Fipronil Triggers Immunotoxicity Through Reactive Oxygen Species-Driven Mitochondrial Apoptosis in Thymocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Animals
2.3. Protocol of Sub-Chronic Animal Experiment and Preparation of Thymocytes from Mice Thymus
2.4. RNA Isolation and Quantitative Polymerase Chain Reaction (qPCR)
2.5. Evaluation of Mitochondrial Function
2.6. Evaluation of Cytokines by Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Assessment of Apoptotic/Necrotic Indicators
2.8. Measurement of Mitochondrial Depolarization
2.9. Measurement of Intracellular Calcium
2.10. Detection of Glutathione (GSH) Activity
2.11. Quantification of Intracellular ROS Levels
2.12. Evaluation of Lipid Peroxidation (LPO)
2.13. Statistical Analysis
3. Results
3.1. Deregulation of BCL-2 Protein Family Gene Expression by FPN In Vivo, Ex Vivo, and In Vitro
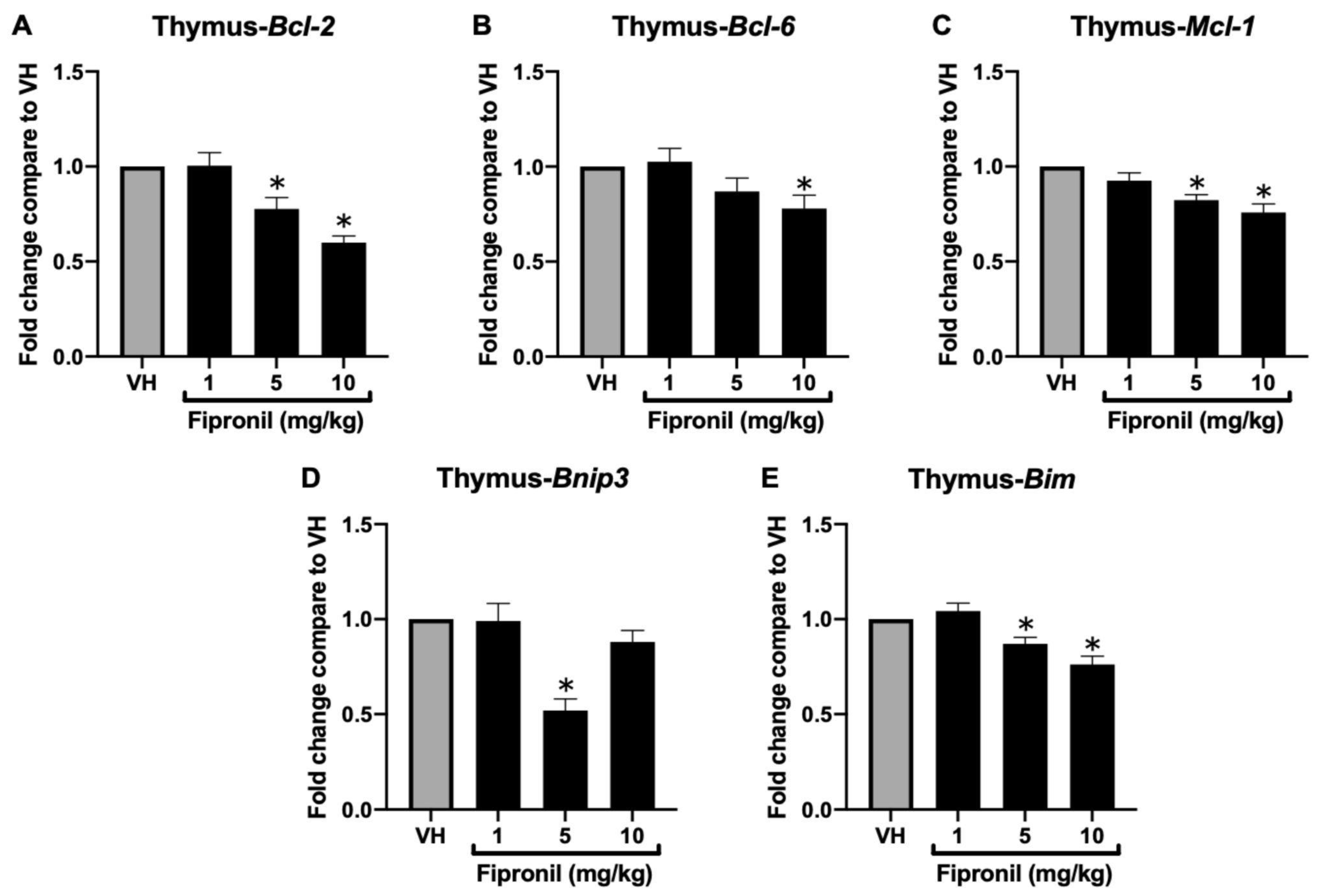
3.1.1. FPN Significantly Attenuated Bcl-2 Family mRNA Expression in the Thymus
3.1.2. FPN Significantly Attenuated Bcl-2 Family mRNA Expression in ConA-Stimulated Thymocytes Ex Vivo
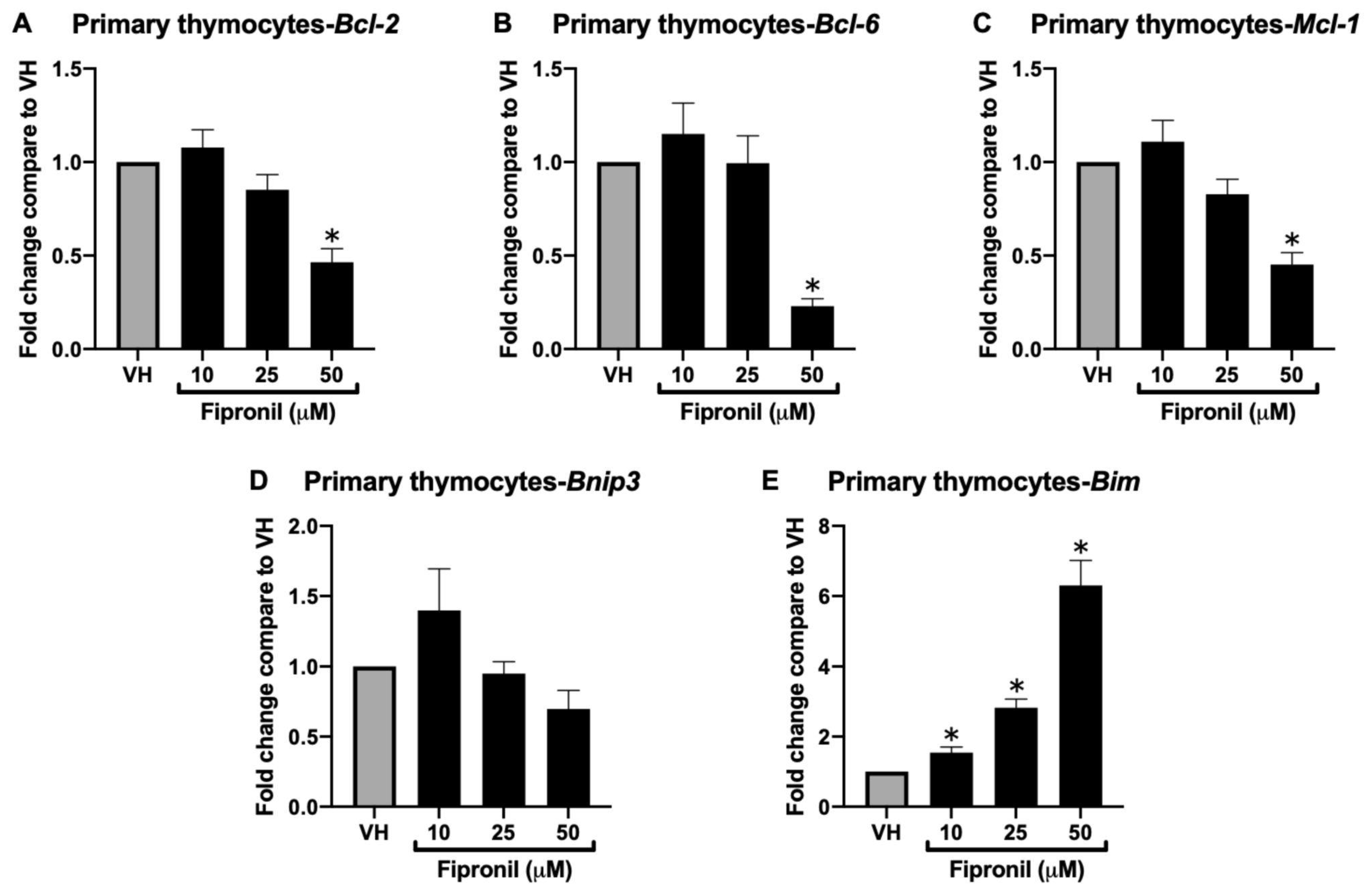
3.1.3. Acute Exposure of FPN Significantly Attenuated Anti-Apoptotic mRNA Expression in the Primary Thymocytes In Vitro
3.2. Cytotoxic and Apoptotic Effects by FPN in an Acute In Vitro Model
3.2.1. Cytotoxic and Immunosuppressive Effects of FPN on Primary Thymocytes
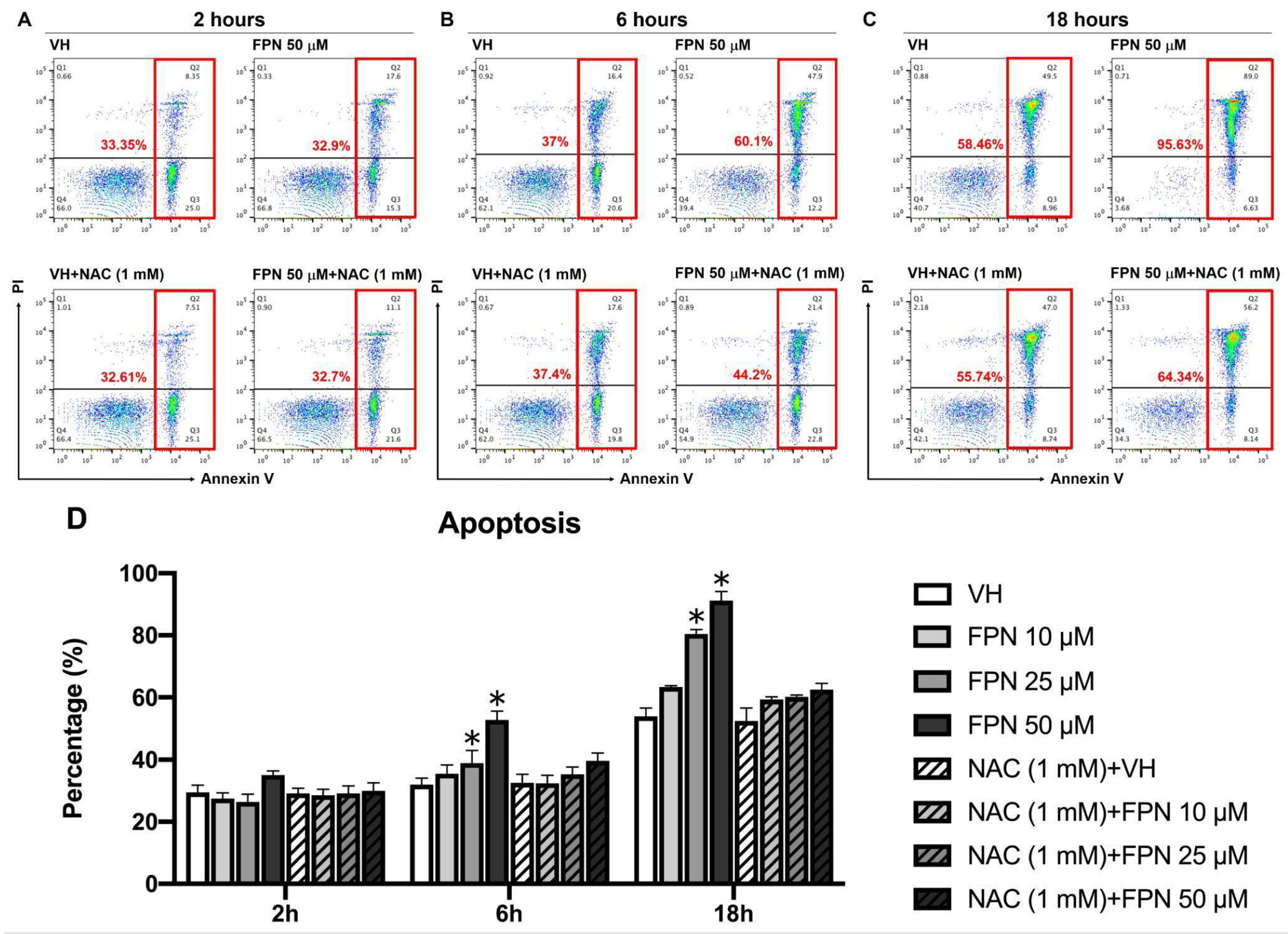
3.2.2. Effects of FPN Treatment on Apoptosis in the Primary Thymocytes In Vitro
3.3. Mitochondrial Dysfunction and Oxidative Stress Dysregulation Induced by FPN Exposure in an Acute In Vitro Model
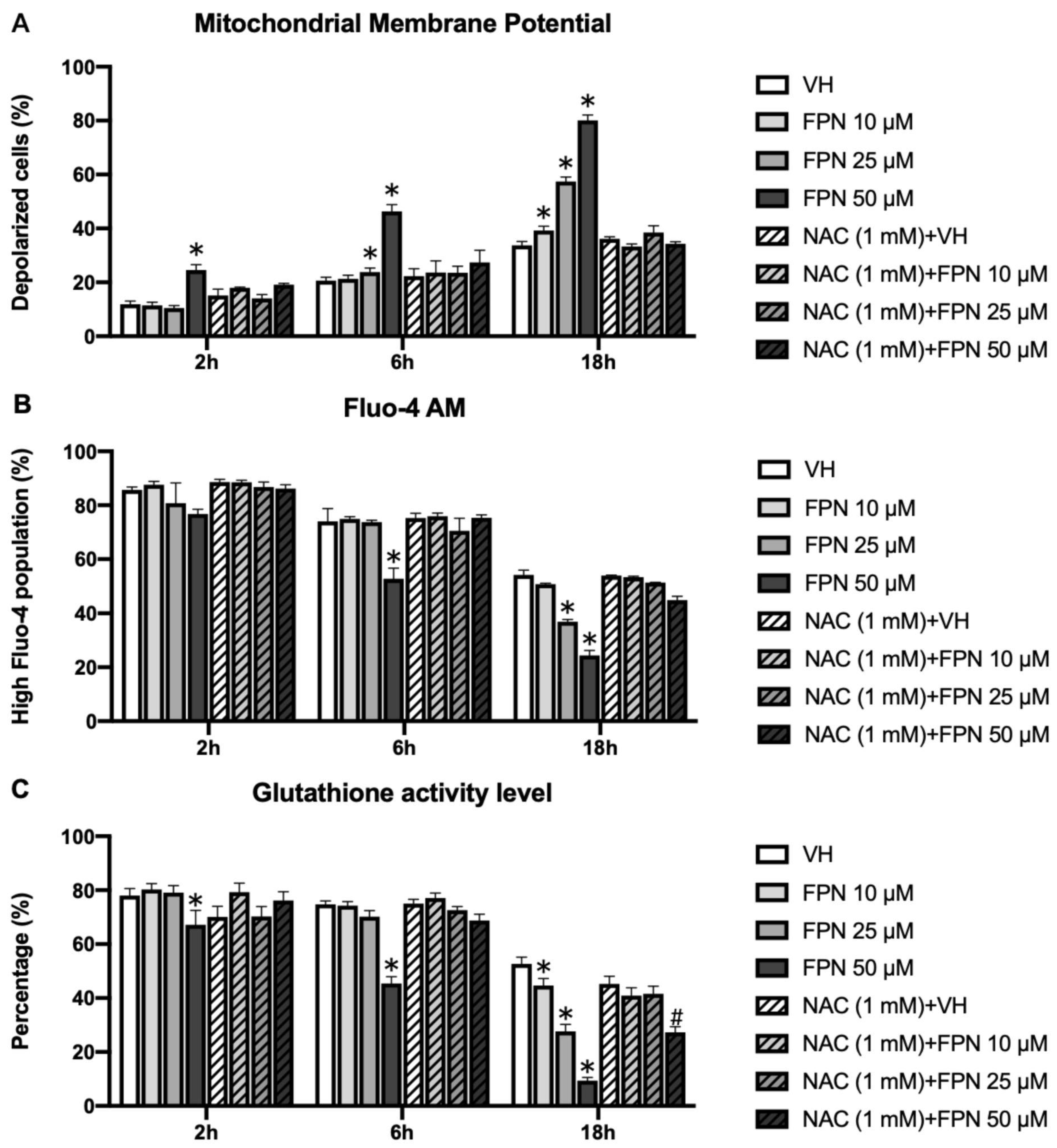
3.3.1. Induction of Mitochondrial Depolarization on Primary Thymocytes by Fipronil
3.3.2. Depletion of Intracellular Calcium on Primary Thymocytes by Fipronil
3.3.3. Reduction of Glutathione by Fipronil on Primary Thymocytes
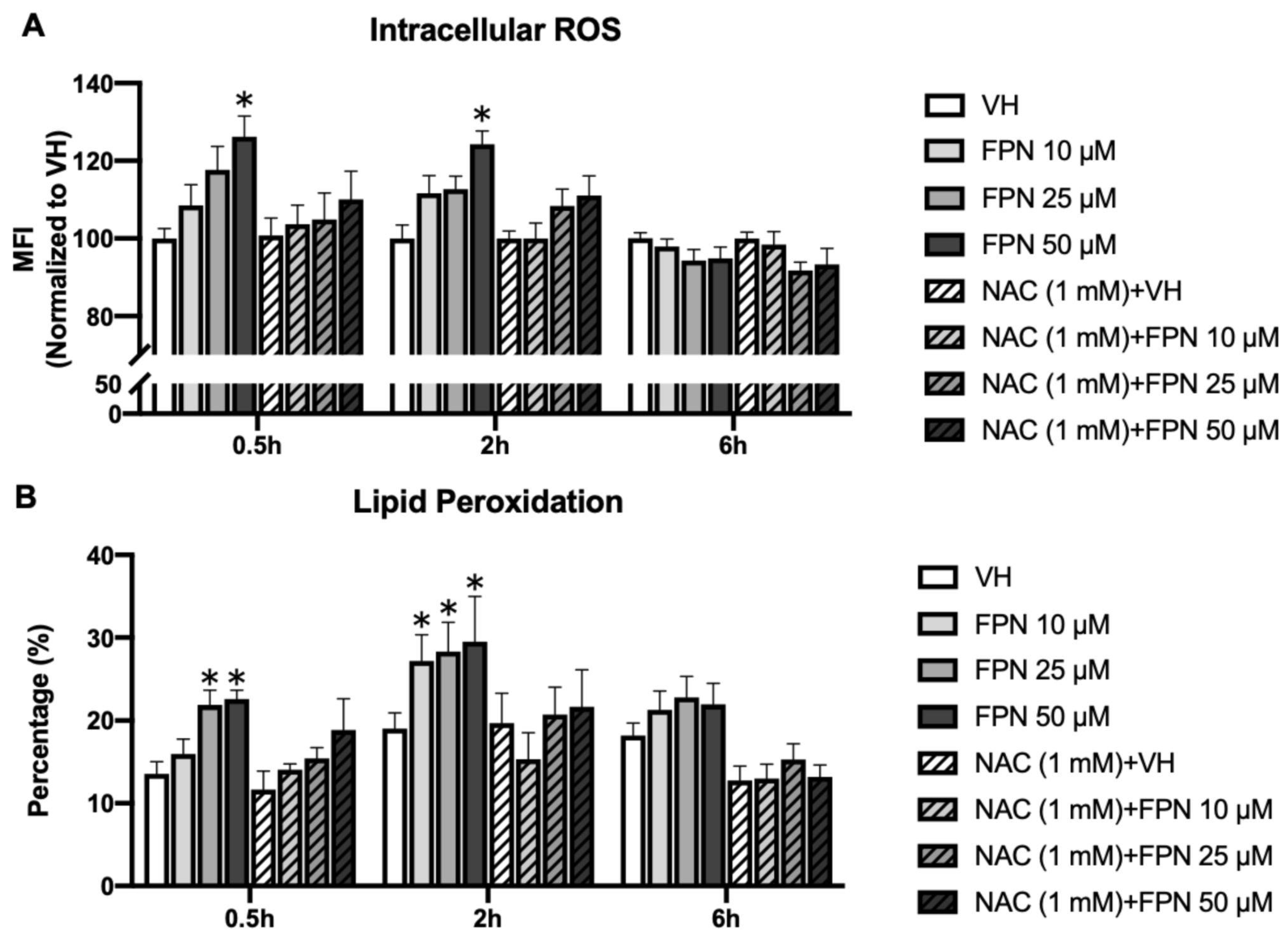
3.3.4. Accumulation of Intracellular ROS by Fipronil on Primary Thymocytes
3.3.5. Fipronil Exposure Elevates Lipid Peroxidation in Primary Thymocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FPN | Fipronil |
| ConA | Concanavalin A |
| PMA/Iono | Phorbol 12-myristate 13-acetate/Ionomycin |
| NAC | N-acetylcysteine |
| ROS | Reactive oxygen species |
| RNS | Reactive nitrogen species |
| MMP | Mitochondrial membrane potential |
| GSH | Glutathione |
| LPO | Lipid peroxidation |
References
- Kim, Y.A.; Yoon, Y.S.; Kim, H.S.; Jeon, S.J.; Cole, E.; Lee, J.; Kho, Y.; Cho, Y.H. Distribution of Fipronil in Humans, and Adverse Health Outcomes of in Utero Fipronil Sulfone Exposure in Newborns. Int. J. Hyg. Environ. Health 2019, 222, 524–532. [Google Scholar] [CrossRef]
- Mohamed, F.; Senarathna, L.; Percy, A.; Abeyewardene, M.; Eaglesham, G.; Cheng, R.; Azher, S.; Hittarage, A.; Dissanayake, W.; Sheriff, M.R.; et al. Acute Human Self-Poisoning with the N-Phenylpyrazole Insecticide Fipronil–A GABAA-Gated Chloride Channel Blocker. J. Toxicol. Clin. Toxicol. 2004, 42, 955–963. [Google Scholar] [CrossRef]
- Cam, M.; Durieu, E.; Bodin, M.; Manousopoulou, A.; Koslowski, S.; Vasylieva, N.; Barnych, B.; Hammock, B.D.; Bohl, B.; Koch, P.; et al. Induction of Amyloid-Β42 Production by Fipronil and Other Pyrazole Insecticides. J. Alzheimer’s Dis. 2018, 62, 1663–1681. [Google Scholar] [CrossRef] [PubMed]
- Environmental Fate and Toxicology of Fipronil. Available online: https://www.jstage.jst.go.jp/article/jpestics/32/3/32_3_189/_article?form=MG0AV3 (accessed on 24 January 2025).
- Stehr, C.M.; Linbo, T.L.; Incardona, J.P.; Scholz, N.L. The Developmental Neurotoxicity of Fipronil: Notochord Degeneration and Locomotor Defects in Zebrafish Embryos and Larvae. Toxicol. Sci. 2006, 92, 270–278. [Google Scholar] [CrossRef]
- Khan, S.; Jan, M.H.; Kumar, D.; Telang, A.G. Firpronil Induced Spermotoxicity Is Associated with Oxidative Stress, DNA Damage and Apoptosis in Male Rats. Pestic. Biochem. Physiol. 2015, 124, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, Z.; Ju, B.; Wang, Y.; Wang, J.; Bai, D. Apoptotic Effect of Organophosphorus Insecticide Chlorpyrifos on Mouse Retina in Vivo via Oxidative Stress and Protection of Combination of Vitamins C and E. Exp. Toxicol. Pathol. 2008, 59, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Mossa, A.-T.H.; Swelam, E.S.; Mohafrash, S.M.M. Sub-Chronic Exposure to Fipronil Induced Oxidative Stress, Biochemical and Histopathological Changes in the Liver and Kidney of Male Albino Rats. Toxicol. Rep. 2015, 2, 775–784. [Google Scholar] [CrossRef]
- Tingle, C.C.D.; Rother, J.A.; Dewhurst, C.F.; Lauer, S.; King, W.J. Fipronil: Environmental Fate, Ecotoxicology, and Human Health Concerns. In Reviews of Environmental Contamination and Toxicology: Continuation of Residue Reviews; Ware, G.W., Ed.; Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2003; pp. 1–66. ISBN 978-1-4899-7283-5. [Google Scholar]
- Wu, J.; Lu, J.; Lu, H.; Lin, Y.; Chris Wilson, P. Occurrence and Ecological Risks from Fipronil in Aquatic Environments Located within Residential Landscapes. Sci. Total Environ. 2015, 518–519, 139–147. [Google Scholar] [CrossRef]
- Ratra, G.S.; Casida, J.E. GABA Receptor Subunit Composition Relative to Insecticide Potency and Selectivity. Toxicol. Lett. 2001, 122, 215–222. [Google Scholar] [CrossRef]
- Cole, L.M.; Nicholson, R.A.; Casida, J.E. Action of Phenylpyrazole Insecticides at the GABA-Gated Chloride Channel. Pestic. Biochem. Physiol. 1993, 46, 47–54. [Google Scholar] [CrossRef]
- Kuo, J.-F.; Cheng, Y.-H.; Tung, C.-W.; Wang, C.-C. Fipronil Disturbs the Antigen-Specific Immune Responses and GABAergic Gene Expression in the Ovalbumin-Immunized BALB/c Mice. BMC Vet. Res. 2024, 20, 30. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.-F.; Wu, H.-Y.; Tung, C.-W.; Huang, W.-H.; Lin, C.-S.; Wang, C.-C. Induction of Thymus Atrophy and Disruption of Thymocyte Development by Fipronil through Dysregulation of IL-7-Associated Genes. Chem. Res. Toxicol. 2024, 37, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, Y.; Ayaki, T.; Urushitani, M.; Ito, H.; Takahashi, R. Activated Caspase-9 Immunoreactivity in Glial and Neuronal Cytoplasmic Inclusions in Multiple System Atrophy. Neurosci. Lett. 2016, 628, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Murali, A.K.; Mehrotra, S. Apoptosis—An Ubiquitous T Cell Immunomodulator. J. Clin. Cell Immunol. 2011, 2 (Suppl. S3), 1–10. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of Apoptosis by the BCL-2 Protein Family: Implications for Physiology and Therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Bano, F.; Mohanty, B. Thyroid Disrupting Pesticides Mancozeb and Fipronil in Mixture Caused Oxidative Damage and Genotoxicity in Lymphoid Organs of Mice. Environ. Toxicol. Pharmacol. 2020, 79, 103408. [Google Scholar] [CrossRef]
- Vidau, C.; González-Polo, R.A.; Niso-Santano, M.; Gómez-Sánchez, R.; Bravo-San Pedro, J.M.; Pizarro-Estrella, E.; Blasco, R.; Brunet, J.-L.; Belzunces, L.P.; Fuentes, J.M. Fipronil Is a Powerful Uncoupler of Oxidative Phosphorylation That Triggers Apoptosis in Human Neuronal Cell Line SHSY5Y. NeuroToxicology 2011, 32, 935–943. [Google Scholar] [CrossRef]
- Awad, M.A.; Ahmed, Z.S.O.; AbuBakr, H.O.; Elbargeesy, G.A.E.-F.H.; Moussa, M.H.G. Fipronil Induced Oxidative Stress in Neural Tissue of Albino Rat with Subsequent Apoptosis and Tissue Reactivity. Acta Histochem. 2021, 123, 151764. [Google Scholar] [CrossRef]
- Wang, X.; Martínez, M.A.; Wu, Q.; Ares, I.; Martínez-Larrañaga, M.R.; Anadón, A.; Yuan, Z. Fipronil Insecticide Toxicology: Oxidative Stress and Metabolism. Crit. Rev. Toxicol. 2016, 46, 876–899. [Google Scholar] [CrossRef]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress Versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef]
- Badgujar, P.C.; Chandratre, G.A.; Pawar, N.N.; Telang, A.G.; Kurade, N.P. Fipronil Induced Oxidative Stress Involves Alterations in SOD1 and Catalase Gene Expression in Male Mice Liver: Protection by Vitamins E and C. Environ. Toxicol. 2016, 31, 1147–1158. [Google Scholar] [CrossRef]
- Badgujar, P.C.; Pawar, N.N.; Chandratre, G.A.; Telang, A.G.; Sharma, A.K. Fipronil Induced Oxidative Stress in Kidney and Brain of Mice: Protective Effect of Vitamin E and Vitamin C. Pestic. Biochem. Physiol. 2015, 118, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Green, D.R. Metabolic Checkpoints in Activated T Cells. Nat. Immunol. 2012, 13, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L.; Pearce, E.J. Metabolic Pathways in Immune Cell Activation and Quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef]
- Sena, L.A.; Li, S.; Jairaman, A.; Prakriya, M.; Ezponda, T.; Hildeman, D.A.; Wang, C.-R.; Schumacker, P.T.; Licht, J.D.; Perlman, H.; et al. Mitochondria Are Required for Antigen-Specific T Cell Activation through Reactive Oxygen Species Signaling. Immunity 2013, 38, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-Y.; Lucavs, J.; Ballard, D.; Das, J.K.; Kumar, A.; Wang, L.; Ren, Y.; Xiong, X.; Song, J. Metabolic Reprogramming and Reactive Oxygen Species in T Cell Immunity. Front. Immunol. 2021, 12, 652687. [Google Scholar] [CrossRef]
- Das, P.C.; Cao, Y.; Cherrington, N.; Hodgson, E.; Rose, R.L. Fipronil Induces CYP Isoforms and Cytotoxicity in Human Hepatocytes. Chem. Biol. Interact. 2006, 164, 200–214. [Google Scholar] [CrossRef]
- Caballero, M.V.; Ares, I.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Fipronil Induces CYP Isoforms in Rats. Food Chem. Toxicol. 2015, 83, 215–221. [Google Scholar] [CrossRef]
- Aldayel, T.S.; Abdel-Rahman, H.G.; Gad EL-Hak, H.N.; Abdelrazek, H.M.A.; Mohamed, R.M.; El-Sayed, R.M. Assessment of Modulatory Activity of Uncaria Tomentosa Extract against Fipronil Immunotoxicity in Male Rats. Ecotoxicol. Environ. Saf. 2021, 224, 112674. [Google Scholar] [CrossRef]
- Chen, D.; Li, J.; Zhao, Y.; Wu, Y. Human Exposure of Fipronil Insecticide and the Associated Health Risk. J. Agric. Food Chem. 2022, 70, 63–71. [Google Scholar] [CrossRef]
- Zhang, N.; Hartig, H.; Dzhagalov, I.; Draper, D.; He, Y.W. The Role of Apoptosis in the Development and Function of T Lymphocytes. Cell Res. 2005, 15, 749–769. [Google Scholar] [CrossRef] [PubMed]
- Veis, D.J.; Sorenson, C.M.; Shutter, J.R.; Korsmeyer, S.J. Bcl-2-Deficient Mice Demonstrate Fulminant Lymphoid Apoptosis, Polycystic Kidneys, and Hypopigmented Hair. Cell 1993, 75, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Nakayama, K.; Negishi, I.; Kuida, K.; Sawa, H.; Loh, D.Y. Targeted Disruption of Bcl-2 Alpha Beta in Mice: Occurrence of Gray Hair, Polycystic Kidney Disease, and Lymphocytopenia. Proc. Natl. Acad. Sci. USA 1994, 91, 3700–3704. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, Y.; Nakayama, K.; Nakayama, K.; Tomita, T.; Isoda, M.; Loh, D.Y.; Nakauchi, H. Role of Bcl-2 in the Development of Lymphoid Cells from the Hematopoietic Stem Cell. Blood 1997, 89, 853–862. [Google Scholar] [CrossRef]
- Akashi, K.; Kondo, M.; von Freeden-Jeffry, U.; Murray, R.; Weissman, I.L. Bcl-2 Rescues T Lymphopoiesis in Interleukin-7 Receptor–Deficient Mice. Cell 1997, 89, 1033–1041. [Google Scholar] [CrossRef]
- Maraskovsky, E.; O’Reilly, L.A.; Teepe, M.; Corcoran, L.M.; Peschon, J.J.; Strasser, A. Bcl-2 Can Rescue T Lymphocyte Development in Interleukin-7 Receptor–Deficient Mice but Not in Mutant Rag-1−/− Mice. Cell 1997, 89, 1011–1019. [Google Scholar] [CrossRef]
- Opferman, J.T.; Letai, A.; Beard, C.; Sorcinelli, M.D.; Ong, C.C.; Korsmeyer, S.J. Development and Maintenance of B and T Lymphocytes Requires Antiapoptotic MCL-1. Nature 2003, 426, 671–676. [Google Scholar] [CrossRef]
- Opferman, J.T.; Iwasaki, H.; Ong, C.C.; Suh, H.; Mizuno, S.; Akashi, K.; Korsmeyer, S.J. Obligate Role of Anti-Apoptotic MCL-1 in the Survival of Hematopoietic Stem Cells. Science 2005, 307, 1101–1104. [Google Scholar] [CrossRef]
- Wang, J.; He, N.; Zhang, N.; Quan, D.; Zhang, S.; Zhang, C.; Yu, R.T.; Atkins, A.R.; Zhu, R.; Yang, C.; et al. NCoR1 Restrains Thymic Negative Selection by Repressing Bim Expression to Spare Thymocytes Undergoing Positive Selection. Nat. Commun. 2017, 8, 959. [Google Scholar] [CrossRef]
- Wan, J.; Martinvalet, D.; Ji, X.; Lois, C.; Kaech, S.M.; Von Andrian, U.H.; Lieberman, J.; Ahmed, R.; Manjunath, N. The Bcl-2 Family pro-Apoptotic Molecule, BNIP3 Regulates Activation-Induced Cell Death of Effector Cytotoxic T Lymphocytes. Immunology 2003, 110, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Rikka, S.; Quinsay, M.N.; Thomas, R.L.; Kubli, D.A.; Zhang, X.; Murphy, A.N.; Gustafsson, Å.B. Bnip3 Impairs Mitochondrial Bioenergetics and Stimulates Mitochondrial Turnover. Cell Death Differ. 2011, 18, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, Å.B. Bnip3 as a Dual Regulator of Mitochondrial Turnover and Cell Death in the Myocardium. Pediatr. Cardiol. 2011, 32, 267–274. [Google Scholar] [CrossRef]
- Fischer, S.F.; Bouillet, P.; O’Donnell, K.; Light, A.; Tarlinton, D.M.; Strasser, A. Proapoptotic BH3-Only Protein Bim Is Essential for Developmentally Programmed Death of Germinal Center-Derived Memory B Cells and Antibody-Forming Cells. Blood 2007, 110, 3978–3984. [Google Scholar] [CrossRef]
- Mérino, D.; Giam, M.; Hughes, P.D.; Siggs, O.M.; Heger, K.; O’Reilly, L.A.; Adams, J.M.; Strasser, A.; Lee, E.F.; Fairlie, W.D.; et al. The Role of BH3-Only Protein Bim Extends beyond Inhibiting Bcl-2–like Prosurvival Proteins. J. Cell Biol. 2009, 186, 355. [Google Scholar] [CrossRef] [PubMed]
- Shaki, F.; Hosseini, M.-J.; Ghazi-Khansari, M.; Pourahmad, J. Toxicity of Depleted Uranium on Isolated Rat Kidney Mitochondria. Biochim. Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 1940–1950. [Google Scholar] [CrossRef]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The Mitochondrial Membrane Potential (Δψm) in Apoptosis: An Update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Bhosale, G.; Sharpe, J.A.; Sundier, S.Y.; Duchen, M.R. Calcium Signaling as a Mediator of Cell Energy Demand and a Trigger to Cell Death. Ann. N. Y. Acad. Sci. 2015, 1350, 107–116. [Google Scholar] [CrossRef]
- Pathak, T.; Trebak, M. Mitochondrial Ca2+ Signaling. Pharmacol. Ther. 2018, 192, 112–123. [Google Scholar] [CrossRef]
- Aboul-Enein, F.; Rauschka, H.; Kornek, B.; Stadelmann, C.; Stefferl, A.; Brück, W.; Lucchinetti, C.; Schmidbauer, M.; Jellinger, K.; Lassmann, H. Preferential Loss of Myelin-Associated Glycoprotein Reflects Hypoxia-Like White Matter Damage in Stroke and Inflammatory Brain Diseases. J. Neuropathol. Exp. Neurol. 2003, 62, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ki, Y.-W.; Lee, J.E.; Park, J.H.; Shin, I.C.; Koh, H.C. Reactive Oxygen Species and Mitogen-Activated Protein Kinase Induce Apoptotic Death of SH-SY5Y Cells in Response to Fipronil. Toxicol. Lett. 2012, 211, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, Y.S.; Lee, J.-B.; Park, K.-H.; Paik, M.; Jeong, M.; Koh, H.C. Meloxicam Inhibits Fipronil-Induced Apoptosis via Modulation of the Oxidative Stress and Inflammatory Response in SH-SY5Y Cells. J. Appl. Toxicol. 2016, 36, 10–23. [Google Scholar] [CrossRef]
- Romero, A.; Ramos, E.; Ares, I.; Castellano, V.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Fipronil Sulfone Induced Higher Cytotoxicity than Fipronil in SH-SY5Y Cells: Protection by Antioxidants. Toxicol. Lett. 2016, 252, 42–49. [Google Scholar] [CrossRef]
- Yadav, A.; Mishra, P.C. Modeling the Activity of Glutathione as a Hydroxyl Radical Scavenger Considering Its Neutral Non-Zwitterionic Form. J. Mol. Model. 2012, 19, 767–777. [Google Scholar] [CrossRef]
- Aydin, B. Effects of Thiacloprid, Deltamethrin and Their Combination on Oxidative Stress in Lymphoid Organs, Polymorphonuclear Leukocytes and Plasma of Rats. Pestic. Biochem. Physiol. 2011, 100, 165–171. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhong, S.; Zhang, H.; Wang, X.; Qi, P.; Xu, H. Oxidative Injury Is Involved in Fipronil-Induced G2/M Phase Arrest and Apoptosis in Spodoptera frugiperda (Sf9) Cell Line. Pestic. Biochem. Physiol. 2013, 105, 122–130. [Google Scholar] [CrossRef]
- der Paal, J.V.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A. Effect of Lipid Peroxidation on Membrane Permeability of Cancer and Normal Cells Subjected to Oxidative Stress. Chem. Sci. 2015, 7, 489–498. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, M.; Li, H.; Wei, T.; Tang, C.; Zhou, Y.; Long, X. Effects of Low-Level Lipid Peroxidation on the Permeability of Nitroaromatic Molecules across a Membrane: A Computational Study. ACS Omega 2020, 5, 4798–4806. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, J.; Luo, Z.; Li, Y.; Huang, Y. Emerging Mechanisms of Lipid Peroxidation in Regulated Cell Death and Its Physiological Implications. Cell Death Dis. 2024, 15, 859. [Google Scholar] [CrossRef]



| Gene Name | Primers (5′ to 3′) |
|---|---|
| Bcl-2 | F: CCTGTGGATGACTGAGTACCTG R: AGCCAGGAGAAATCAAACAGAGG |
| Mcl-1 | F: AGCTTCATCGAACCATTAGCAGAA R: CCTTCTAGGTCCTGTACGTGGA |
| Bcl-6 | F: CAGAGATGTGCCTCCATACTGC R: CTCCTCAGAGAAACGGCAGTCA |
| Bnip3 | F: GCTCCAAGAGTTCTCACTGTGAC R: GTTTTTCTCGCCAAAGCTGTGGC |
| Bim | F: GGAGATACGGATTGCACAGGAG R: CTCCATACCAGACGGAAGATAAAG |
| Hprt | F: TCAGTCAACGGGGGACATAAA R: GGGGCTGTACTGCTTAACCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, J.-F.; Hsiao, Y.-P.; Wang, Y.-D.; Weng, H.-P.; Wang, C.-C. Fipronil Triggers Immunotoxicity Through Reactive Oxygen Species-Driven Mitochondrial Apoptosis in Thymocytes. Toxics 2025, 13, 204. https://doi.org/10.3390/toxics13030204
Kuo J-F, Hsiao Y-P, Wang Y-D, Weng H-P, Wang C-C. Fipronil Triggers Immunotoxicity Through Reactive Oxygen Species-Driven Mitochondrial Apoptosis in Thymocytes. Toxics. 2025; 13(3):204. https://doi.org/10.3390/toxics13030204
Chicago/Turabian StyleKuo, Jui-Fang, Yai-Ping Hsiao, Yao-De Wang, Hsin-Pei Weng, and Chia-Chi Wang. 2025. "Fipronil Triggers Immunotoxicity Through Reactive Oxygen Species-Driven Mitochondrial Apoptosis in Thymocytes" Toxics 13, no. 3: 204. https://doi.org/10.3390/toxics13030204
APA StyleKuo, J.-F., Hsiao, Y.-P., Wang, Y.-D., Weng, H.-P., & Wang, C.-C. (2025). Fipronil Triggers Immunotoxicity Through Reactive Oxygen Species-Driven Mitochondrial Apoptosis in Thymocytes. Toxics, 13(3), 204. https://doi.org/10.3390/toxics13030204







