Effects of the UV Filter Octocrylene and Its Degradation Product Benzophenone on Pacific Oyster (Magallana gigas) Larvae: A Call for Reassessment of Environmental Hazards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Octocrylene and Benzophenone Solutions
2.2. Obtaining Oyster Gametes
2.3. Fertilization
2.4. Embryo–Larval Toxicity Tests
2.4.1. DNA Damage
2.4.2. Embryogenesis Success
2.4.3. Larvae Swimming Velocity
2.5. Statistical Analysis
3. Results
3.1. DNA Damage
3.2. Embryogenesis Success
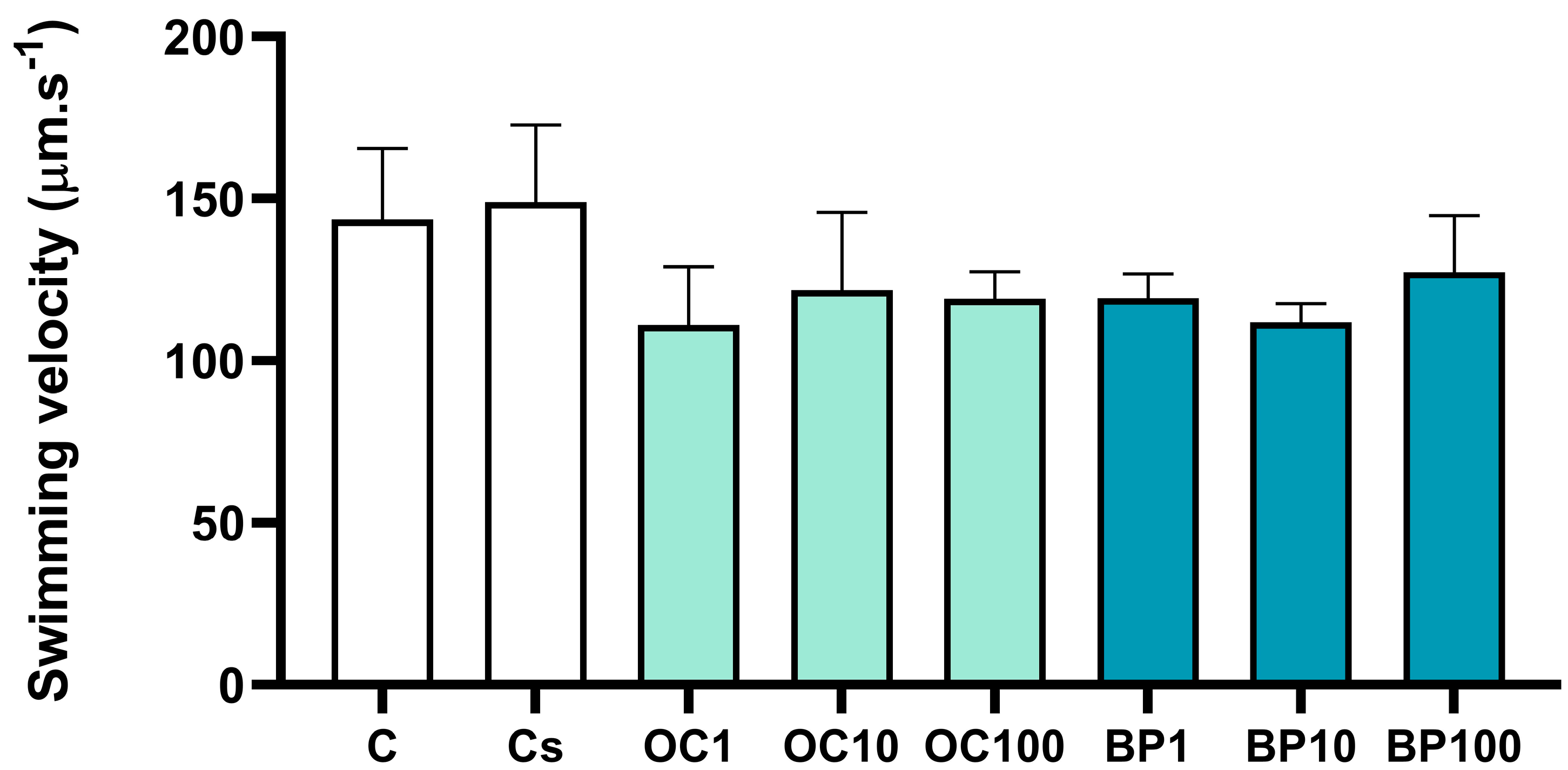
3.3. Swimming Velocity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurihara, H.; Kato, S.; Ishimatsu, A. Effects of increased seawater pCO2 on early development of the oyster Crassostrea gigas. Aquat. Biol. 2007, 1, 91–98. [Google Scholar] [CrossRef]
- Kurihara, H. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser. 2008, 373, 275–284. [Google Scholar] [CrossRef]
- Ross, P.M.; Parker, L.; O’Connor, W.A.; Bailey, E.A. The impact of ocean acidification on reproduction, early development and settlement of marine organisms. Water 2011, 3, 1005–1030. [Google Scholar] [CrossRef]
- Mohammed, A. Why are Early Life Stages of Aquatic Organisms more Sensitive to Toxicants than Adults? In New Insights into Toxicity and Drug Testing; Gowder, S., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Coglianese, M.P.; Martin, M. Individual and interactive effects of environmental stress on the embryonic development of the Pacific oyster, Crassostrea gigas. Mar. Environ. Res. 1981, 5, 13–27. [Google Scholar] [CrossRef]
- His, E.; Beiras, R.; Seaman, M.N.L. The Assessment of Marine Pollution Bioassays with Bivalve Embryos and Larvae; Elsevier Masson SAS: Amsterdam, The Netherlands, 1999; Volume 37, ISBN 0120261375. [Google Scholar]
- Bringer, A.; Thomas, H.; Prunier, G.; Dubillot, E.; Bossut, N.; Churlaud, C.; Clérandeau, C.; Le Bihanic, F.; Cachot, J. High density polyethylene (HDPE) microplastics impair development and swimming activity of Pacific oyster D-larvae, Crassostrea gigas, depending on particle size. Environ. Pollut. 2020, 260, 113978. [Google Scholar] [CrossRef]
- Tallec, K.; Huvet, A.; Di Poi, C.; González-Fernández, C.; Lambert, C.; Petton, B.; Le Goïc, N.; Berchel, M.; Soudant, P.; Paul-Pont, I. Nanoplastics impaired oyster free living stages, gametes and embryos. Environ. Pollut. 2018, 242, 1226–1235. [Google Scholar] [CrossRef]
- Mai, H.; Cachot, J.; Brune, J.; Geffard, O.; Belles, A.; Budzinski, H.; Morin, B. Embryotoxic and genotoxic effects of heavy metals and pesticides on early life stages of Pacific oyster (Crassostrea gigas). Mar. Pollut. Bull. 2012, 64, 2663–2670. [Google Scholar] [CrossRef]
- Mai, H.; Gonzalez, P.; Pardon, P.; Tapie, N.; Budzinski, H.; Cachot, J.; Morin, B. Comparative responses of sperm cells and embryos of Pacific oyster (Crassostrea gigas) to exposure to metolachlor and its degradation products. Aquat. Toxicol. 2014, 147, 48–56. [Google Scholar] [CrossRef]
- Mai, H.; Morin, B.; Pardon, P.; Gonzalez, P.; Budzinski, H.; Cachot, J.Ô. Environmental concentrations of irgarol, diuron and S-metolachlor induce deleterious effects on gametes and embryos of the Pacific oyster, Crassostrea gigas. Mar. Environ. Res. 2013, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Levallois, A.; Costil, K.; Caplat, C.; Basuyaux, O.; Lebel, J.M.; Guegan, C.; Serpentini, A. Comparative effects of trace metal elements released from dissolution of aluminum-based galvanic anodes, aluminum chloride, zinc chloride, and their mixture on the development of the Pacific oyster D-larvae, Crassostrea gigas. Environ. Sci. Pollut. Res. 2023, 30, 101535–101545. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, R.; Montagna, M.; Balbi, T.; Raffo, E.; Palumbo, F.; Canesi, L. Adaptation of the bivalve embryotoxicity assay for the high throughput screening of emerging contaminants in Mytilus galloprovincialis. Mar. Environ. Res. 2014, 99, 1–8. [Google Scholar] [CrossRef]
- Di Poi, C.; Costil, K.; Bouchart, V.; Halm-Lemeille, M.P. Toxicity assessment of five emerging pollutants, alone and in binary or ternary mixtures, towards three aquatic organisms. Environ. Sci. Pollut. Res. 2018, 25, 6122–6134. [Google Scholar] [CrossRef] [PubMed]
- Rolton, A.; Champeau, O.; Barrick, A.; Boundy, M.; Tremblay, L.A.; Vignier, J. Characterization of the effects of triclosan on sperm and embryos of Mytilus and Perna mussel species. Aquat. Toxicol. 2022, 245, 106107. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Miglioli, A.; Balbi, T.; Fabbri, E. Physiological Roles of Serotonin in Bivalves: Possible Interference by Environmental Chemicals Resulting in Neuroendocrine Disruption. Front. Endocrinol. 2022, 13, 792589. [Google Scholar] [CrossRef]
- Bachelot, M.; Li, Z.; Munaron, D.; Le Gall, P.; Casellas, C.; Fenet, H.; Gomez, E. Organic UV filter concentrations in marine mussels from French coastal regions. Sci. Total Environ. 2012, 420, 273–279. [Google Scholar] [CrossRef]
- Castro, M.; Fernandes, J.O.; Pena, A.; Cunha, S.C. Occurrence, profile and spatial distribution of UV-filters and musk fragrances in mussels from Portuguese coastline. Mar. Environ. Res. 2018, 138, 110–118. [Google Scholar] [CrossRef]
- Gadelha, J.R.; Rocha, A.C.; Camacho, C.; Eljarrat, E.; Peris, A.; Aminot, Y.; Readman, J.W.; Boti, V.; Nannou, C.; Kapsi, M.; et al. Persistent and emerging pollutants assessment on aquaculture oysters (Crassostrea gigas) from NW Portuguese coast (Ria De Aveiro). Sci. Total Environ. 2019, 666, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Mitchelmore, C.L.; He, K.; Gonsior, M.; Hain, E.; Heyes, A.; Clark, C.; Younger, R.; Schmitt-Kopplin, P.; Feerick, A.; Conway, A.; et al. Occurrence and distribution of UV-filters and other anthropogenic contaminants in coastal surface water, sediment, and coral tissue from Hawaii. Sci. Total Environ. 2019, 670, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Pintado-Herrera, M.G.; Allan, I.J.; González-Mazo, E.; Lara-Martín, P.A. Passive Samplers vs Sentinel Organisms: One-Year Monitoring of Priority and Emerging Contaminants in Coastal Waters. Environ. Sci. Technol. 2020, 54, 6693–6702. [Google Scholar] [CrossRef]
- Juliano, C.; Magrini, G.A. Cosmetic Ingredients as Emerging Pollutants of Environmental and Health Concern. A Mini-Review. Cosmetics 2017, 4, 11. [Google Scholar] [CrossRef]
- Molins-Delgado, D.; Muñoz, R.; Nogueira, S.; Alonso, M.B.; Torres, J.P.; Malm, O.; Ziolli, R.L.; Hauser-Davis, R.A.; Eljarrat, E.; Barceló, D.; et al. Occurrence of organic UV filters and metabolites in Lebranche mullet (Mugil liza) from Brazil. Sci. Total Environ. 2018, 618, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Agawin, N.S.R.; Sunyer-Caldú, A.; Díaz-Cruz, M.S.; Frank-Comas, A.; García-Márquez, M.G.; Tovar-Sánchez, A. Mediterranean seagrass Posidonia oceanica accumulates sunscreen UV filters. Mar. Pollut. Bull. 2022, 176, 113417. [Google Scholar] [CrossRef]
- Blüthgen, N.; Meili, N.; Chew, G.; Odermatt, A.; Fent, K. Accumulation and effects of the UV- filter octocrylene in adult and embryonic zebrafish (Danio rerio). Sci. Total Environ. 2014, 476–477, 207–217. [Google Scholar] [CrossRef]
- Bratkovics, S.; Sapozhnikova, Y. Determination of seven commonly used organic UV filters in fresh and saline waters by liquid chromatography-tandem mass spectrometry. Anal. Methods 2011, 3, 2943–2950. [Google Scholar] [CrossRef]
- Cadena-Aizaga, M.I.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Occurrence and environmental hazard of organic UV filters in seawater and wastewater from Gran Canaria Island (Canary Islands, Spain). Environ. Pollut. 2022, 300, 118843. [Google Scholar] [CrossRef] [PubMed]
- Langford, K.H.; Thomas, K.V. Inputs of chemicals from recreational activities into the Norwegian coastal zone. J. Environ. Monit. 2008, 10, 894–898. [Google Scholar] [CrossRef]
- Tsui, M.M.P.; Leung, H.W.; Wai, T.C.; Yamashita, N.; Taniyasu, S.; Liu, W.; Lam, P.K.S.; Murphy, M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries. Water Res. 2014, 67, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Rodríguez, A.; Rodrigo Sanz, M.; Betancort Rodríguez, J.R. Occurrence of eight UV filters in beaches of Gran Canaria (Canary Islands). An approach to environmental risk assessment. Chemosphere 2015, 131, 85–90. [Google Scholar] [CrossRef]
- Gayathri, M.; Sutha, J.; Mohanthi, S.; Ramesh, M.; Poopal, R.K. Ecotoxicological evaluation of the UV-filter octocrylene (OC) in embryonic zebrafish (Danio rerio): Developmental, biochemical and cellular biomarkers. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 271, 109688. [Google Scholar] [CrossRef]
- Falfushynska, H.; Sokolov, E.P.; Fisch, K.; Gazie, H.; Schulz-Bull, D.E.; Sokolova, I.M. Biomarker-based assessment of sublethal toxicity of organic UV filters (ensulizole and octocrylene) in a sentinel marine bivalve Mytilus edulis. Sci. Total Environ. 2021, 798, 149171. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, L.; Wu, S.; Lu, L.; Xu, Y.; Zhu, Y.; Guo, M.; Zhuang, S. Recent advances on endocrine disrupting effects of UV filters. Int. J. Environ. Res. Public Health 2016, 13, 782. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Ma, X.Y.; Wang, X.C.; Ngo, H.H. Assessment of multiple hormone activities of a UV-filter (octocrylene) in zebrafish (Danio rerio). Chemosphere 2016, 159, 433–441. [Google Scholar] [CrossRef]
- Yan, S.; Liang, M.; Chen, R.; Hong, X.; Zha, J. Reproductive toxicity and estrogen activity in Japanese medaka (Oryzias latipes) exposed to environmentally relevant concentrations of octocrylene. Environ. Pollut. 2020, 261, 114104. [Google Scholar] [CrossRef]
- European Commission. COMMISSION REGULATION (EU) 2022/1176 Amending Regulation (EC) No 1223/2009 of the European Parliament and of the Council as Regards the Use of Certain UV Filters in Cosmetic Products; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Downs, C.A.; Dinardo, J.C.; Stien, D.; Rodrigues, A.M.S.; Lebaron, P. Benzophenone Accumulates over Time from the Degradation of Octocrylene in Commercial Sunscreen Products. Chem. Res. Toxicol. 2021, 34, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Foubert, K.; Dendooven, E.; Theunis, M.; Naessens, T.; Ivanova, B.; Pieters, L.; Gilissen, L.; Huygens, S.; De Borggraeve, W.; Lambert, J.; et al. The presence of benzophenone in sunscreens and cosmetics containing the organic UV filter octocrylene: A laboratory study. Contact Dermat. 2021, 85, 69–77. [Google Scholar] [CrossRef]
- Medici, A.; Saviano, L.; Siciliano, A.; Libralato, G.; Guida, M.; Previtera, L.; Di Fabio, G.; Zarrelli, A. Octocrylene: From Sunscreens to the Degradation Pathway during Chlorination Processes: Formation of Byproducts and Their Ecotoxicity Assessment. Molecules 2022, 27, 5286. [Google Scholar] [CrossRef]
- Canada Gazette. Canada Gazette, Part I: Order Adding a Toxic Substance to Schedule 1 to the Canadian Environmental Protection Act, 1999; Government of Canada: Ottawa, ON, Canada, 2022; Volume 156, p. 14. [Google Scholar]
- IARC (International Agency for Research on Cancer). Some chemicals present in industrial and consumer products, food and drinking-water. IARC Monogr. 2013, 101, 9. [Google Scholar]
- Alvarez, D.A.; Maruya, K.A.; Dodder, N.G.; Lao, W.; Furlong, E.T.; Smalling, K.L. Occurrence of contaminants of emerging concern along the California coast (2009–10) using passive sampling devices. Mar. Pollut. Bull. 2014, 81, 347–354. [Google Scholar] [CrossRef]
- Shetty, N.; Schalka, S.; Lim, H.W.; Mohammad, T.F. The effects of UV filters on health and the environment. Photochem. Photobiol. Sci. 2023, 22, 2463–2471. [Google Scholar] [CrossRef]
- Carve, M.; Nugegoda, D.; Allinson, G.; Shimeta, J. A systematic review and ecological risk assessment for organic ultraviolet filters in aquatic environments. Environ. Pollut. 2021, 268, 115894. [Google Scholar] [CrossRef] [PubMed]
- Scheele, A.; Sutter, K.; Karatum, O.; Danley-Thomson, A.A.; Redfern, L.K. Environmental impacts of the ultraviolet filter oxybenzone. Sci. Total Environ. 2023, 863, 160966. [Google Scholar] [CrossRef]
- Lozano, C.; Lee, C.; Wattiez, R.; Lebaron, P.; Matallana-Surget, S. Unraveling the molecular effects of oxybenzone on the proteome of an environmentally relevant marine bacterium. Sci. Total Environ. 2021, 793, 148431. [Google Scholar] [CrossRef]
- Morgan, M.B.; Ross, J.; Ellwanger, J.; Phrommala, R.M.; Youngblood, H.; Qualley, D.; Williams, J. Sea Anemones Responding to Sex Hormones, Oxybenzone, and Benzyl Butyl Phthalate: Transcriptional Profiling and in Silico Modelling Provide Clues to Decipher Endocrine Disruption in Cnidarians. Front. Genet. 2022, 12, 793306. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens Cause Coral Bleaching by Promoting Viral Infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Cocci, P.; Mosconi, G.; Palermo, F.A. Organic UV Filters Induce Toll-like-Receptors and Related Signaling Pathways in Peripheral Blood Mononuclear Cells of Juvenile Loggerhead Sea Turtles (Caretta caretta). Animals 2022, 12, 594. [Google Scholar] [CrossRef]
- Downs, C.A.; Bishop, E.; Diaz-Cruz, M.S.; Haghshenas, S.A.; Stien, D.; Rodrigues, A.M.S.; Woodley, C.M.; Sunyer-Caldú, A.; Doust, S.N.; Espero, W.; et al. Oxybenzone contamination from sunscreen pollution and its ecological threat to Hanauma Bay, Oahu, Hawaii, U.S.A. Chemosphere 2022, 291, 132880. [Google Scholar] [CrossRef] [PubMed]
- Gamain, P.; Gonzalez, P.; Cachot, J.; Pardon, P.; Tapie, N.; Gourves, P.Y.; Budzinski, H.; Morin, B. Combined effects of pollutants and salinity on embryo-larval development of the Pacific oyster, Crassostrea gigas. Mar. Environ. Res. 2016, 113, 31–38. [Google Scholar] [CrossRef]
- OECD—Organisation for Economic Co-operation and Development. Detailed Review Paper (DRP) on Molluscs Life-Cycle Toxicity Testing (Series on Testing and Assessment No. 121). OECD Guidel. Test. Chem. 2010, 121, 182. [Google Scholar]
- Leverett, D.; Thain, J. Oyster embryo-larval bioassay (revised). ICES Tech. Mar. Environ. Sci. 2013, 54, 34. [Google Scholar]
- Boukadida, K.; Cachot, J.; Morin, B.; Clerandeau, C.; Banni, M. Moderate temperature elevation increase susceptibility of early-life stage of the Mediterranean mussel, Mytilus galloprovincialis to metal-induced genotoxicity. Sci. Total Environ. 2019, 663, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Gamain, P.; Roméro-Ramirez, A.; Gonzalez, P.; Mazzella, N.; Gourves, P.Y.; Compan, C.; Morin, B.; Cachot, J. Assessment of swimming behavior of the Pacific oyster D-larvae (Crassostrea gigas) following exposure to model pollutants. Environ. Sci. Pollut. Res. 2019, 27, 3675–3685. [Google Scholar] [CrossRef] [PubMed]
- Beiras, R.; His, E. Effects of dissolved mercury on embryogenesis, survival and growth of Mytilus galloprovincialis mussel larvae. Mar. Ecol. Prog. Ser. 1995, 126, 185–189. [Google Scholar] [CrossRef]
- Marçal, R.; Sousa, P.; Marques, A.; Pereira, V.; Guilherme, S.; Barreto, A.; Costas, B.; Rocha, R.J.M.; Pacheco, M. Exploring the Antioxidant and Genoprotective Potential of Salicornia ramosissima Incorporation in the Diet of the European Seabass (Dicentrarchus labrax). Animals 2024, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Rocha, T.; Qualhato, G.; Oliveira, L.; Amaral, C.; Conceição, E.; Sabóia-Morais, S.; Bailão, E. Acute exposure to environmentally relevant concentrations of benzophenone-3 induced genotoxicity in Poecilia reticulata. Aquat. Toxicol. 2019, 216, 105293. [Google Scholar] [CrossRef] [PubMed]
- Anido-Varela, L.; Seoane, M.; Esperanza, M.; Cid, Á.; Rioboo, C. Cytotoxicity of BP-3 and BP-4: Blockage of extrusion pumps, oxidative damage and programmed cell death on Chlamydomonas reinhardtii. Aquat. Toxicol. 2022, 251, 106285. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.; Chen, X.; Liu, X.; Li, N.; Nie, Y.; Lu, G. Comparison of developmental toxicity of benzophenone-3 and its metabolite benzophenone-8 in zebrafish. Aquat. Toxicol. 2023, 258, 106515. [Google Scholar] [CrossRef]
- Nogueira, D.J.; Mattos, J.J.; Dybas, P.R.; Flores-Nunes, F.; Sasaki, S.T.; Taniguchi, S.; Schmidt, É.C.; Bouzon, Z.L.; Bícego, M.C.; Melo, C.M.R.; et al. Effects of phenanthrene on early development of the Pacific oyster Crassostrea gigas (Thunberg, 1789). Aquat. Toxicol 2017, 191, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Pu, F.; Li, L.; You, W.; Ke, C.; Feng, D. Functional analysis of a tyrosinase gene involved in early larval shell biogenesis in Crassostrea angulata and its response to ocean acidification. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2017, 206, 8–15. [Google Scholar] [CrossRef]
- Miglioli, A.; Dumollard, R.; Balbi, T.; Besnardeau, L.; Canesi, L. Characterization of the main steps in first shell formation in Mytilus galloprovincialis: Possible role of tyrosinase. Proc. R. Soc. B Biol. Sci. 2019, 286, 20192043. [Google Scholar] [CrossRef]
- Huan, P.; Liu, G.; Wang, H.; Liu, B. Identification of a tyrosinase gene potentially involved in early larval shell biogenesis of the Pacific oyster Crassostrea gigas. Dev. Genes Evol. 2013, 223, 389–394. [Google Scholar] [CrossRef]
- Waldbusser, G.G.; Brunner, E.L.; Haley, B.A.; Hales, B.; Langdon, C.J.; Prahl, F.G. A developmental and energetic basis linking larval oyster shell formation to acidification sensitivity. Geophys. Res. Lett. 2013, 40, 2171–2176. [Google Scholar] [CrossRef]
- Lasota, R.; Gierszewska, K.; Viard, F.; Wolowicz, M.; Dobrzyn, K.; Comtet, T. Abnormalities in bivalve larvae from the Puck Bay (Gulf of Gdansk, southern Baltic Sea) as an indicator of environmental pollution. Mar. Pollut. Bull. 2018, 126, 363–371. [Google Scholar] [CrossRef]
- Ruszkiewicz, J.A.; Pinkas, A.; Ferrer, B.; Peres, T.V.; Tsatsakis, A.; Aschner, M. Neurotoxic effect of active ingredients in sunscreen products, a contemporary review. Toxicol. Rep. 2017, 4, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Beiras, R.; Widdows, J. Effect of the neurotransmitters dopamine, serotonin and norepinephrine on the ciliary activity of mussel (Mytilus edulis) larvae. Mar. Biol. 1995, 122, 597–603. [Google Scholar] [CrossRef]
- Thorel, E.; Clergeaud, F.; Rodrigues, A.M.S.; Lebaron, P.; Stien, D. A Comparative Metabolomics Approach Demonstrates That Octocrylene Accumulates in Stylophora pistillata Tissues as Derivatives and That Octocrylene Exposure Induces Mitochondrial Dysfunction and Cell Senescence. Chem. Res. Toxicol. 2022, 35, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Mouneyrac, C.; Amiard-Triquet, C. Biomarkers of Ecological Relevance in Ecotoxicology. In Encyclopedia of Aquatic Ecotoxicology; Férard, J.-F., Blaise, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 221–236. ISBN 9789400757042. [Google Scholar]
- Rohr, J.R.; Salice, C.J.; Nisbet, R.M. The pros and cons of ecological risk assessment based on data from different levels of biological organization. Crit. Rev. Toxicol. 2016, 46, 756–784. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalhais, A.; Lippa, R.; Oliveira, I.B.; Di Lorenzo, G.; Mieiro, C.; Pacheco, M. Effects of the UV Filter Octocrylene and Its Degradation Product Benzophenone on Pacific Oyster (Magallana gigas) Larvae: A Call for Reassessment of Environmental Hazards. Toxics 2025, 13, 177. https://doi.org/10.3390/toxics13030177
Carvalhais A, Lippa R, Oliveira IB, Di Lorenzo G, Mieiro C, Pacheco M. Effects of the UV Filter Octocrylene and Its Degradation Product Benzophenone on Pacific Oyster (Magallana gigas) Larvae: A Call for Reassessment of Environmental Hazards. Toxics. 2025; 13(3):177. https://doi.org/10.3390/toxics13030177
Chicago/Turabian StyleCarvalhais, Ana, Romina Lippa, Isabel Benta Oliveira, Gaetano Di Lorenzo, Cláudia Mieiro, and Mário Pacheco. 2025. "Effects of the UV Filter Octocrylene and Its Degradation Product Benzophenone on Pacific Oyster (Magallana gigas) Larvae: A Call for Reassessment of Environmental Hazards" Toxics 13, no. 3: 177. https://doi.org/10.3390/toxics13030177
APA StyleCarvalhais, A., Lippa, R., Oliveira, I. B., Di Lorenzo, G., Mieiro, C., & Pacheco, M. (2025). Effects of the UV Filter Octocrylene and Its Degradation Product Benzophenone on Pacific Oyster (Magallana gigas) Larvae: A Call for Reassessment of Environmental Hazards. Toxics, 13(3), 177. https://doi.org/10.3390/toxics13030177









