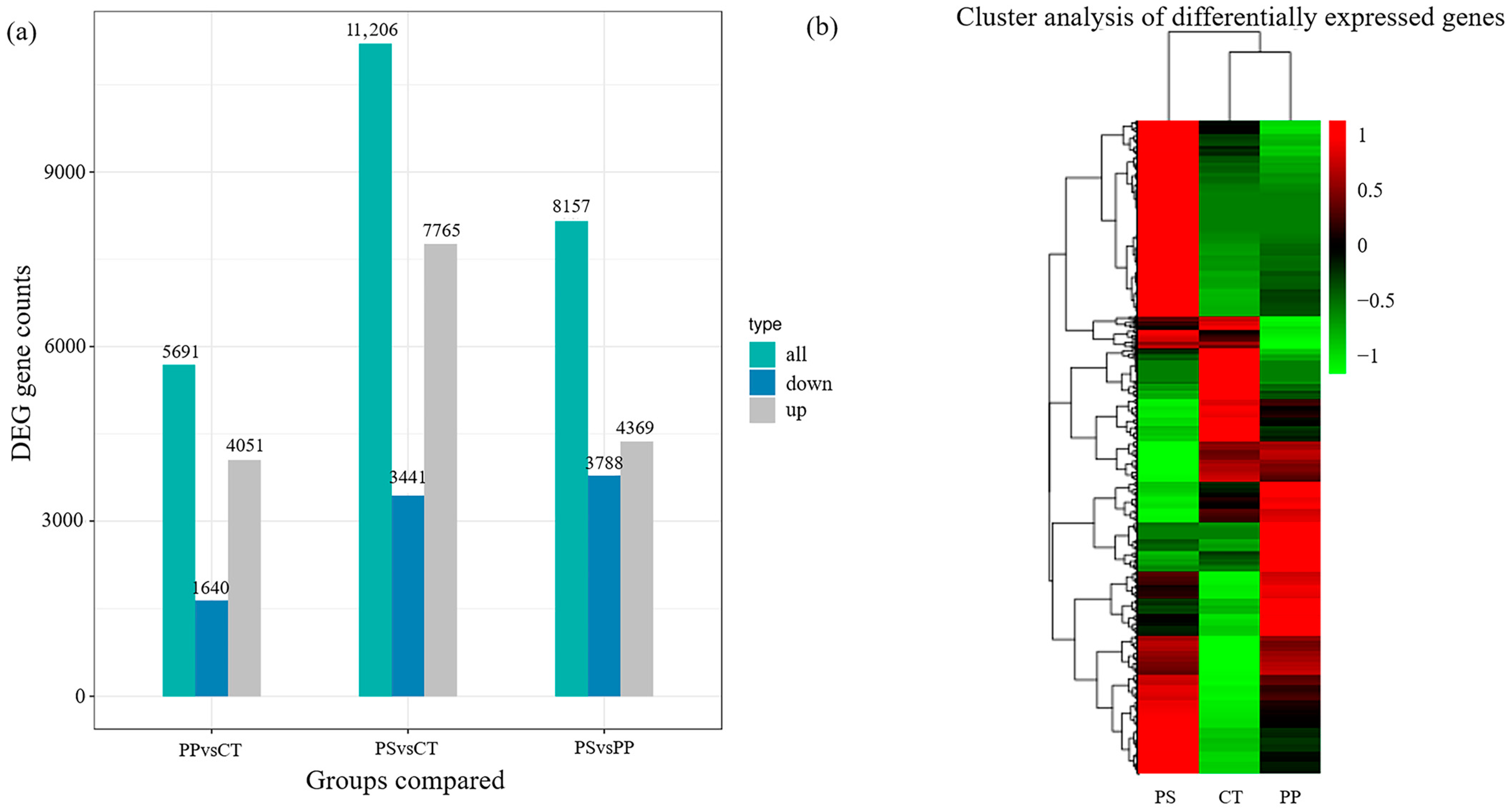
3.4. Correlation Analysis and Identification of Differentially Expressed Genes (DEGs)
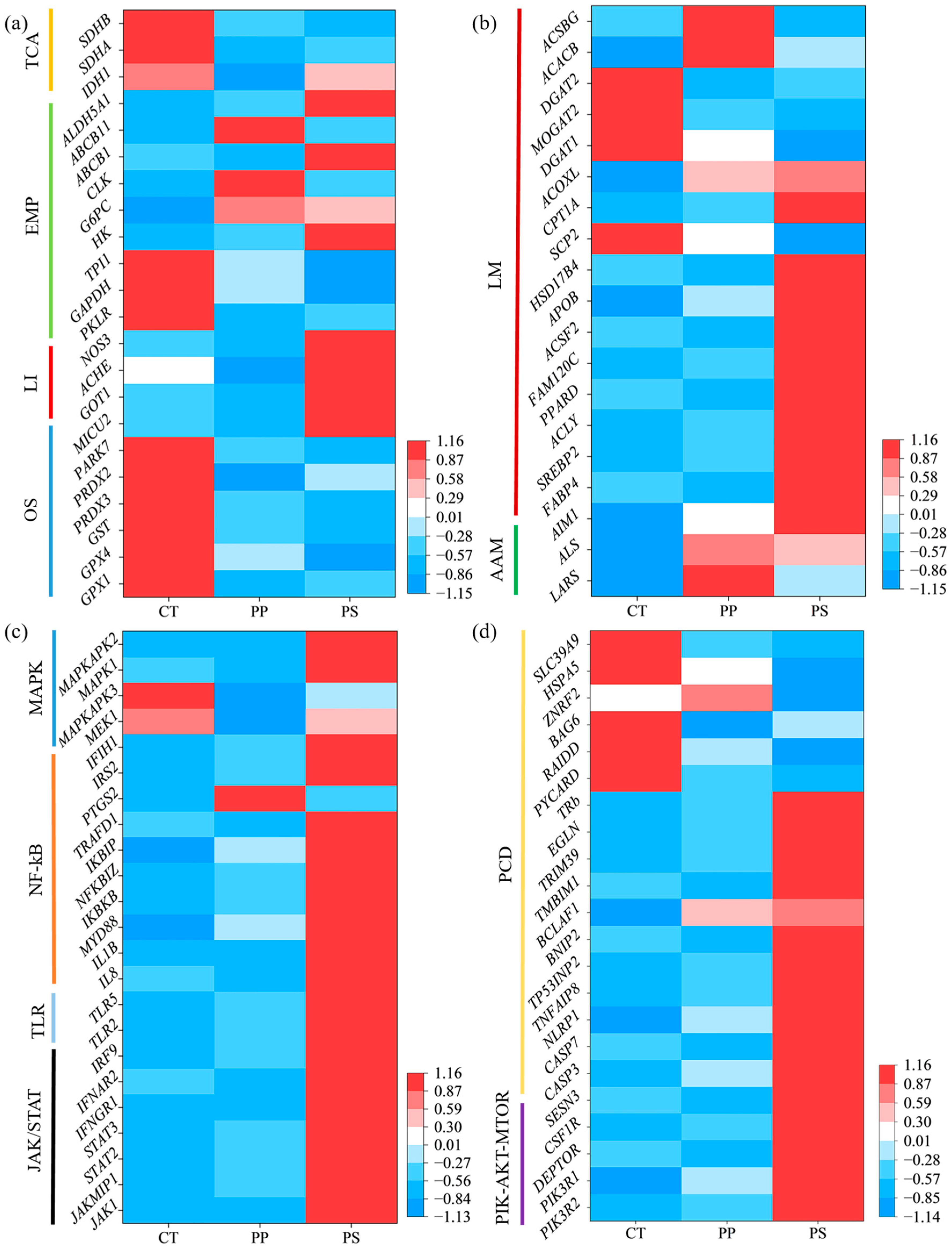
3.5. Transcriptional Expression Profiles of Genes
4. Discussion
4.1. Effects of MPs on Mortality, Metamorphosis Rate, and Morphology of Tadpoles
4.2. PE and PS Exposure Damaged the Liver Tissue of Tadpoles
4.3. PS Exposure Induced Dysregulation of Intestinal Microbiota of Tadpoles
4.4. PP and PS Exposure Altered Transcription Levels of Liver Genes in Tadpoles
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Q.; Li, J.; Wang, C.; Chen, A.; You, Y.; Yang, S.; Liu, H.; Jiang, G.; Wu, Y.; Li, Y. Research Progress on Distribution, Sources, Identification, Toxicity, and Biodegradation of Microplastics in the Ocean, Freshwater, and Soil Environment. Front. Environ. Sci. Eng. 2021, 16, 1. [Google Scholar] [CrossRef]
- Cheung, P.K.; Fok, L. Characterisation of Plastic Microbeads in Facial Scrubs and Their Estimated Emissions in Mainland China. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Jho, E.H. Effect of Different Types and Shapes of Microplastics on the Growth of Lettuce. Chemosphere 2023, 339, 139660. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hao, R.; Shi, X.; Zhang, S.; Sun, B.; Zhao, S.; Huotari, J. Application of a Microplastic Trap to the Determination of the Factors Controlling the Lakebed Deposition of Microplastics. Sci. Total Environ. 2022, 843, 156883. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Xu, L.; Yang, M.; Qin, Z.; Zhang, Q.; Zhang, L. Spatial Distribution Characteristics and Migration of Microplastics in Surface Water, Groundwater and Sediment in Karst Areas: The Case of Yulong River in Guilin, Southwest China. Sci. Total Environ. 2023, 868, 161578. [Google Scholar] [CrossRef]
- Tamminga, M.; Fischer, E.K. Microplastics in a Deep, Dimictic Lake of the North German Plain with Special Regard to Vertical Distribution Patterns. Environ. Pollut. 2020, 267, 115507. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.-S.; Singh, S. Microplastic Pollution: Threats and Impacts on Global Marine Ecosystems. Sustainability 2023, 15, 13252. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, S.-H.; Luo, G.; Kang, Y.; Zhang, L.; Pan, Y.; Zhou, X.; Fan, L.; Liang, B.; Wang, A. The Contamination of Microplastics in China’s Aquatic Environment: Occurrence, Detection and Implications for Ecological Risk. Environ. Pollut. 2022, 296, 118737. [Google Scholar] [CrossRef]
- Gurumoorthi, K.; Luis, A.J. Recent Trends on Microplastics Abundance and Risk Assessment in Coastal Antarctica: Regional Meta-Analysis. Environ. Pollut. 2023, 324, 121385. [Google Scholar] [CrossRef]
- Hu, L.; Chernick, M.; Hinton, D.E.; Shi, H. Microplastics in Small Waterbodies and Tadpoles from Yangtze River Delta, China. Environ. Sci. Technol. 2018, 52, 8885–8893. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Niu, X.; Tang, M.; Zhang, B.-T.; Wang, G.; Yue, W.; Kong, X.; Zhu, J. Distribution of Microplastics in Surface Water of the Lower Yellow River near Estuary. Sci. Total Environ. 2020, 707, 135601. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in Freshwaters and Drinking Water: Critical Review and Assessment of Data Quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.; Maulida, N.; Setyowati, W.N.; Fairus, S.; Puspito, D.A. Microplastic Pollution in the Water and Sediment of Krukut River, Jakarta, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2022, 986, 012084. [Google Scholar] [CrossRef]
- Lin, H.-T.; Schneider, F.; Aziz, M.A.; Wong, K.Y.; Arunachalam, K.D.; Praveena, S.M.; Sethupathi, S.; Chong, W.C.; Nafisyah, A.L.; Parthasarathy, P.; et al. Microplastics in Asian Rivers: Geographical Distribution, Most Detected Types, and Inconsistency in Methodologies. Environ. Pollut. 2024, 349, 123985. [Google Scholar] [CrossRef] [PubMed]
- Le, V.-G.; Nguyen, M.-K.; Ngo, H.H.; Barceló, D.; Nguyen, H.-L.; Um, M.J.; Nguyen, D.D. Microplastics in Aquaculture Environments: Current Occurrence, Adverse Effects, Ecological Risk, and Nature-Based Mitigation Solutions. Mar. Pollut. Bull. 2024, 209, 117168. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Hu, X.; Yang, B.; Zhang, G.; Wang, J.; Ling, W. Distribution, Abundance and Risks of Microplastics in the Environment. Chemosphere 2020, 249, 126059. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, X.; Sun, Y.; Deng, Q.; Wu, Q.; Wen, Z.; Chen, H. Harmful Effects of Microplastics on Respiratory System of Aquatic Animals: A Systematic Review and Meta-Analysis. Aquat. Toxicol. 2024, 273, 107003. [Google Scholar] [CrossRef] [PubMed]
- Usman, S.; Abdull Razis, A.F.; Shaari, K.; Amal, M.N.A.; Saad, M.Z.; Mat Isa, N.; Nazarudin, M.F. Polystyrene Microplastics Exposure: An Insight into Multiple Organ Histological Alterations, Oxidative Stress and Neurotoxicity in Javanese Medaka Fish (Oryzias javanicus Bleeker, 1854). Int. J. Environ. Res. Public Health 2021, 18, 9449. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Zhang, T.; Chen, X.; Zhu, B.; Zhao, M.; Fu, K.; Zhang, Y.; Tang, H.; Pang, H.; Guo, Y.; et al. Behavioral Impairments and Disrupted Mitochondrial Energy Metabolism Induced by Polypropylene Microplastics in Zebrafish Larvae. Sci. Total Environ. 2024, 947, 174541. [Google Scholar] [CrossRef] [PubMed]
- da Costa Araújo, A.P.; de Melo, N.F.S.; de Oliveira Junior, A.G.; Rodrigues, F.P.; Fernandes, T.; de Andrade Vieira, J.E.; Rocha, T.L.; Malafaia, G. How Much Are Microplastics Harmful to the Health of Amphibians? A Study with Pristine Polyethylene Microplastics and Physalaemus cuvieri. J. Hazard. Mater. 2020, 382, 121066. [Google Scholar] [CrossRef] [PubMed]
- de Brito, R.R.; de Oliveira Ferreira, R.; Soares, W.R.; Guimarães, A.T.B.; de Lima Rodrigues, A.S.; da Luz, T.M.; Gomes, A.R.; de Matos, L.P.; Malafaia, G. Persistent Effects of Naturally Aged Polyethylene Terephthalate Microplastics on Physalaemus cuvieri Tadpoles: The Toxic Legacy beyond Exposure. Water Air Soil Pollut. 2025, 236, 122. [Google Scholar] [CrossRef]
- Lu, H.; Hu, Y.; Kang, C.; Meng, Q.; Lin, Z. Cadmium-Induced Toxicity to Amphibian Tadpoles Might Be Exacerbated by Alkaline Not Acidic pH Level. Ecotoxicol. Environ. Saf. 2021, 218, 112288. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Pang, Y.; Xiao, S.; Xie, L.; Zhang, Y. Toxicity of Polystyrene Microplastics with Cadmium on the Digestive System of Rana zhenhaiensis Tadpoles. Toxics 2024, 12, 854. [Google Scholar] [CrossRef]
- Tian, R.; Chen, L.; Zhao, Z.; Gao, T.; Zong, L.; Chang, J.; Zhang, J. Mechanism Insights into the Histopathological Changes of Polypropylene Microplastics Induced Gut and Liver in Zebrafish. Ecotox. Environ. Safe. 2024, 280, 116537. [Google Scholar] [CrossRef] [PubMed]
- da Costa Araújo, A.P.; Gomes, A.R.; Malafaia, G. Hepatotoxicity of Pristine Polyethylene Microplastics in Neotropical Physalaemus cuvieri Tadpoles (Fitzinger, 1826). J. Hazard. Mater. 2020, 386, 121992. [Google Scholar] [CrossRef] [PubMed]
- Gosner, K.L. A Simplified Table for Staging Anuran Embryos and Larvae with Notes on Identification. Herpetologica 1960, 16, 183–190. [Google Scholar]
- Jiang, K.; Li, J.-T. Method for external measurement of adult anuran specimens. Bio-101 2021, e1010675. [Google Scholar]
- Wu, C.; Zhang, Y.; Chai, L.; Wang, H. Histological Changes, Lipid Metabolism and Oxidative Stress in the Liver of Bufo gargarizans Exposed to Cadmium Concentrations. Chemosphere 2017, 179, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lv, Y.; Liu, J.; Chang, L.; Chen, Q.; Zhu, L.; Wang, B.; Jiang, J.; Zhu, W. Size Matters Either Way: Differently-Sized Microplastics Affect Amphibian Host and Symbiotic Microbiota Discriminately. Environ. Pollut. 2023, 328, 121634. [Google Scholar] [CrossRef] [PubMed]
- Balestrieri, A.; Winkler, A.; Scribano, G.; Gazzola, A.; Lastrico, G.; Grioni, A.; Pellitteri-Rosa, D.; Tremolada, P. Differential Effects of Microplastic Exposure on Anuran Tadpoles: A Still Underrated Threat to Amphibian Conservation? Environ. Pollut. 2022, 303, 119137. [Google Scholar] [CrossRef]
- Boyero, L.; López-Rojo, N.; Bosch, J.; Alonso, A.; Correa-Araneda, F.; Pérez, J. Microplastics Impair Amphibian Survival, Body Condition and Function. Chemosphere 2020, 244, 125500. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Zhang, X.; Liu, X.; Liao, X.; Zhang, H.; Yan, C.; Lin, Y.; Huang, Q. Polystyrene Microplastics Induce Metabolic Disturbances in Marine Medaka (Oryzias melastigmas) Liver. Sci. Total Environ. 2021, 782, 146885. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, Y.; Li, X.; Chai, L.; Wang, H. Exposure to Nitrate Alters the Histopathology and Gene Expression in the Liver of Bufo gargarizans Tadpoles. Chemosphere 2019, 217, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Zhao, C.; Cai, R.; Wu, H.; Wei, L.; Zhou, C.; Chai, J.; Teng, X.; Liu, T. Vital Role of Oxidative Stress in Tadpole Liver Damage Caused by Polystyrene Nanoparticles. Ecotoxicol. Environ. Saf. 2024, 277, 116331. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-Y.; Fu, J.-J.; Shi, J.-B.; Zhou, Q.-F.; Yuan, C.-G.; Jiang, G.-B. Methylmercury Accumulation, Histopathology Effects, and Cholinesterase Activity Alterations in Medaka (Oryzias latipes) Following Sublethal Exposure to Methylmercury Chloride. Environ. Toxicol. Pharmacol. 2006, 22, 225–233. [Google Scholar] [CrossRef]
- Zheng, R.; Liu, R.; Wu, M.; Wang, H.; Xie, L. Effects of Sodium Perchlorate and Exogenous L-thyroxine on Growth, Development and Leptin Signaling Pathway of Bufo gargarizans Tadpoles during Metamorphosis. Ecotoxicol. Environ. Saf. 2020, 206, 111410. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Zhai, L.; Lu, K. Co-Exposure of Polycarbonate Microplastics Aggravated the Toxic Effects of Imidacloprid on the Liver and Gut Microbiota in Mice. Environ. Toxicol. Pharmacol. 2023, 101, 104194. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Chen, X.; Ren, C.; Teng, Y.; Shen, Y.; Wu, M.; Wang, H.; Huang, M. Comparison of the Characteristics of Intestinal Microbiota Response in Bufo gargarizans Tadpoles: Exposure to the Different Environmental Chemicals (Cu, Cr, Cd and NO3–N). Chemosphere 2020, 247, 125925. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, Y.; Gao, J.; Li, X.; Wang, H. Nitrate Exposure Induces Intestinal Microbiota Dysbiosis and Metabolism Disorder in Bufo gargarizans Tadpoles. Environ. Pollut. 2020, 264, 114712. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, H.; Shi, Y.; Tian, H.; Yu, F.; Liu, M.; Gao, L. Exposure to Nitrate Induced Growth, Intestinal Histology and Microbiota Alterations of Bufo raddei Strauch Tadpoles. Aquat. Toxicol. 2023, 258, 106477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Huang, M.; Liu, Y.; Wan, Y.; Duan, R.; Wu, L. Effects of Atrazine Short-Term Exposure on Jumping Ability and Intestinal Microbiota Diversity in Male Pelophylax nigromaculatus Adults. Environ. Sci. Pollut. Res. 2021, 28, 36122–36132. [Google Scholar] [CrossRef]
- Umamaheswari, S.; Priyadarshinee, S.; Bhattacharjee, M.; Kadirvelu, K.; Ramesh, M. Exposure to Polystyrene Microplastics Induced Gene Modulated Biological Responses in Zebrafish (Danio rerio). Chemosphere 2021, 281, 128592. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jia, X.; Zhu, H.; Zhang, Q.; He, Y.; Shen, Y.; Xu, X.; Li, J. The Effects of Exposure to Microplastics on Grass Carp (Ctenopharyngodon idella) at the Physiological, Biochemical, and Transcriptomic Levels. Chemosphere 2022, 286, 131831. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, Y.; Qu, Y.; Chai, L.; Li, X.; Wang, H. Effects of Nitrate on Development and Thyroid Hormone Signaling Pathway during Bufo gargarizans Embryogenesis. Chemosphere 2019, 235, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, W.; Wang, B.; Wang, Y.; Li, N.; Li, R.; Yan, Y.; Sun, Y.; He, J. Potato Glycoside Alkaloids Exhibit Antifungal Activity by Regulating the Tricarboxylic Acid Cycle Pathway of Fusarium solani. Front. Microbiol. 2024, 15, 1390269. [Google Scholar] [CrossRef]
- Liaud, M.-F.; Lichtl, C.; Apt, K.; Martin, W.; Cerff, R. Compartment-Specific Isoforms of TPI and GAPDH Are Imported into Diatom Mitochondria as a Fusion Protein: Evidence in Favor of a Mitochondrial Origin of the Eukaryotic Glycolytic Pathway. Mol. Biol. Evol. 2000, 17, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Moreno, D.G.; Hernández-Aguirre, L.E.; Peregrino-Uriarte, A.B.; Leyva-Carrillo, L.; Gómez-Jiménez, S.; Contreras-Vergara, C.; Hernández-López, J.; Yepiz-Plascencia, G. Changes of Glycolysis and Gluconeogenesis Key Enzymes in the Muscle of the Shrimp Penaeus vannamei in Response to Hypoxia and Reoxygenation. J. Exp. Mar. Biol. Ecol. 2024, 580, 152052. [Google Scholar] [CrossRef]
- Gu, R.; Zhang, F.; Chen, G.; Han, C.; Liu, J.; Ren, Z.; Zhu, Y.; Waddington, J.L.; Zheng, L.T.; Zhen, X. Clk1 Deficiency Promotes Neuroinflammation and Subsequent Dopaminergic Cell Death through Regulation of Microglial Metabolic Reprogramming. Brain Behav. Immun. 2017, 60, 206–219. [Google Scholar] [CrossRef]
- Xu, S.-Y.; Mo, Y.-H.; Liu, Y.-J.; Wang, X.; Li, H.-Y.; Yang, W.-D. Physiological and Genetic Responses of the Benthic Dinoflagellate Prorocentrum lima to Polystyrene Microplastics. Harmful Algae 2024, 136, 102652. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bao, Z.; Wan, Z.; Fu, Z.; Jin, Y. Polystyrene Microplastic Exposure Disturbs Hepatic Glycolipid Metabolism at the Physiological, Biochemical, and Transcriptomic Levels in Adult Zebrafish. Sci. Total Environ. 2020, 710, 136279. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Du, Z.; Chen, B.; Ren, M.; Yang, Q.; Sui, Y.; Wang, Q.; Wang, A.; Zhao, H.; Qin, Y.; et al. Association of SREBP2 Gene Polymorphisms with the Risk of Osteonecrosis of the Femoral Head Relates to Gene Expression and Lipid Metabolism Disorders. Mol. Med. Rep. 2017, 16, 7145–7153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, Y.-C.; Lee, D.S.; Jung, Y.; Bin, C.E.; An, J.; Sanghyeob, L.; Hwan, L.J. Transcriptome Analysis of a Transgenic Arabidopsis Plant Overexpressing CsBCAT7 Reveals the Relationship between CsBCAT7 and Branched-Chain Amino Acid Catabolism. J. Plant Biotechnol. 2021, 48, 228–235. [Google Scholar] [CrossRef]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to Polystyrene Microplastics Causes Reproductive Toxicity through Oxidative Stress and Activation of the P38 MAPK Signaling Pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef] [PubMed]
- Swaraj, S.; Tripathi, S. Interference without Interferon: Interferon-Independent Induction of Interferon-Stimulated Genes and Its Role in Cellular Innate Immunity. mBio 2024, 15, e02582-24. [Google Scholar] [CrossRef] [PubMed]
- Tsurumi, A.; Zhao, C.; Li, W.X. Canonical and Non-Canonical JAK/STAT Transcriptional Targets May Be Involved in Distinct and Overlapping Cellular Processes. BMC Genom. 2017, 18, 718. [Google Scholar] [CrossRef]
- Zuo, H.; Weng, K.; Luo, M.; Yang, L.; Weng, S.; He, J.; Xu, X. A MicroRNA-1-Mediated Inhibition of the NF-κB Pathway by the JAK-STAT Pathway in the Invertebrate Litopenaeus Vannamei. J. Immunol. 2020, 204, 2918–2930. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Liu, Z.; Wu, D.; Chen, M.; Lv, W.; Zhao, Y. Accumulation of Polystyrene Microplastics in Juvenile Eriocheir sinensis and Oxidative Stress Effects in the Liver. Aquat. Toxicol. 2018, 200, 28–36. [Google Scholar] [CrossRef]
- Fu, C.; Ye, S.; Liu, Y.; Li, S. Role of CARD Region of MDA5 Gene in Canine Influenza Virus Infection. Viruses 2020, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Zhang, H.; Shi, H.; Peng, Y.; Han, M.; Hu, T.; Liao, X.; Liu, Y.; Zhang, J.; Xu, G. Enhanced Hepatotoxicity in Zebrafish Due to Co-Exposure of Microplastics and Sulfamethoxazole: Insights into ROS-Mediated MAPK Signaling Pathway Regulation. Ecotoxicol. Environ. Saf. 2024, 278, 116415. [Google Scholar] [CrossRef]
- Cao, J.; Xu, R.; Geng, Y.; Xu, S.; Guo, M. Exposure to Polystyrene Microplastics Triggers Lung Injury via Targeting Toll-like Receptor 2 and Activation of the NF-κB Signal in Mice. Environ. Pollut. 2023, 320, 121068. [Google Scholar] [CrossRef]
- Xu, T.; Cui, J.; Xu, R.; Cao, J.; Guo, M. Microplastics Induced Inflammation and Apoptosis via Ferroptosis and the NF-κB Pathway in Carp. Aquat. Toxicol. 2023, 262, 106659. [Google Scholar] [CrossRef]
- Martinez-Jacobo, L.; Ancer-Arellano, C.I.; Ortiz-Lopez, R.; Salinas-Santander, M.; Villarreal-Villarreal, C.D.; Ancer-Rodriguez, J.; Camacho-Zamora, B.; Zomosa-Signoret, V.; Medina-De la Garza, C.E.; Ocampo-Candiani, J.; et al. Evaluation of the Expression of Genes Associated with Inflammation and Apoptosis in Androgenetic Alopecia by Targeted RNA-Seq. Ski. Appendage Disord. 2017, 4, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Bo, X.; Mu, D.; Wu, M.; Xiao, H.; Wang, H. The Morphological Changes and Molecular Biomarker Responses in the Liver of Fluoride-Exposed Bufo gargarizans Larvae. Ecotoxicol. Environ. Saf. 2018, 151, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, D.; Li, Y.; Luo, Y.; Liang, B.; Yu, K.; Xiong, W.; Zuo, D. Overexpression of TP53INP2 Promotes Apoptosis in Clear Cell Renal Cell Cancer via Caspase-8/TRAF6 Signaling Pathway. J. Immunol. Res. 2022, 2022, 1260423. [Google Scholar] [CrossRef]
- Pan, Y.; Cheng, A.; Zhang, X.; Wang, M.; Chen, S.; Zhu, D.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y.; et al. Transcriptome Analysis of Duck Embryo Fibroblasts for the Dynamic Response to Duck Tembusu Virus Infection and Dual Regulation of Apoptosis Genes. Aging 2020, 12, 17503–17527. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Chen, H.; Gao, X.; He, X.; Jin, R.; Wei, Y.; Li, S.; Xie, L.; Zhang, Y. Effects of Exposure to Different Types of Microplastics on the Growth and Development of Rana zhenhaiensis Tadpoles. Toxics 2025, 13, 165. https://doi.org/10.3390/toxics13030165
Xiao S, Chen H, Gao X, He X, Jin R, Wei Y, Li S, Xie L, Zhang Y. Effects of Exposure to Different Types of Microplastics on the Growth and Development of Rana zhenhaiensis Tadpoles. Toxics. 2025; 13(3):165. https://doi.org/10.3390/toxics13030165
Chicago/Turabian StyleXiao, Shimin, Hao Chen, Xiyao Gao, Xinni He, Rongzhou Jin, Yunqi Wei, Shuran Li, Lei Xie, and Yongpu Zhang. 2025. "Effects of Exposure to Different Types of Microplastics on the Growth and Development of Rana zhenhaiensis Tadpoles" Toxics 13, no. 3: 165. https://doi.org/10.3390/toxics13030165
APA StyleXiao, S., Chen, H., Gao, X., He, X., Jin, R., Wei, Y., Li, S., Xie, L., & Zhang, Y. (2025). Effects of Exposure to Different Types of Microplastics on the Growth and Development of Rana zhenhaiensis Tadpoles. Toxics, 13(3), 165. https://doi.org/10.3390/toxics13030165





